Acute Effects of Hesperidin in Oxidant/Antioxidant State Markers and Performance in Amateur Cyclists
Abstract
1. Introduction
2. Methodology
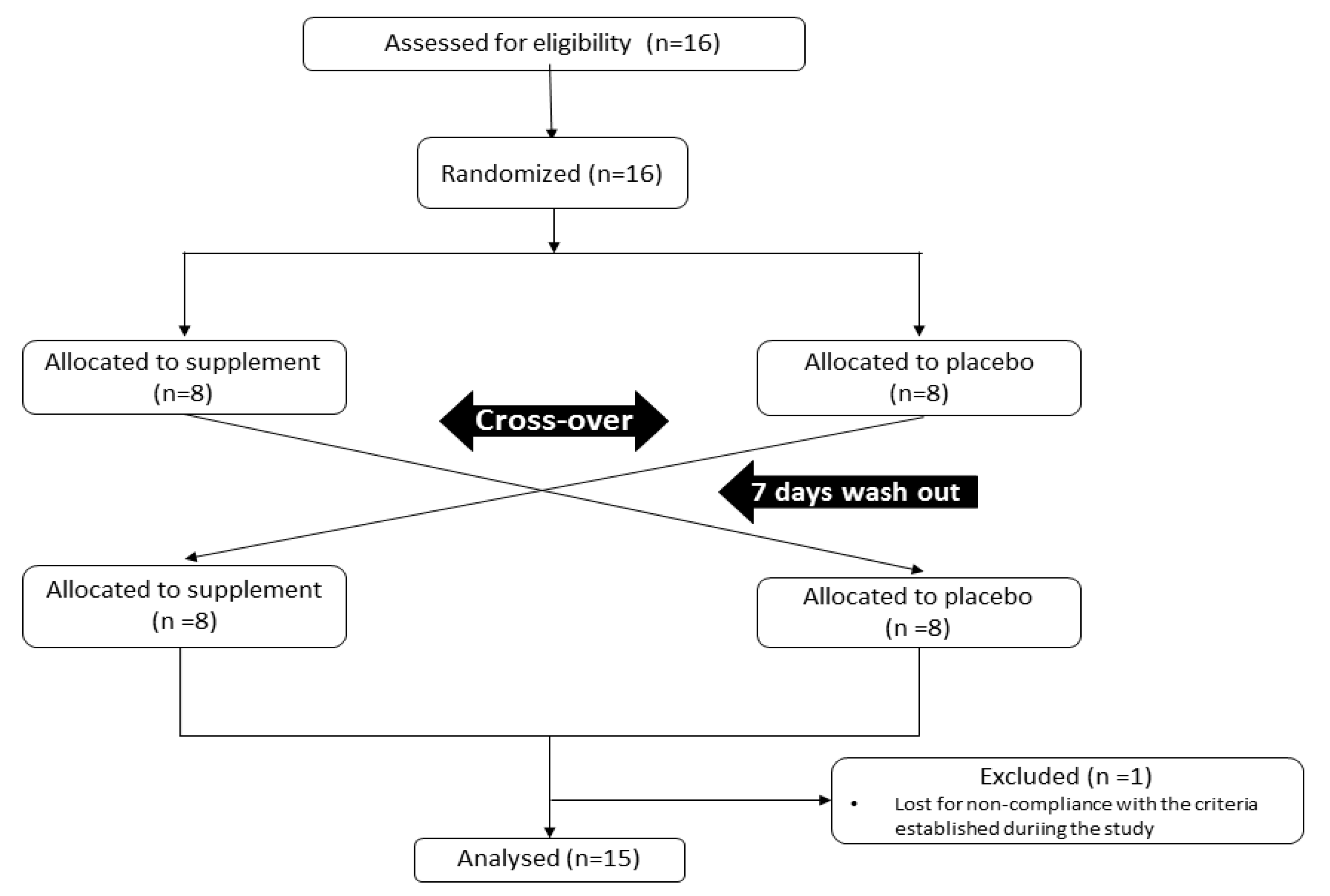
2.1. Experimental Design
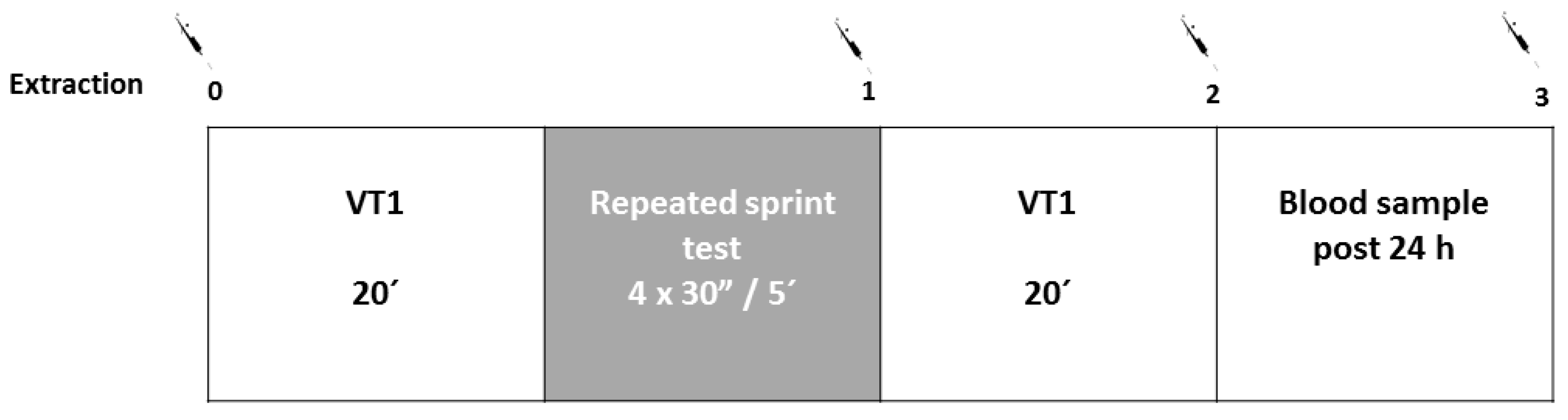
2.2. Procedures
2.3. Tests
2.3.1. Medical Exam
2.3.2. Anthropometry
2.3.3. Maximal Test
2.3.4. Rectangular Test
2.3.5. Repeated Sprints Test
2.3.6. Blood and Urine Analysis
2.3.7. Hesperidin Metabolites Urine
2.4. Markers of Oxidative Stress and Antioxidant Status
2.4.1. TBARS
2.4.2. Catalase
2.4.3. SOD
2.4.4. Glutathion
2.5. Statistical Analyses
3. Results
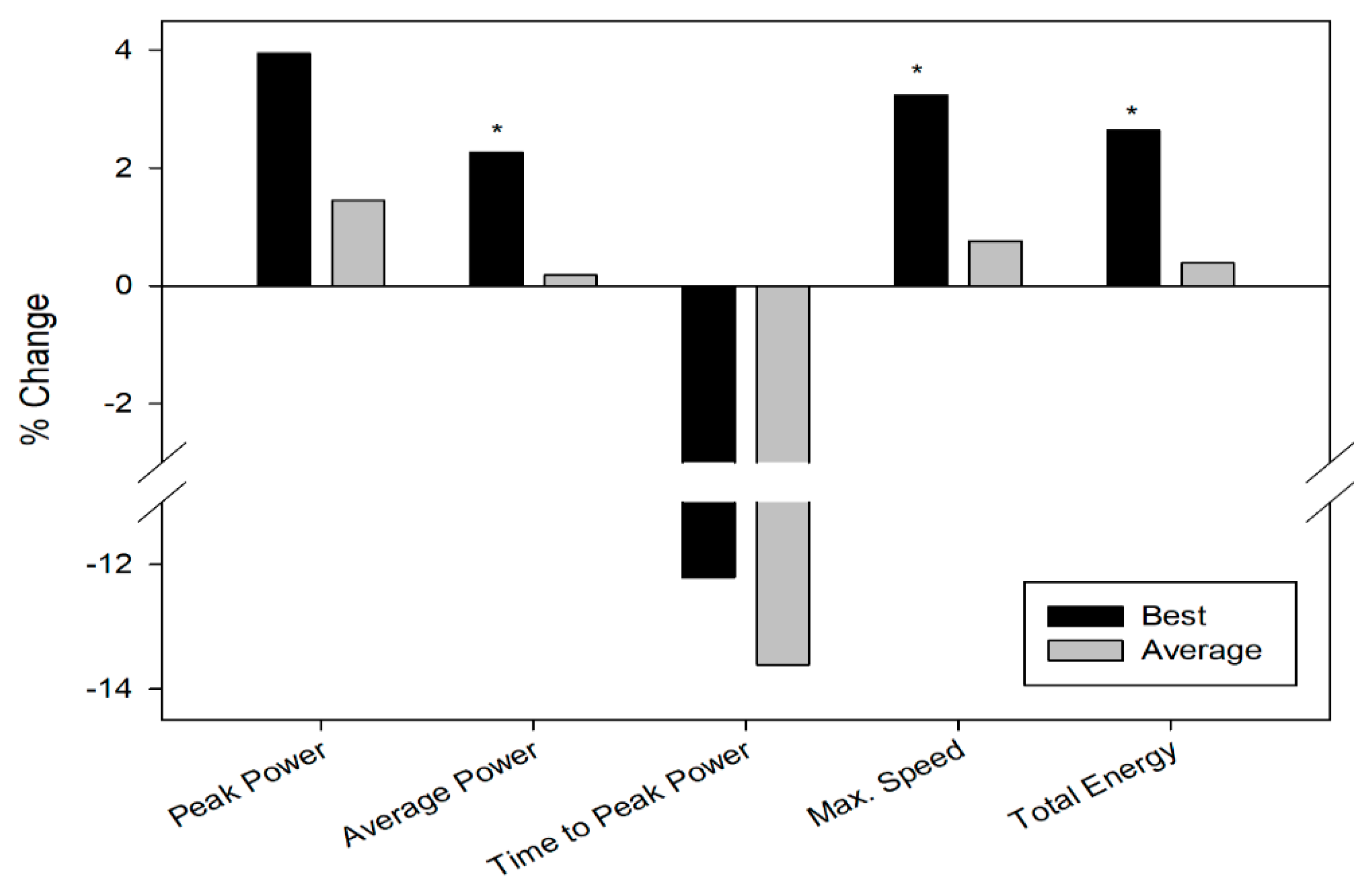
3.1. Repeated Sprint Test
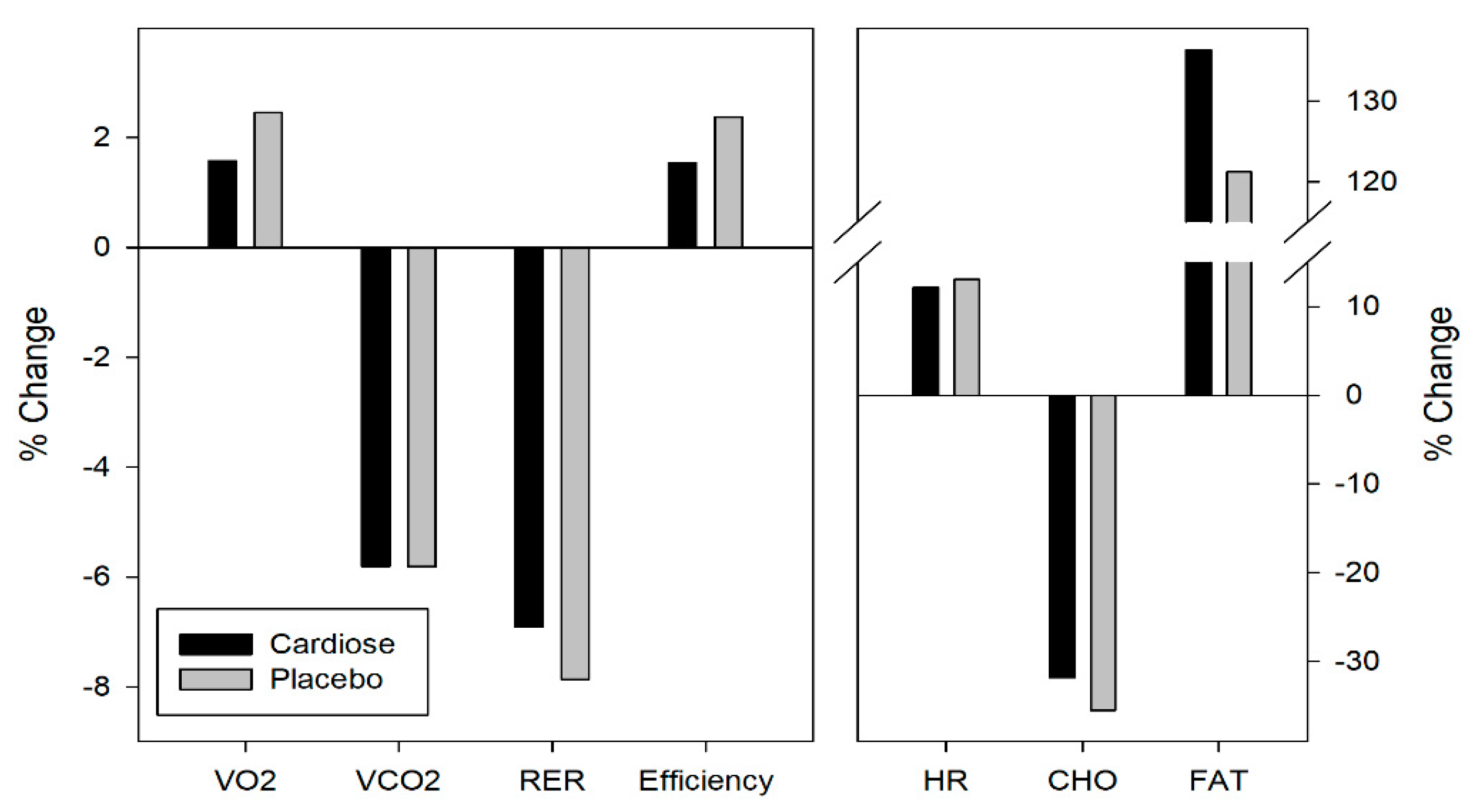
3.2. Metabolic Parameters
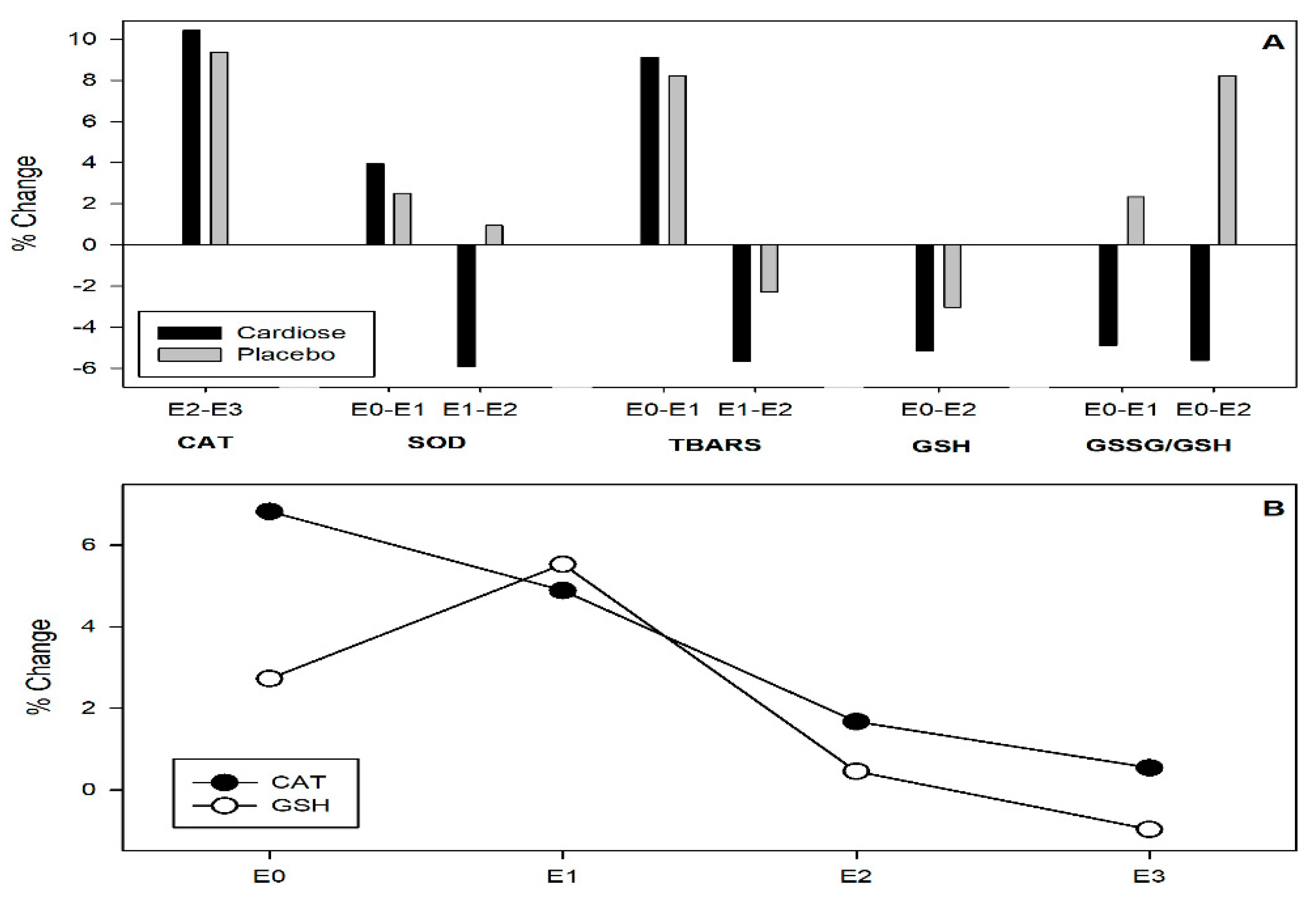
3.3. Antioxidant Parameters
3.4. Hesperidin Metabolites Urine
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pipe, A.; Ayotte, C. Nutritional supplements and doping. Clin. J. Sport Med. 2002, 12, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Greenhaff, P.L.; Hespel, P. Dietary supplements for athletes: emerging trends and recurring themes. J. Sports Sci. 2011, 29, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J. Nutritional ergogenic aids and exercise performance. Nutr. Res. Rev. 1999, 12, 255–280. [Google Scholar] [CrossRef] [PubMed]
- Garthe, I.; Maughan, R.J. Athletes and Supplements: Prevalence and Perspectives. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Froiland, K.; Koszewski, W.; Hingst, J.; Kopecky, L. Nutritional supplement use among college athletes and their sources of information. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 104–120. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Read, R.S.D. Dietary Supplements in Sport. J. Sports Sci. 1993, 15, 43–65. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.; Deakin, V. Clinical Sports Nutrition; McGraw-Hill: Sydney, Australia, 2010. [Google Scholar]
- Somerville, V.; Bringans, C.; Braakhuis, A. Polyphenols and performance: A systematic review and meta-analysis. J. Sports Sci. 2017, 47, 1589–1599. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Systematic analysis of the content of 502 polyphenols in 452 foods and beverages: an application of the phenol-explorer database. J. Agric. Food Chem. 2010, 58, 4959–4969. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Zarfeshany, A.; Asgary, S.; Javanmard, S.H. Potent health effects of pomegranate. Adv. Biomed. Res. 2014, 3, 100. [Google Scholar] [CrossRef] [PubMed]
- Cases, J.; Romain, C.; Marín-Pagán, C.; Chung, L.H.; Rubio-Pérez, J.M.; Laurent, C.; Gaillet, S.; Prost-Camus, E.; Prost, M.; Alcaraz, P.E. Supplementation with a polyphenol-rich extract, PerfLoad®, improves physical performance during high-intensity exercise: A randomized, double blind, crossover trial. Nutrients 2017, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Romain, C.; Freitas, T.T.; Martínez-Noguera, F.J.; Laurent, C.; Gaillet, S.; Chung, L.H.; Alcaraz, P.E.; Cases, J. Supplementation with a Polyphenol-Rich Extract, TensLess®, Attenuates Delayed Onset Muscle Soreness and Improves Muscle Recovery from Damages After Eccentric Exercise. Phytother. Res. 2017, 31, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Sumner, M.D.; Elliott-Eller, M.; Weidner, G.; Daubenmier, J.J.; Chew, M.H.; Marlin, R.; Raisin, C.J.; Ornish, D. Effects of pomegranate juice consumption on myocardial perfusion in patients with coronary heart disease. Am. J. Cardiol. 2005, 96, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Ganio, M.S.; Armstrong, L.E.; Johnson, E.C.; Klau, J.F.; Ballard, K.D.; Michniak-Kohn, B.; Kaushik, D.; Maresh, C.M. Effect of quercetin supplementation on maximal oxygen uptake in men and women. J. Sports Sci. 2010, 28, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Carlstedt, C.J.; Chen, S.; Carmichael, M.D.; Murphy, E.A. The dietary flavonoid quercetin increases VO2max and endurance capacity. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P. Flavonoids as antagonists at A1 adenosine receptors. Phytother. Res. 2006, 20, 1009–1012. [Google Scholar] [CrossRef]
- Peschek, K.; Pritchett, R.; Bergman, E.; Pritchett, K. The effects of acute post exercise consumption of two cocoa-based beverages with varying flavanol content on indices of muscle recovery following downhill treadmill running. Nutrients 2013, 6, 50–62. [Google Scholar] [CrossRef]
- Decroix, L.; Soares, D.D.; Meeusen, R.; Heyman, E.; Tonoli, C. Cocoa Flavanol Supplementation and Exercise: A Systematic Review. J. Sports Sci. 2018, 48, 867–892. [Google Scholar] [CrossRef]
- Cavarretta, E.; Peruzzi, M.; Del Vescovo, R.; Di Pilla, F.; Gobbi, G.; Serdoz, A.; Ferrara, R.; Schirone, L.; Sciarretta, S.; Nocella, C. Dark Chocolate Intake Positively Modulates Redox Status and Markers of Muscular Damage in Elite Football Athletes: A Randomized Controlled Study. Oxidative Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Stevenson, D.E. Polyphenols as Adaptogens—The Real Mechanism of the Antioxidant Effect? Infect. Immun. 2012, 60, 2828–2834. [Google Scholar]
- Vlavcheski, F.; Naimi, M.; Murphy, B.; Hudlicky, T.; Tsiani, E. Rosmarinic Acid, a Rosemary Extract Polyphenol, Increases Skeletal Muscle Cell Glucose Uptake and Activates AMPK. Molecules 2017, 22, 1669. [Google Scholar] [CrossRef] [PubMed]
- Svensson, K.; Schnyder, S.; Albert, V.; Cardel, B.; Quagliata, L.; Terracciano, L.M.; Handschin, C. Resveratrol and SRT1720 Elicit Differential Effects in Metabolic Organs and Modulate Systemic Parameters Independently of Skeletal Muscle Peroxisome Proliferator-activated Receptor γ Co-activator 1α (PGC-1α). J. Biol. Chem. 2015, 290, 16059–16076. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Tejada, S.; Bibiloni Mdel, M.; Tur, J.A.; Pons, A. Polyphenols: Well beyond the antioxidant capacity: Polyphenol supplementation and exercise-induced oxidative stress and inflammation. Curr. Pharm. Biotechnol. 2014, 15, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Malaguti, M.; Angeloni, C.; Hrelia, S. Polyphenols in exercise performance and prevention of exercise-induced muscle damage. Oxidative Med. Cell. Longev. 2013, 2013. [Google Scholar] [CrossRef]
- Vina, J.; Gimeno, A.; Sastre, J.; Desco, C.; Asensi, M.; Pallardó, F.V.; Cuesta, A.; Ferrero, J.A.; Terada, L.S.; Repine, J.E. Mechanism of free radical production in exhaustive exercise in humans and rats; role of xanthine oxidase and protection by allopurinol. IUBMB Life. 2000, 49, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Sastre, J.; Asensi, M.; Gasco, E.; Pallardo, F.V.; Ferrero, J.; Furukawa, T.; Vina, J. Exhaustive physical exercise causes oxidation of glutathione status in blood: prevention by antioxidant administration. Am. J. Physiol. 1992, 263, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Taylor, A.W.; Ohno, H.; Goto, S. Adaptation to exercise-induced oxidative stress: From muscle to brain. Exerc. Immunol. Rev. 2001, 7, 90–107. [Google Scholar] [PubMed]
- Mastaloudis, A.; Leonard, S.W.; Traber, M.G. Oxidative stress in athletes during extreme endurance exercise. Free Radicals Biol. Med. 2001, 31, 911–922. [Google Scholar] [CrossRef]
- Gelabert-Rebato, M.; Wiebe, J.C.; Martin-Rincon, M.; Galvan-Alvarez, V.; Curtelin, D.; Perez-Valera, M.; Habib, J.J.; Perez-Lopez, A.; Vega, T.; Morales-Alamo, D.; et al. Enhancement of Exercise Performance by 48 Hours, and 15-Day Supplementation with Mangiferin and Luteolin in Men. Nutrients 2019, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Overdevest, E.; Wouters, J.A.; Wolfs, K.H.; van Leeuwen, J.J.; Possemiers, S. Citrus Flavonoid Supplementation Improves Exercise Performance in Trained Athletes. J. Sport. Sci. Med. 2018, 17, 24. [Google Scholar]
- Tomás-Barberán, F.A.; Clifford, M.N. Flavanones, chalcones and dihydrochalcones—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1073–1080. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Yanez, J.A.; Andrews, P.K.; Davies, N.M. Methods of analysis and separation of chiral flavonoids. J. Chromatogr. B. 2007, 848, 159–181. [Google Scholar] [CrossRef]
- Aturki, Z.; Brandi, V.; Sinibaldi, M. Separation of flavanone-7-O-glycoside diastereomers and analysis in citrus juices by multidimensional liquid chromatography coupled with mass spectrometry. J. Agric. Food Chem. 2004, 52, 5303–5308. [Google Scholar] [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef]
- Tejada, S.; Pinya, S.; Martorell, M.; Capo, X.; Tur, J.A.; Pons, A.; Sureda, A. Potential Anti-inflammatory Effects of Hesperidin from the Genus Citrus. Curr. Med. Chem. 2018, 25, 4929–4945. [Google Scholar] [CrossRef]
- Li, C.; Schluesener, H. Health-promoting effects of the citrus flavanone hesperidin. Crit. Rev. Food Sci. Nutr. 2017, 57, 613–631. [Google Scholar] [CrossRef]
- Rizza, S.; Muniyappa, R.; Iantorno, M.; Kim, J.A.; Chen, H.; Pullikotil, P.; Senese, N.; Tesauro, M.; Lauro, D.; Cardillo, C.; et al. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J. Clin. Endocrinol. Metab. 2011, 96, 782–792. [Google Scholar] [CrossRef]
- Besco, R.; Sureda, A.; Tur, J.A.; Pons, A. The effect of nitric-oxide-related supplements on human performance. J. Sports Sci. 2012, 42, 99–117. [Google Scholar] [CrossRef]
- Schreuder, T.H.; Eijsvogels, T.M.; Greyling, A.; Draijer, R.; Hopman, M.T.; Thijssen, D.H. Effect of black tea consumption on brachial artery flow-mediated dilation and ischaemia-reperfusion in humans. Appl. Physiol., Nutr., Metab. 2014, 39, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Morand, C.; Dubray, C.; Milenkovic, D.; Lioger, D.; Martin, J.F.; Scalbert, A.; Mazur, A. Hesperidin contributes to the vascular protective effects of orange juice: a randomized crossover study in healthy volunteers. Am. J. Clin. Nutr. 2011, 93, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Engler, M.B.; Engler, M.M.; Chen, C.Y.; Malloy, M.J.; Browne, A.; Chiu, E.Y.; Kwak, H.K.; Milbury, P.; Paul, S.M.; Blumberg, J.; et al. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J. Am. Coll. Nutr. 2004, 23, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Yang, X.; Croft, K.D.; Considine, M.J.; Ward, N.C.; Rich, L.; Puddey, I.B.; Swinny, E.; Mubarak, A.; Hodgson, J.M. Flavonoid-rich apples and nitrate-rich spinach augment nitric oxide status and improve endothelial function in healthy men and women: A randomized controlled trial. Free Radical Biol. Med. 2012, 52, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Barona, J.; Aristizabal, J.C.; Blesso, C.N.; Volek, J.S.; Fernandez, M.L. Grape polyphenols reduce blood pressure and increase flow-mediated vasodilation in men with metabolic syndrome. J. Nutr. 2012, 142, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Salden, B.N.; Troost, F.J.; de Groot, E.; Stevens, Y.R.; Garces-Rimon, M.; Possemiers, S.; Winkens, B.; Masclee, A.A. Randomized clinical trial on the efficacy of hesperidin 2S on validated cardiovascular biomarkers in healthy overweight individuals. Am. J. Clin. Nutr. 2016, 104, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Biesemann, N.; Ried, J.S.; Ding-Pfennigdorff, D.; Dietrich, A.; Rudolph, C.; Hahn, S.; Hennerici, W.; Asbrand, C.; Leeuw, T.; Strubing, C. High throughput screening of mitochondrial bioenergetics in human differentiated myotubes identifies novel enhancers of muscle performance in aged mice. Sci. Rep. 2018, 8, 9408. [Google Scholar] [CrossRef] [PubMed]
- Pittaluga, M.; Sgadari, A.; Tavazzi, B.; Fantini, C.; Sabatini, S.; Ceci, R.; Amorini, A.M.; Parisi, P.; Caporossi, D. Exercise-induced oxidative stress in elderly subjects: the effect of red orange supplementation on the biochemical and cellular response to a single bout of intense physical activity. Free Radical Res. 2013, 47, 202–211. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.M.; Dourado, G.K.; Cesar, T.B. Hesperidin associated with continuous and interval swimming improved biochemical and oxidative biomarkers in rats. J. Int. Soc. Sports Nutr. 2013, 10, 27. [Google Scholar] [CrossRef]
- Aptekmann, N.P.; Cesar, T.B. Orange juice improved lipid profile and blood lactate of overweight middle-aged women subjected to aerobic training. Maturitas. 2010, 67, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Estruel-Amades, S.; Massot-Cladera, M.; Garcia-Cerda, P.; Perez-Cano, F.J.; Franch, A.; Castell, M.; Camps-Bossacoma, M. Protective Effect of Hesperidin on the Oxidative Stress Induced by an Exhausting Exercise in Intensively Trained Rats. Nutrients 2019, 11, 783. [Google Scholar] [CrossRef] [PubMed]
- MacRae, H.S.; Mefferd, K.M. Dietary antioxidant supplementation combined with quercetin improves cycling time trial performance. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.D.; Roberts, M.G.; Tarpey, M.D.; Weekes, J.C.; Thomas, C.H. The effect of a decaffeinated green tea extract formula on fat oxidation, body composition and exercise performance. J. Int. Soc. Sports Nutr. 2015, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Scribbans, T.D.; Ma, J.K.; Edgett, B.A.; Vorobej, K.A.; Mitchell, A.S.; Zelt, J.G.; Simpson, C.A.; Quadrilatero, J.; Gurd, B.J. Resveratrol supplementation does not augment performance adaptations or fibre-type-specific responses to high-intensity interval training in humans. Appl. Physiol., Nutr., Metab. 2014, 39, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Skarpanska-Stejnborn, A.; Pilaczynska-Szczesniak, L.; Basta, P.; Deskur-Smielcka, E.; Horoszkiewicz-Hassan, M. The influence of supplementation with artichoke (Cynara scolymus L.) extract on selected redox parameters in rowers. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 313–327. [Google Scholar] [CrossRef]
- General Assembly of the World Medical Association. WMA Declaration of Helsinki-Ethical principles for medical research involving human subjects. J. Am. Coll. Dent. 2014, 81, 14–18. [Google Scholar]
- Marfell-Jones, M.J.; Stewart, A.; De Ridder, J. International Standards for Anthropometric Assessment; ISAK: Lower Hutt, New Zealand, 2012. [Google Scholar]
- Faulkner, J.A. Physiology of Swimming. Res Q 1966, 37, 41–54. [Google Scholar] [CrossRef]
- Norton, K.; Whittingham, N.; Carter, L.; Kerr, D.; Gore, C.; Marfell-Jones, M. Measurement techniques in anthropometry. Anthropometrica 1996, 1, 25–75. [Google Scholar]
- Edvardsen, E.; Hem, E.; Anderssen, S.A. End criteria for reaching maximal oxygen uptake must be strict and adjusted to sex and age: a cross-sectional study. PLoS ONE 2014, 9, 85276. [Google Scholar] [CrossRef]
- Howley, E.T.; Bassett, D.R., Jr.; Welch, H.G. Criteria for maximal oxygen uptake: review and commentary. Med. Sci. Sports Exerc. 1995, 27, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, K.; Beaver, W.L.; Whipp, B.J. Gas exchange theory and the lactic acidosis (anaerobic) threshold. Circulation 1990, 81, 14–30. [Google Scholar]
- Schisterman, E.F.; Faraggi, D.; Browne, R.; Freudenheim, J.; Dorn, J.; Muti, P.; Armstrong, D.; Reiser, B.; Trevisan, M. TBARS and cardiovascular disease in a population-based Newsample. J Cardiovasc. Risk. 2001, 8, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J. Determination of malondialdehyde as dithiobarbituric acid adduct in biological samples by HPLC with fluorescence detection: comparison with ultraviolet-visible spectrophotometry. Clin. Chem. 2001, 47, 1725–1727. [Google Scholar] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- RANDOX Radicales Libres; Randox Laboratories Ltd.: Crumlin, UK, 1996; pp. 1–16.
- Akerboom, T.P.; Sies, H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981, 77, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Asensi, M.; Sastre, J.; Pallardo, F.V.; Estrela, J.M.; Vina, J. Determination of oxidized glutathione in blood: High-performance liquid chromatography. Methods Enzymol. 1994, 234, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Navarro, M.; Vallejo, F.; Sentandreu, E.; Navarro, J.L.; Tomas-Barberan, F.A. Volunteer stratification is more relevant than technological treatment in orange juice flavanone bioavailability. J. Agric. Food Chem. 2014, 62, 24–27. [Google Scholar] [CrossRef]
- Bishop, D.; Girard, O.; Mendez-Villanueva, A. Repeated-sprint ability—Part II: Recommendations for training. J. Sports Sci. 2011, 41, 741–756. [Google Scholar] [CrossRef]
- McLeay, Y.; Stannard, S.; Houltham, S.; Starck, C. Dietary thiols in exercise: oxidative stress defence, exercise performance, and adaptation. J. Int. Soc. Sports Nutr. 2017, 14, 12. [Google Scholar] [CrossRef]
- Jackson, M.J. Exercise and oxygen radical production by muscle. In Handbook of Oxidants and Antioxidants in Exercise; Sen, C., Packer, L., Hiinnincn, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Peternelj, T.-T.; Coombes, J.S. Antioxidant supplementation during exercise training. J. Sports Sci. 2011, 41, 1043–1069. [Google Scholar] [CrossRef] [PubMed]
- Elavarasan, J.; Velusamy, P.; Ganesan, T.; Ramakrishnan, S.K.; Rajasekaran, D.; Periandavan, K. Hesperidin-mediated expression of Nrf2 and upregulation of antioxidant status in senescent rat heart. J. Pharm. Pharmacol. 2012, 64, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Huerta, O.D.; Aguilera, C.M.; Martin, M.V.; Soto, M.J.; Rico, M.C.; Vallejo, F.; Tomas-Barberan, F.; Perez-de-la-Cruz, A.J.; Gil, A.; Mesa, M.D. Normal or High Polyphenol Concentration in Orange Juice Affects Antioxidant Activity, Blood Pressure, and Body Weight in Obese or Overweight Adults. J. Nutr. 2015, 145, 1808–1816. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Parmar, H.S. Evaluation of antioxidative and anti-inflammatory potential of hesperidin and naringin on the rat air pouch model of inflammation. Inflamm Res. 2011, 60, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, D.Z.; Cubrilo, D.G.; Puzovic, V.S.; Vuletic, M.S.; Zivkovic, V.I.; Barudzic, N.S.; Radovanovic, D.S.; Djuric, D.M.; Jakovljevic, V.L. Changes in athlete’s redox state induced by habitual and unaccustomed exercise. Oxid. Med. Cell. Longevity 2012, 2012, 805850. [Google Scholar] [CrossRef] [PubMed]
- Cavia-Saiz, M.; Busto, M.D.; Pilar-Izquierdo, M.C.; Ortega, N.; Perez-Mateos, M.; Muniz, P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: a comparative study. J. Sci. Food Agric. 2010, 90, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Wilmsen, P.K.; Spada, D.S.; Salvador, M. Antioxidant activity of the flavonoid hesperidin in chemical and biological systems. J. Agric. Food Chem. 2005, 53, 4757–4761. [Google Scholar] [CrossRef] [PubMed]
- Spirlandeli, A.L.; Deminice, R.; Jordao, A.A. Plasma malondialdehyde as biomarker of lipid peroxidation: effects of acute exercise. Int. J. Sports Med. 2014, 35, 14–18. [Google Scholar] [CrossRef]
- Gasparovic, A.C.; Jaganjac, M.; Mihaljevic, B.; Sunjic, S.B.; Zarkovic, N. Assays for the measurement of lipid peroxidation. Methods Mol. Biol. 2013, 965, 283–296. [Google Scholar] [CrossRef]
- Sies, H. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 1999, 27, 916–921. [Google Scholar] [CrossRef]
- Flohe, L. The fairytale of the GSSG/GSH redox potential. Biochim. Biophys. Acta. 2013, 1830, 3139–3142. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Miler, M.; Zivanovic, J.; Ajdzanovic, V.; Orescanin-Dusic, Z.; Milenkovic, D.; Konic-Ristic, A.; Blagojevic, D.; Milosevic, V.; Sosic-Jurjevic, B. Citrus flavanones naringenin and hesperetin improve antioxidant status and membrane lipid compositions in the liver of old-aged Wistar rats. Exp. Gerontol. 2016, 84, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhu, J.; Zhou, Y.; Wei, Y.; Xi, L.; Qin, H.; Rao, Z.; Han, M.; Ma, Y.; Wu, X. Hesperidin Alleviates Oxidative Stress and Upregulates the Multidrug Resistance Protein 2 in Isoniazid and Rifampicin-Induced Liver Injury in Rats. J. Biochem. Mol. Toxicol. 2016, 30, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vargas, J.E.; Zarco, N.; Shibayama, M.; Segovia, J.; Tsutsumi, V.; Muriel, P. Hesperidin prevents liver fibrosis in rats by decreasing the expression of nuclear factor-kappaB, transforming growth factor-beta and connective tissue growth factor. Pharmacology. 2014, 94, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Eynon, N.; Alves, A.J.; Sagiv, M.; Yamin, C.; Sagiv, M.; Meckel, Y. Interaction between SNPs in the NRF2 gene and elite endurance performance. Physiol. Genomics. 2009, 41, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F. Polyphenols in Sport: Facts or Fads? In Antioxidants in Sport Nutrition; Lamprecht, M., Ed.; Taylor Francis Group: Abingdon, UK, 2015. [Google Scholar]
- Tadaishi, M.; Miura, S.; Kai, Y.; Kano, Y.; Oishi, Y.; Ezaki, O. Skeletal muscle-specific expression of PGC-1α-b, an exercise-responsive isoform, increases exercise capacity and peak oxygen uptake. PLoS ONE 2011, 6, 28290. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Nuclear respiratory factors and the pathways of nuclear-mitochondrial interaction. Trends Cardiovasc. Med. 1996, 6, 39–45. [Google Scholar] [CrossRef]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef]
- Miura, S.; Kai, Y.; Kamei, Y.; Ezaki, O. Isoform-specific increases in murine skeletal muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) mRNA in response to beta2-adrenergic receptor activation and exercise. Endocrinology 2008, 149, 4527–4533. [Google Scholar] [CrossRef]
- Chinsomboon, J.; Ruas, J.; Gupta, R.K.; Thom, R.; Shoag, J.; Rowe, G.C.; Sawada, N.; Raghuram, S.; Arany, Z. The transcriptional coactivator PGC-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proc. Natl. Acad. Sci. USA 2009, 106, 21401–21406. [Google Scholar] [CrossRef] [PubMed]
| Age (years) | Height (cm) | Weight (kg) | BMI (kg/m2) | BF (%) | VO2max (mL·kg−1·min−1) | VT1 (%) | VT2 (%) |
|---|---|---|---|---|---|---|---|
| 33.3 ± 7.9 | 174.9 ± 4.2 | 69.4 ± 4.5 | 22.7 ± 1.2 | 11.2 ± 2.2 | 61.6 ± 7.4 | 53.0 ± 6.1 | 86.0 ± 4.7 |
| Parameters | Best Sprint Data | Average (All Sprints) | ||
|---|---|---|---|---|
| Cardiose® | Placebo | Cardiose® | Placebo | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| PeakPower (w) | 835.50 ± 96.08 | 803.79 ± 110.43 | 740.16 ± 74.52 | 729.55 ± 91.36 |
| Poweraverage (w) | 567.84 ± 55.44 * | 555.25 ± 51.81 * | 511.71 ± 52.68 | 510.78 ± 52.99 |
| Time to peakpower (ms) | 2840.69 ± 715.99 | 3235.85 ± 1516.06 | 3003.13 ± 950.28 | 3476.14 ± 1546.57 |
| Max speed(rpm) | 132.86 ± 9.59 * | 128.70 ± 9.24 * | 120.83 ± 7.79 | 119.92 ± 9.79 |
| Totalenergy (J) | 16246.29 ± 1600.37 * | 15827.79 ± 1505.86 * | 14874.79 ± 1570.83 | 14818.36 ± 1608.24 |
| Metabolic parameters | Cardiose® | Placebo | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| VO2 (L/min) | Pre | 2.11 ± 0.39 | 2.06 ± 0.39 |
| Post | 2.15 ± 0.45 | 2.13 ± 0.51 | |
| VCO2 (L/min) | Pre | 1.93 ± 0.41 | 1.86 ± 0.35 |
| Post | 1.83 ± 0.43 | 1.77 ± 0.43 | |
| RER | Pre | 0.91 ± 0.03 | 0.90 ± 0.02 |
| Post | 0.85 ± 0.03 | 0.83 ± 0.02 | |
| Efficiency (mL/Kg/W) | Pre | 3.97 ± 0.48 | 3.85 ± 0.39 |
| Post | 4.02 ± 0.49 | 3.94 ± 0.51 | |
| HR (pul/min) | Pre | 128.55 ± 9.53 | 128.04 ± 8.95 |
| Post | 144.15 ± 12.50 | 144.74 ± 11.31 | |
| Carbohydrates | Pre | 105.5 ± 33.3 | 97.6 ± 19.7 |
| Post | 72.9 ± 28.5 | 63.6 ± 17.7 | |
| Fat | Pre | 14.1 ± 6.0 | 15.9 ± 6.1 |
| Post | 28.5 ± 4.0 | 31.8 ± 8.7 | |
| Antioxidant/Oxidant Status Markers | Cardiose® Mean ± SD | Placebo Mean ± SD | ||||||
|---|---|---|---|---|---|---|---|---|
| E0 | E1 | E2 | E3 | E0 | E1 | E2 | E3 | |
| CAT (U/g Hb) | 25.66 ± 4.74 | 53.93 ± 13.41 * | 27.53 ± 6.54 * | 24.66 ± 4.27 | 24.02 ± 3.13 | 51.41 ± 16.41 * | 27.07 ± 4.63 * | 24.53 ± 4.18 |
| SOD (U/g Hb) | 1298.00 ± 261.75 | 1349.13 ± 225.31 | 1269.27 ± 271.13 | 1228.33 ± 229.77 | 1319.00 ± 145.54 | 1352.13 ± 201.31 | 1364.80 ± 272.74 | 1337.67 ± 193.97 |
| GSH (nmol/mg protein) | 25.02 ± 2.80 | 24.89 ± 2.90 | 23.73 ± 2.10 | 24.36 ± 2.75 | 24.36 ± 2.24 | 23.59 ± 3.37 | 23.62 ± 3.19 | 24.60 ± 1.72 |
| GSSG (nmol/mg protein) | 0.351 ± 0.073 | 0.334 ± 0.075 | 0.315 ± 0.067 | 0.378 ± 0.152 | 0.325 ± 0.073 | 0.316 ± 0.078 | 0.336 ± 0.068 | 0.388 ± 0.130 |
| % GSSG/GSH | 1.42 ± 0.32 | 1.35 ± 0.31 | 1.34 ± 0.31 | 1.54 ± 0.54 | 1.34 ± 0.28 | 1.37 ± 0.41 | 1.45 ± 0.37 | 1.57 ± 0.48 |
| TBARS (nmol/mg protein) | 2.49 ± 0.34 | 2.71 ± 0.45 | 2.56 ± 0.44 | 2.63 ± 0.26 | 2.43 ± 0.22 | 2.63 ± 0.36 | 2.57 ± 0.38 | 2.58 ± 0.32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Noguera, F.J.; Marín-Pagán, C.; Carlos-Vivas, J.; Rubio-Arias, J.A.; Alcaraz, P.E. Acute Effects of Hesperidin in Oxidant/Antioxidant State Markers and Performance in Amateur Cyclists. Nutrients 2019, 11, 1898. https://doi.org/10.3390/nu11081898
Martínez-Noguera FJ, Marín-Pagán C, Carlos-Vivas J, Rubio-Arias JA, Alcaraz PE. Acute Effects of Hesperidin in Oxidant/Antioxidant State Markers and Performance in Amateur Cyclists. Nutrients. 2019; 11(8):1898. https://doi.org/10.3390/nu11081898
Chicago/Turabian StyleMartínez-Noguera, Francisco Javier, Cristian Marín-Pagán, Jorge Carlos-Vivas, Jacobo A. Rubio-Arias, and Pedro E. Alcaraz. 2019. "Acute Effects of Hesperidin in Oxidant/Antioxidant State Markers and Performance in Amateur Cyclists" Nutrients 11, no. 8: 1898. https://doi.org/10.3390/nu11081898
APA StyleMartínez-Noguera, F. J., Marín-Pagán, C., Carlos-Vivas, J., Rubio-Arias, J. A., & Alcaraz, P. E. (2019). Acute Effects of Hesperidin in Oxidant/Antioxidant State Markers and Performance in Amateur Cyclists. Nutrients, 11(8), 1898. https://doi.org/10.3390/nu11081898




