Dietary Fatty Acids and Host–Microbial Crosstalk in Neonatal Enteric Infection
Abstract
1. Introduction
2. Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
3. Microbial Programming of the Infant Immune System
4. Microbiome and Immune Development
5. Microbiota and Dietary Fatty Acids
6. Fatty Acids and Inflammation
7. Dietary Fatty Acids, the Microbiome and Enteric Disease in Infants
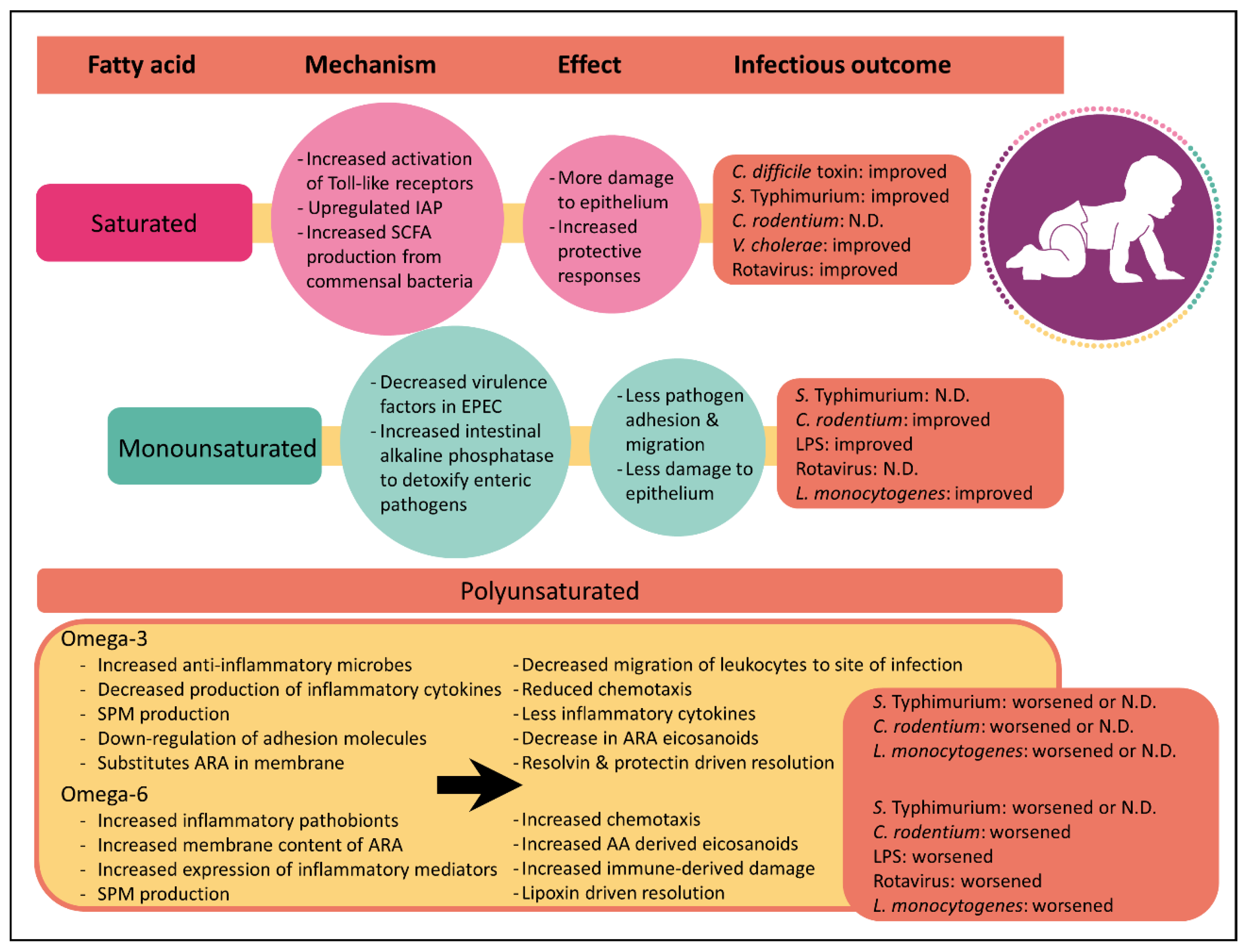
8. Saturated Fatty Acids
9. Monounsaturated Fatty Acids
10. Polyunsaturated Fatty Acids
11. Omega-6 Polyunsaturated Fatty Acids
12. Omega-3 Polyunsaturated Fatty Acids
13. Future Directions for Infant Formula
14. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Newburg, D.S. Bioactive components of human milk: Evolution, efficiency, and protection. Adv. Exp. Med. Biol. 2001, 501, 3–10. [Google Scholar] [PubMed]
- Grulee, C.; Sanford, H.; Herron, P. Breast and artificial feeding influence on morbidity and mortality of twenty thousand infants. JAMA 1934, 103, 735–739. [Google Scholar] [CrossRef]
- Fomon, S. Infant feeding in the 20th century: Formula and beikost. J. Nutr. 2001, 131, 409S–420S. [Google Scholar] [CrossRef] [PubMed]
- Stevens, E.E.; Patrick, T.E.; Pickler, R. A history of infant feeding. J. Perinat. Educ. 2009, 18, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Unicef. Infant and Young Child Feeding. Available online: https://data.unicef.org/topic/nutrition/infant-and-young-child-feeding/ (accessed on 22 August 2018).
- Quigley, M.A.; Kelly, Y.J.; Sacker, A. Breastfeeding and hospitalization for diarrheal and respiratory infection in the United Kingdom Millennium Cohort Study. Pediatrics 2007, 119, e837–e842. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.S.; Kakuma, R. The optimal duration of exclusive breastfeeding: A systematic review. Adv. Exp. Med. Biol. 2004, 554, 63–77. [Google Scholar] [PubMed]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Delplanque, B.; Gibson, R.; Koletzko, B.; Lapillonne, A.; Strandvik, B. Lipid Quality in Infant Nutrition: Current Knowledge and Future Opportunities. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 8–17. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef]
- DeCoffe, D.; Quin, C.; Gill, S.K.; Tasnim, N.; Brown, K.; Godovannyi, A.; Dai, C.; Abulizi, N.; Chan, Y.K.; Ghosh, S.; et al. Dietary Lipid Type, Rather Than Total Number of Calories, Alters Outcomes of Enteric Infection in Mice. J. Infect. Dis. 2016, 213, 1846–1856. [Google Scholar] [CrossRef]
- McNeil, N.I. The contribution of the large intestine to energy supplies in man. Am. J. Clin. Nutr. 1984, 39, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; Littman, D.R. Modulation of immune homeostasis by commensal bacteria. Curr. Opin. Microbiol. 2011, 14, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.L.; Shi, H.N.; Walker, W.A. The role of microbes in developmental immunologic programming. Pediatr. Res. 2011, 69, 465–472. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, S.; Hornef, M.W.; Chassin, C. Establishment of intestinal homeostasis during the neonatal period. Cell Mol. Life Sci. 2011, 68, 3699–3712. [Google Scholar] [CrossRef] [PubMed]
- Levy, O. Innate immunity of the newborn: Basic mechanisms and clinical correlates. Nat. Rev. Immunol. 2007, 7, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Bouskra, D.; Brezillon, C.; Berard, M.; Werts, C.; Varona, R.; Boneca, I.G.; Eberl, G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 2008, 456, 507–510. [Google Scholar] [CrossRef]
- Abrams, G.D.; Bauer, H.; Sprinz, H. Influence of the normal flora on mucosal morphology and cellular renewal in the ileum. A comparison of germ-free and conventional mice. Lab. Invest. 1963, 12, 355–364. [Google Scholar]
- Macpherson, A.J.; Harris, N.L. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 2004, 4, 478–485. [Google Scholar] [CrossRef]
- Umesaki, Y.; Setoyama, H.; Matsumoto, S.; Okada, Y. Expansion of alpha beta T-cell receptor-bearing intestinal intraepithelial lymphocytes after microbial colonization in germ-free mice and its independence from thymus. Immunology 1993, 79, 32–37. [Google Scholar]
- Crabbe, P.A.; Nash, D.R.; Bazin, H.; Eyssen, H.; Heremans, J.F. Immunohistochemical observations on lymphoid tissues from conventional and germ-free mice. Lab. Invest. 1970, 22, 448–457. [Google Scholar] [PubMed]
- Chung, H.; Pamp, S.J.; Hill, J.A.; Surana, N.K.; Edelman, S.M.; Troy, E.B.; Reading, N.C.; Villablanca, E.J.; Wang, S.; Mora, J.R.; et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell 2012, 149, 1578–1593. [Google Scholar] [CrossRef] [PubMed]
- El Aidy, S.; Hooiveld, G.; Tremaroli, V.; Backhed, F.; Kleerebezem, M. The gut microbiota and mucosal homeostasis: Colonized at birth or at adulthood, does it matter? Gut Microb. 2013, 4, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, E. Mucosal immunology: Any old bugs won’t do. Nat. Rev. Immunol. 2012, 12, 554–555. [Google Scholar] [CrossRef] [PubMed]
- Cash, H.L.; Whitham, C.V.; Behrendt, C.L.; Hooper, L.V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 2006, 313, 1126–1130. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Chen, C.T.; Gordon, J.I. Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host. PLoS Biol. 2006, 4, e413. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, E.M.; Poroyko, V.; Caplan, M.S.; Alverdy, J.; Morowitz, M.J.; Liu, D. Murine gut microbiota and transcriptome are diet dependent. Annal. Surg. 2013, 257, 287–294. [Google Scholar] [CrossRef]
- Fanaro, S.; Chierici, R.; Guerrini, P.; Vigi, V. Intestinal microflora in early infancy: Composition and development. Acta Paediatr. Suppl. 2003, 91, 48–55. [Google Scholar] [CrossRef]
- Donovan, S.M.; Wang, M.; Li, M.; Friedberg, I.; Schwartz, S.L.; Chapkin, R.S. Host-microbe interactions in the neonatal intestine: Role of human milk oligosaccharides. Adv. Nutr. 2012, 3, 450S–455S. [Google Scholar] [CrossRef]
- Harmsen, H.J.; Wildeboer-Veloo, A.C.; Raangs, G.C.; Wagendorp, A.A.; Klijn, N.; Bindels, J.G.; Welling, G.W. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 61–67. [Google Scholar] [CrossRef]
- Koenig, J.E.; Spor, A.; Scalfone, N.; Fricker, A.D.; Stombaugh, J.; Knight, R.; Angenent, L.T.; Ley, R.E. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA 2011, 108, 4578–4585. [Google Scholar] [CrossRef] [PubMed]
- Boesten, R.; Schuren, F.; Ben Amor, K.; Haarman, M.; Knol, J.; de Vos, W.M. Bifidobacterium population analysis in the infant gut by direct mapping of genomic hybridization patterns: Potential for monitoring temporal development and effects of dietary regimens. Microb. Biotechnol. 2011, 4, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Konya, T.; Maughan, H.; Guttman, D.S.; Field, C.J.; Chari, R.S.; Sears, M.R.; Becker, A.B.; Scott, J.A.; Kozyrskyj, A.L.; et al. Gut microbiota of healthy Canadian infants: Profiles by mode of delivery and infant diet at 4 months. CMAJ 2013, 185, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Mackie, R.I.; Sghir, A.; Gaskins, H.R. Developmental microbial ecology of the neonatal gastrointestinal tract. Am. J. Clin. Nutr. 1999, 69, 1035s–1045s. [Google Scholar] [CrossRef] [PubMed]
- Long, K.Z.; Wood, J.W.; Vasquez Gariby, E.; Weiss, K.M.; Mathewson, J.J.; de la Cabada, F.J.; DuPont, H.L.; Wilson, R.A. Proportional hazards analysis of diarrhea due to enterotoxigenic Escherichia coli and breast feeding in a cohort of urban Mexican children. Am. J. Epidemiol. 1994, 139, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.L.; Bauer, M.; Naylor, A.; Sutcliffe, E.; Clark, L. Increasing Breastfeeding Rates to Reduce Infant Illness at the Community Level. Pediatrics 1998, 101, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, M.; Pang, X.; Zhao, Y.; Wang, L.; Zhao, L. Structural resilience of the gut microbiota in adult mice under high-fat dietary perturbations. ISME J. 2012, 6, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill-Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009, 137, e1711–e1712. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R. Use of high-fat diets to study rodent obesity as a model of human obesity. Int. J. Obes. 2019. [Google Scholar] [CrossRef]
- Abulizi, N.; Quin, C.; Brown, K.; Chan, Y.K.; Gill, S.K.; Gibson, D.L. Gut mucosal proteins and bacteriome are shaped by the saturation index of dietary lipids. Nutrients 2019, 11, 418. [Google Scholar] [CrossRef]
- Ghosh, S.; DeCoffe, D.; Brown, K.; Rajendiran, E.; Estaki, M.; Dai, C.; Yip, A.; Gibson, D.L. Fish oil attenuates omega-6 polyunsaturated fatty acid-induced dysbiosis and infectious colitis but impairs LPS dephosphorylation activity causing sepsis. PLoS ONE 2013, 8, e55468. [Google Scholar] [CrossRef] [PubMed]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Le Huerou-Luron, I.; Bouzerzour, K.; Ferret-Bernard, S.; Menard, O.; Le Normand, L.; Perrier, C.; Le Bourgot, C.; Jardin, J.; Bourlieu, C.; Carton, T.; et al. A mixture of milk and vegetable lipids in infant formula changes gut digestion, mucosal immunity and microbiota composition in neonatal piglets. Eur. J. Nutr. 2018, 57, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Bhinder, G.; Allaire, J.M.; Garcia, C.; Lau, J.T.; Chan, J.M.; Ryz, N.R.; Bosman, E.S.; Graef, F.A.; Crowley, S.M.; Celiberto, L.S.; et al. Milk Fat Globule Membrane Supplementation in Formula Modulates the Neonatal Gut Microbiome and Normalizes Intestinal Development. Sci. Rep. 2017, 7, 45274. [Google Scholar] [CrossRef] [PubMed]
- Zavaleta, N.; Kvistgaard, A.S.; Graverholt, G.; Respicio, G.; Guija, H.; Valencia, N.; Lonnerdal, B. Efficacy of an MFGM-enriched complementary food in diarrhea, anemia, and micronutrient status in infants. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 561–568. [Google Scholar] [PubMed]
- Simopoulos, A.P. Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 1999, 70, 560S–569S. [Google Scholar] [CrossRef] [PubMed]
- Sham, H.P.; Walker, K.H.; Abdulnour, R.E.; Krishnamoorthy, N.; Douda, D.N.; Norris, P.C.; Barkas, I.; Benito-Figueroa, S.; Colby, J.K.; Serhan, C.N.; et al. 15-epi-Lipoxin A4, Resolvin D2, and Resolvin D3 Induce NF-kappaB Regulators in Bacterial Pneumonia. J. Immunol. 2018, 200, 2757–2766. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dalli, J.; Levy, B.D. Lipid mediators in the resolution of inflammation. Cold Spring Harb. Perspect. Biol. 2014, 7, a016311. [Google Scholar] [CrossRef]
- Caughey, G.E.; Mantzioris, E.; Gibson, R.A.; Cleland, L.G.; James, M.J. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am. J. Clin. Nutr. 1996, 63, 116–122. [Google Scholar] [CrossRef]
- Endres, S.; Ghorbani, R.; Kelley, V.E.; Georgilis, K.; Lonnemann, G.; van der Meer, J.W.; Cannon, J.G.; Rogers, T.S.; Klempner, M.S.; Weber, P.C.; et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N. Engl. J. Med. 1989, 320, 265–271. [Google Scholar] [CrossRef]
- Zhao, Y.; Joshi-Barve, S.; Barve, S.; Chen, L.H. Eicosapentaenoic acid prevents LPS-induced TNF-alpha expression by preventing NF-kappaB activation. J. Am. Coll. Nutr. 2004, 23, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.B.; Pedersen, J.O.; Ekelund, S.; Grunnet, N.; Jersild, C.; Dyerberg, J. Cod liver oil inhibits neutrophil and monocyte chemotaxis in healthy males. Atherosclerosis 1989, 77, 53–57. [Google Scholar] [CrossRef]
- Schmidt, E.B.; Varming, K.; Pedersen, J.O.; Lervang, H.H.; Grunnet, N.; Jersild, C.; Dyerberg, J. Long-term supplementation with n-3 fatty acids, II: Effect on neutrophil and monocyte chemotaxis. Scand. J. Clin. Lab. Invest. 1992, 52, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.I.; Benincaso, A.I.; Knoell, C.T.; Larkin, J.K.; Austen, K.F.; Robinson, D.R. Dietary omega-3 polyunsaturated fatty acids inhibit phosphoinositide formation and chemotaxis in neutrophils. J. Clin. Investig. 1993, 91, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.A.; Pinder, A.C.; Piper, Z.; Johnson, I.T.; Lund, E.K. Fish oil supplementation inhibits the expression of major histocompatibility complex class II molecules and adhesion molecules on human monocytes. Am. J. Clin. Nutr. 1996, 63, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Arita, M.; Yoshida, M.; Hong, S.; Tjonahen, E.; Glickman, J.N.; Petasis, N.A.; Blumberg, R.S.; Serhan, C.N. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc. Natl. Acad. Sci. USA 2005, 102, 7671–7676. [Google Scholar] [CrossRef]
- Bisicchia, E.; Sasso, V.; Catanzaro, G.; Leuti, A.; Besharat, Z.M.; Chiacchiarini, M.; Molinari, M.; Ferretti, E.; Viscomi, M.T.; Chiurchiu, V. Resolvin D1 Halts Remote Neuroinflammation and Improves Functional Recovery after Focal Brain Damage Via ALX/FPR2 Receptor-Regulated MicroRNAs. Mol. Neurobiol. 2018, 55, 6894–6905. [Google Scholar] [CrossRef]
- Lima-Garcia, J.F.; Dutra, R.C.; da Silva, K.; Motta, E.M.; Campos, M.M.; Calixto, J.B. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br. J. Pharmacol. 2011, 164, 278–293. [Google Scholar] [CrossRef]
- WHO. Causes of Child Mortality, by Country, 2000‒2010. Available online: http://www.who.int/gho/child_health/mortality/mortality_causes_text/en/ (accessed on 22 August 2018).
- WHO. Diarrhoeal Disease. Available online: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (accessed on 28 November 2018).
- Petri, W.A., Jr.; Miller, M.; Binder, H.J.; Levine, M.M.; Dillingham, R.; Guerrant, R.L. Enteric infections, diarrhea, and their impact on function and development. J. Clin. Investig. 2008, 118, 1277–1290. [Google Scholar] [CrossRef]
- WHO. Diarrhoea: Why Children Are Still Dying and What Can Be Done; UNICEF/WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Keusch, G.T.; Walker, C.F.; Das, J.K.; Horton, S.; Habte, D. Diarrheal Diseases, 3rd ed.; World Bank: Washington, DC, USA, 2016; Volume 2. [Google Scholar]
- Kosek, M.; Bern, C.; Guerrant, R.L. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull. World Health Organ. 2003, 81, 197–204. [Google Scholar] [PubMed]
- Lamberti, L.M.; Fischer Walker, C.L.; Noiman, A.; Victora, C.; Black, R.E. Breastfeeding and the risk for diarrhea morbidity and mortality. BMC Public Health 2011, 11, S15. [Google Scholar] [CrossRef]
- Mendonca, M.A.; Araujo, W.M.C.; Borgo, L.A.; Alencar, E.R. Lipid profile of different infant formulas for infants. PLoS ONE 2017, 12, e0177812. [Google Scholar] [CrossRef]
- Vallance, B.A.; Deng, W.; Jacobson, K.; Finlay, B.B. Host susceptibility to the attaching and effacing bacterial pathogen Citrobacter rodentium. Infect. Immun. 2003, 71, 3443–3453. [Google Scholar] [CrossRef]
- Bell, J.A.; St Charles, J.L.; Murphy, A.J.; Rathinam, V.A.; Plovanich-Jones, A.E.; Stanley, E.L.; Wolf, J.E.; Gettings, J.R.; Whittam, T.S.; Mansfield, L.S. Multiple factors interact to produce responses resembling spectrum of human disease in Campylobacter jejuni infected C57BL/6 IL-10-/- mice. BMC Microbiol. 2009, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Barthel, M.; Hapfelmeier, S.; Quintanilla-Martinez, L.; Kremer, M.; Rohde, M.; Hogardt, M.; Pfeffer, K.; Russmann, H.; Hardt, W.D. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 2003, 71, 2839–2858. [Google Scholar] [CrossRef] [PubMed]
- Barman, M.; Unold, D.; Shifley, K.; Amir, E.; Hung, K.; Bos, N.; Salzman, N. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect. Immun. 2008, 76, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Delplanque, B.; Qin, D.; Jean-Charles, M.; Philippe, G. Lipids for infant formulas. EDP Sci. 2018. [Google Scholar] [CrossRef]
- Fomon, S.J.; Filer, L.J.; Ziegler, E.E.; Bergmann, K.E.; Bergmann, R.L. Skim milk in infant feeding. Acta Paediatr. Scand. 1977, 66, 17–30. [Google Scholar] [CrossRef]
- Filler, L.; Barness, L.; Goldbloom, R.; Haworth, J.; Holliday, M.; Miller, R.; O’Brien, D.; Pearson, H.; Scriver, C.; Weil, W.; et al. American Academy of Pediatrics. Committee on Nutrition: Childhood diet and coronary heart disease. Pediatrics 1972, 49, 305–307. [Google Scholar]
- Koopman, J.S.; Turkisk, V.J.; Monto, A.S.; Thompson, F.E.; Isaacson, R.E. Milk fat and gastrointestinal illness. Am. J. Public. Health 1984, 74, 1371–1373. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G.; et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hougen, H.; Vollmer, A.C.; Hiebert, S.M. Gut bacteria profiles of Mus musculus at the phylum and family levels are influenced by saturation of dietary fatty acids. Anaerobe 2012, 18, 331–337. [Google Scholar] [CrossRef] [PubMed]
- De Wit, N.; Derrien, M.; Bosch-Vermeulen, H.; Oosterink, E.; Keshtkar, S.; Duval, C.; de Vogel-van den Bosch, J.; Kleerebezem, M.; Müller, M.; van der Meer, R. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am. J. Phys. Gastrointest. Liver Phys. 2012, 303, G589–G599. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Walters, W.A.; Xu, Z.; Knight, R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014, 588, 4223–4233. [Google Scholar] [CrossRef]
- Riva, A.; Borgo, F.; Lassandro, C.; Verduci, E.; Morace, G.; Borghi, E.; Berry, D. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Environ. Microbiol. 2017, 19, 95–105. [Google Scholar] [CrossRef]
- Simoes, C.D.; Maukonen, J.; Kaprio, J.; Rissanen, A.; Pietilainen, K.H. Habitual Dietary Intake Is Associated with Stool Microbiota Composition in Monozygotic Twins 1–3. J. Nutr. 2013, 143, 417–423. [Google Scholar] [CrossRef]
- WHO. Estimated Rotavirus Deaths for Children under 5 Years of Age: 2013, 215,000. Available online: https://www.who.int/immunization/monitoring_surveillance/burden/estimates/rotavirus/en/ (accessed on 28 November 2018).
- Thormar, H.; Isaacs, C.E.; Brown, H.R.; Barshatzky, M.R.; Pessolano, T. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 1987, 31, 27–31. [Google Scholar] [CrossRef]
- Sagher, F.A.; Dodge, J.A.; Simpson, D.H.; Evans, P. Kinetics of viral replication in experimental rotavirus infection: Effects of high dietary fat. J. Pediatr. Gastroenterol. Nutr. 1991, 13, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Munir, M. Infantile diarrhoea: Breast and bottle feeding compared with special reference to their clinical role. Paediatr. Indones. 1985, 25, 100–106. [Google Scholar] [PubMed]
- Estaki, M.; DeCoffe, D.; Gibson, D.L. Interplay between intestinal alkaline phosphatase, diet, gut microbes and immunity. World J. Gastroenterol. 2014, 20, 15650–15656. [Google Scholar] [CrossRef] [PubMed]
- Petschow, B.W.; Batema, R.P.; Talbott, R.D.; Ford, L.L. Impact of medium-chain monoglycerides on intestinal colonisation by Vibrio cholerae or enterotoxigenic Escherichia coli. J. Med. Microbiol. 1998, 47, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Christenson, J.C. Salmonella infections. Pediatr. Rev. 2013, 34, 375–383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tennant, S.M.; Levine, M.M. Live attenuated vaccines for invasive Salmonella infections. Vaccine 2015, 33, C36–C41. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.R.; Dulloo, A.G.; Vladoianu, I.R.; Piguet, P.F.; Arsenijevic, D.; Girardier, L.; Pechere, J.C. Fish oil decreases natural resistance of mice to infection with Salmonella typhimurium. Metabolism 1992, 41, 1–2. [Google Scholar] [CrossRef]
- Clouva-Molyvdas, P.; Peck, M.D.; Alexander, J.W. Short-term dietary lipid manipulation does not affect survival in two models of murine sepsis. JPEN J. Parenter. Enteral. Nutr. 1992, 16, 343–347. [Google Scholar] [CrossRef]
- Puri, P.; Rattan, A.; Bijlani, R.L.; Mahapatra, S.C.; Nath, I. Splenic and intestinal lymphocyte proliferation response in mice fed milk or yogurt and challenged with Salmonella typhimurium. Int. J. Food Sci. Nutr. 1996, 47, 391–398. [Google Scholar] [CrossRef]
- Endt, K.; Stecher, B.; Chaffron, S.; Slack, E.; Tchitchek, N.; Benecke, A.; Van Maele, L.; Sirard, J.C.; Mueller, A.J.; Heikenwalder, M.; et al. The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal Salmonella diarrhea. PLoS Pathog. 2010, 6, e1001097. [Google Scholar] [CrossRef]
- Leite, M.S.; Pacheco, P.; Gomes, R.N.; Guedes, A.T.; Castro-Faria-Neto, H.C.; Bozza, P.T.; Koatz, V.L. Mechanisms of increased survival after lipopolysaccharide-induced endotoxic shock in mice consuming olive oil-enriched diet. Shock 2005, 23, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Hekmatdoost, A.; Wu, X.; Morampudi, V.; Innis, S.M.; Jacobson, K. Dietary oils modify the host immune response and colonic tissue damage following Citrobacter rodentium infection in mice. Am. J. Phys. Gastrointest. Liver Phys. 2013, 304, G917–G928. [Google Scholar]
- De Giuseppe, R.; Roggi, C.; Cena, H. n-3 LC-PUFA supplementation: Effects on infant and maternal outcomes. Eur. J. Nutr. 2014, 53, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Noguera, M.F.; Calvache, J.A.; Bonfill Cosp, X.; Kotanidou, E.P.; Galli-Tsinopoulou, A. Supplementation with long chain polyunsaturated fatty acids (LCPUFA) to breastfeeding mothers for improving child growth and development. Cochrane Database Syst. Rev. 2015, 7, CD007901. [Google Scholar] [CrossRef] [PubMed]
- Simmer, K.; Patole, S.K.; Rao, S.C. Long-chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef]
- Quin, C.; Erland, B.M.; Loeppky, J.L.; Gibson, D.L. Omega-3 polyunsaturated fatty acid supplementation during the pre and post-natal period: A meta-analysis and systematic reivew of randomized and semi-randomized controlled trials. NJIM 2016, 5, 34–54. [Google Scholar] [CrossRef]
- Maattanen, P.; Lurz, E.; Botts, S.R.; Wu, R.Y.; Yeung, C.W.; Li, B.; Abiff, S.; Johnson-Henry, K.C.; Lepp, D.; Power, K.A.; et al. Ground flaxseed reverses protection of a reduced-fat diet against Citrobacter rodentium-induced colitis. Am. J. Phys. Gastrointest. Liver Phys. 2018, 315, G788–G798. [Google Scholar]
- Boisrame-Helms, J.; Meyer, G.; Degirmenci, S.E.; Burban, M.; Schini-Kerth, V.; Cynober, L.; De Bandt, J.P.; Hasselmann, M.; Meziani, F. "Immunonutrition" Has Failed to Improve Peritonitis-Induced Septic Shock in Rodents. PLoS ONE 2016, 11, e0147644. [Google Scholar] [CrossRef]
- Rubin, R.H.; Wilkinson, R.A.; Xu, L.; Robinson, D.R. Dietary marine lipid does not alter susceptibility of (NZBxNZW)F1 mice to pathogenic microorganisms. Prostaglandins 1989, 38, 251–262. [Google Scholar]
- Alder, J.D.; Meulbroek, J.; Jarvis, K.; Mitten, M.; Hutch, T., Sr.; Paige, L.; Shipkowitz, N.; Henningfield, M.F.; Clement, J. Enteral formula composition does not affect response to lethal infectious challenge in mice. J. Nutr. 1994, 124, 2156–2162. [Google Scholar] [CrossRef]
- Anderson, M.; Fritsche, K.L. (n-3) Fatty acids and infectious disease resistance. J. Nutr. 2002, 132, 3566–3576. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, K.L.; Shahbazian, L.M.; Feng, C.; Berg, J.N. Dietary fish oil reduces survival and impairs bacterial clearance in C3H/Hen mice challenged with Listeria monocytogenes. Clin. Sci. 1997, 92, 95–101. [Google Scholar] [CrossRef] [PubMed]
- De Pablo, M.A.; Puertollano, M.A.; Galvez, A.; Ortega, E.; Gaforio, J.J.; Alvarez de Cienfuegos, G. Determination of natural resistance of mice fed dietary lipids to experimental infection induced by Listeria monocytogenes. FEMS Immunol. Med. Microbiol. 2000, 27, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Olive, A.P.; Dudley, M.; Harari, Y.; Dudley, A.; Castro, G.A.; Lifschitz, C.H. Fish oil supplementation does not impair the gut immune response to Trichinella spiralis infection in rats. J. Pediatr. Gastroenterol. Nutr. 1999, 28, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Nielsen, D.S.; Lauritzen, L.; Jakobsen, M.; Michaelsen, K.F. Impact of diet on the intestinal microbiota in 10-month-old infants. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 613–618. [Google Scholar] [CrossRef]
- Lu, J.; Jilling, T.; Li, D.; Caplan, M.S. Polyunsaturated fatty acid supplementation alters proinflammatory gene expression and reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Pediatr. Res. 2007, 61, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, Y.; Okada, K.; Yamakawa, Y.; Ikuse, T.; Baba, Y.; Inage, E.; Fujii, T.; Izumi, H.; Oshida, K.; Nagata, S.; et al. omega-3 fatty acids attenuate mucosal inflammation in premature rat pups. J. Pediatr. Surg. 2011, 46, 489–495. [Google Scholar] [CrossRef]
- Caron, E.; Desseyn, J.L.; Sergent, L.; Bartke, N.; Husson, M.O.; Duhamel, A.; Gottrand, F. Impact of fish oils on the outcomes of a mouse model of acute Pseudomonas aeruginosa pulmonary infection. Br. J. Nutr. 2015, 113, 191–199. [Google Scholar] [CrossRef]
- Kremmyda, L.S.; Vlachava, M.; Noakes, P.S.; Diaper, N.D.; Miles, E.A.; Calder, P.C. Atopy risk in infants and children in relation to early exposure to fish, oily fish, or long-chain omega-3 fatty acids: A systematic review. Clin. Rev. Allergy Immunol. 2011, 41, 36–66. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Liu, B.; Li, J.; Luo, C.Q.; Zhang, Q.; Chen, J.L.; Sinha, A.; Li, Z.Y. Fish intake during pregnancy or infancy and allergic outcomes in children: A systematic review and meta-analysis. Pediatr. Allergy Immunol. 2017, 28, 152–161. [Google Scholar] [CrossRef]
- Chiang, N.; Fredman, G.; Backhed, F.; Oh, S.F.; Vickery, T.; Schmidt, B.A.; Serhan, C.N. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 2012, 484, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Kuba, K.; Ichikawa, A.; Nakayama, M.; Katahira, J.; Iwamoto, R.; Watanebe, T.; Sakabe, S.; Daidoji, T.; Nakamura, S.; et al. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell 2013, 153, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; de la Rosa, X.; Libreros, S.; Serhan, C.N. Novel Resolvin D2 Receptor Axis in Infectious Inflammation. J. Immunol. 2017, 198, 842–851. [Google Scholar] [CrossRef] [PubMed]
- WHO. Babies and Mothers Worldwide Failed by Lack of Investment in Breastfeeding. Available online: http://www.who.int/news-room/detail/01-08-2017-babies-and-mothers-worldwide-failed-by-lack-of-investment-in-breastfeeding (accessed on 28 November 2018).
- Sheela, A. Asia Pacific Baby Food Market- Global Indistry Analysis, Size, Share, Growth, Trends and Forecase 2013–2019. Available online: https://www.prweb.com/releases/2013/10/prweb11217817.htm (accessed on 28 November 2018).
- Bandy, L. Toddler Milk Formula: The Hello Kitty of Packaged Food; Eurominitor International: London, UK, 2014. [Google Scholar]
- Adams, C. Fonterra Spreads Infant Formula Sales in China; New Zealand Herald: Auckland, New Zealand, 2014. [Google Scholar]
- Quigley, M.A.; Carson, C.; Sacker, A.; Kelly, Y. Exclusive breastfeeding duration and infant infection. Eur. J. Clin. Nutr. 2016, 70, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- CODEX. Codex Alimentarius. International Food Standards; CODEX: Rome, Italy, 2016. [Google Scholar]
- Eur Union. Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 supplementing Regulation (EU) No 609/2013 of the European Parliament and the Council as regards the specific compositional and infoamtion requirements for infant formula and follow-on formula and as regards requiremnts on information relatign to infant and young child feeding. Off. J. Eur. Union 2015, 29, 35. [Google Scholar]
- Lim, S.X.; Toh, J.Y.; van Lee, L.; Han, W.M.; Shek, L.P.; Tan, K.H.; Yap, F.; Godfrey, K.M.; Chong, Y.S.; Chong, M.F. Food Sources of Energy and Macronutrient Intakes among Infants from 6 to 12 Months of Age: The Growing Up in Singapore Towards Healthy Outcomes (GUSTO) Study. Int. J. Environ. Res. Public Health 2018, 15, 448. [Google Scholar] [CrossRef] [PubMed]
- Gondolf, U.H.; Tetens, I.; Michaelsen, K.F.; Trolle, E. Dietary habits of partly breast-fed and completely weaned infants at 9 months of age. Public Health Nutr. 2012, 15, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Conn, J.A.; Davies, M.J.; Walker, R.B.; Moore, V.M. Food and nutrient intakes of 9-month-old infants in Adelaide, Australia. Public Health Nutr. 2009, 12, 2448–2456. [Google Scholar] [CrossRef] [PubMed]
- Gallier, S.; Vocking, K.; Post, J.A.; Van De Heijning, B.; Acton, D.; Van Der Beek, E.M.; Van Baalen, T. A novel infant milk formula concept: Mimicking the human milk fat globule structure. Colloids Surf. B. Biointerfaces 2015, 136, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Hageman, J.H.; Danielsen, M.; Nieuwenhuizen, A.G.; Feitsma, A.L.; Dalsgaard, T.K. Comparison of bovine milk fat and vegetable fat for infant formula: Implications for infant health. Int. Dairy J. 2019, 92, 37–49. [Google Scholar] [CrossRef]
- Claumarchirant, L.; Cilla, A.; Matencio, E.; Sanchez-Siles, L.M.; Castro-Gomez, P.; Fontecha, J.; Alegria, A.; Lagarda, M.J. Addition of milk fat globule membrane as an ingredient of infant formulas for resembling the polar lipids of human milk. Int. Dairy J. 2016, 61, 228–238. [Google Scholar] [CrossRef]
- Hamdan, I.J.A.; Sanchez-Siles, L.M.; Garcia-Llatas, G.; Lagarda, M.J. Sterols in Infant Formulas: A Bioaccessibility Study. J. Agric. Food Chem. 2018, 66, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Palmano, K.; Rowan, A.; Guillermo, R.; Guan, J.; McJarrow, P. The role of gangliosides in neurodevelopment. Nutrients 2015, 7, 3891–3913. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A. Role of Sphingolipids in Infant Gut Health and Immunity. J. Pediatr. 2016, 173, S53–S59. [Google Scholar] [CrossRef] [PubMed]
- Bar-Yoseph, F.; Lifshitz, Y.; Cohen, T.; Malard, P.; Xu, C. SN2-Palmitate Reduces Fatty Acid Excretion in Chinese Formula-fed Infants. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 341–347. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quin, C.; Gibson, D.L. Dietary Fatty Acids and Host–Microbial Crosstalk in Neonatal Enteric Infection. Nutrients 2019, 11, 2064. https://doi.org/10.3390/nu11092064
Quin C, Gibson DL. Dietary Fatty Acids and Host–Microbial Crosstalk in Neonatal Enteric Infection. Nutrients. 2019; 11(9):2064. https://doi.org/10.3390/nu11092064
Chicago/Turabian StyleQuin, Candice, and Deanna L. Gibson. 2019. "Dietary Fatty Acids and Host–Microbial Crosstalk in Neonatal Enteric Infection" Nutrients 11, no. 9: 2064. https://doi.org/10.3390/nu11092064
APA StyleQuin, C., & Gibson, D. L. (2019). Dietary Fatty Acids and Host–Microbial Crosstalk in Neonatal Enteric Infection. Nutrients, 11(9), 2064. https://doi.org/10.3390/nu11092064





