Role of Endoplasmic Reticulum and Oxidative Stress Parameters in the Pathophysiology of Disease-Related Malnutrition in Leukocytes of an Outpatient Population
Abstract
1. Introduction
2. Material and Methods
2.1. Subjects
2.2. Assessment of Nutritional Status
2.3. Blood Sampling
2.4. Biochemical Determinations
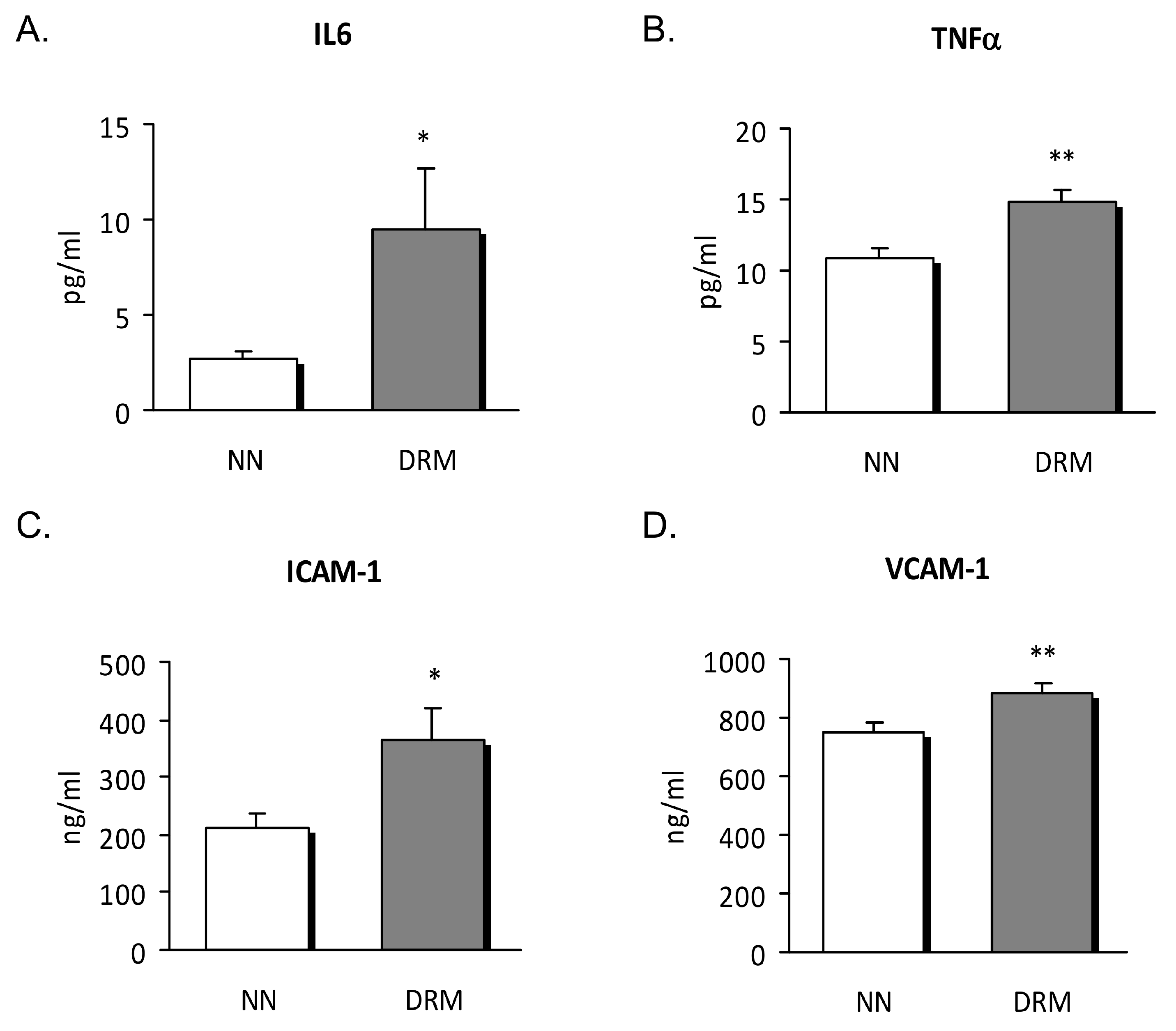
2.5. Soluble Cytokines and Adhesion Molecule Assay
2.6. Leukocyte Isolation
2.7. Static Cytometry
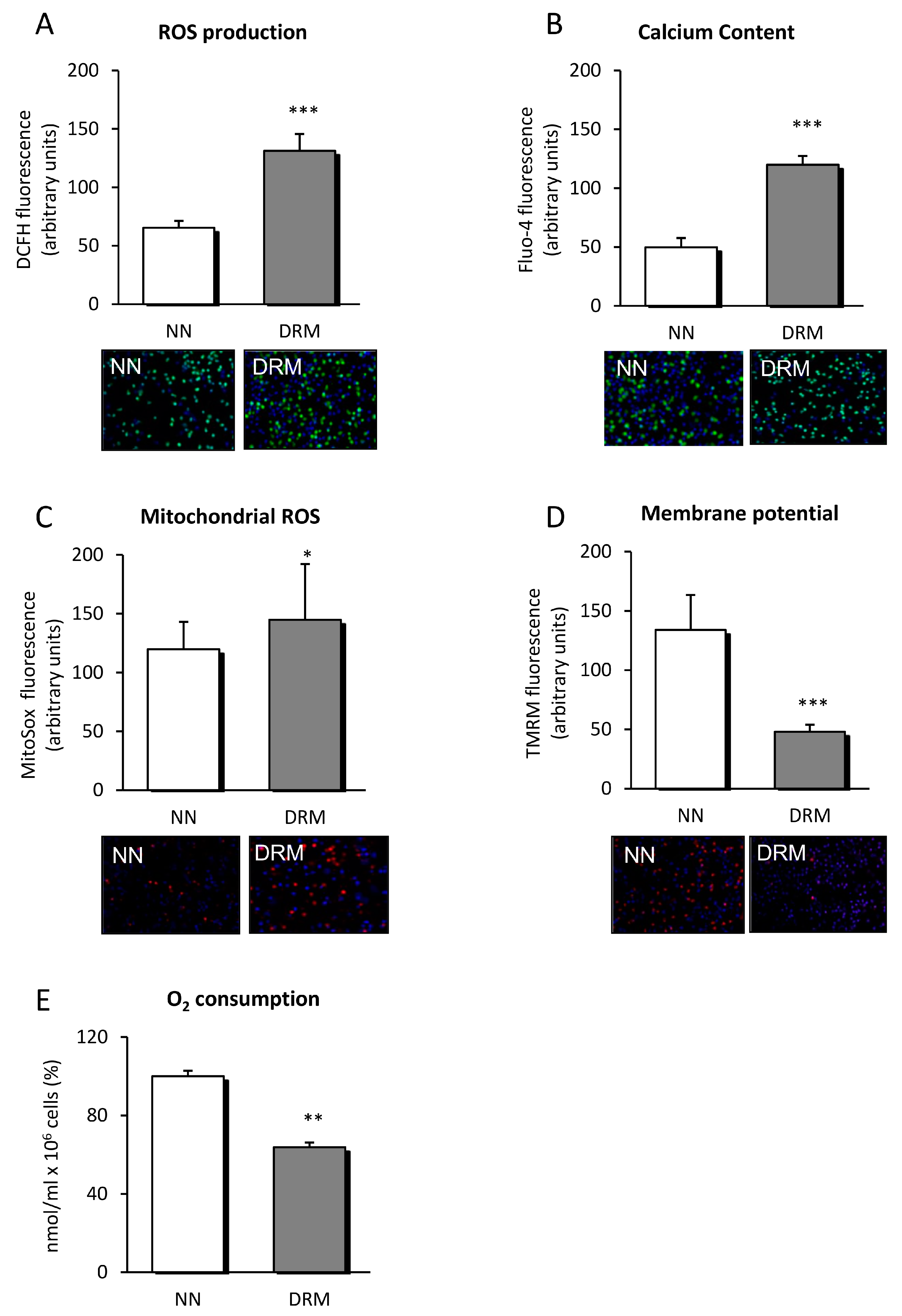
2.8. Mitochondrial Oxygen Consumption Measurements
2.9. Protein Extraction and Quantification
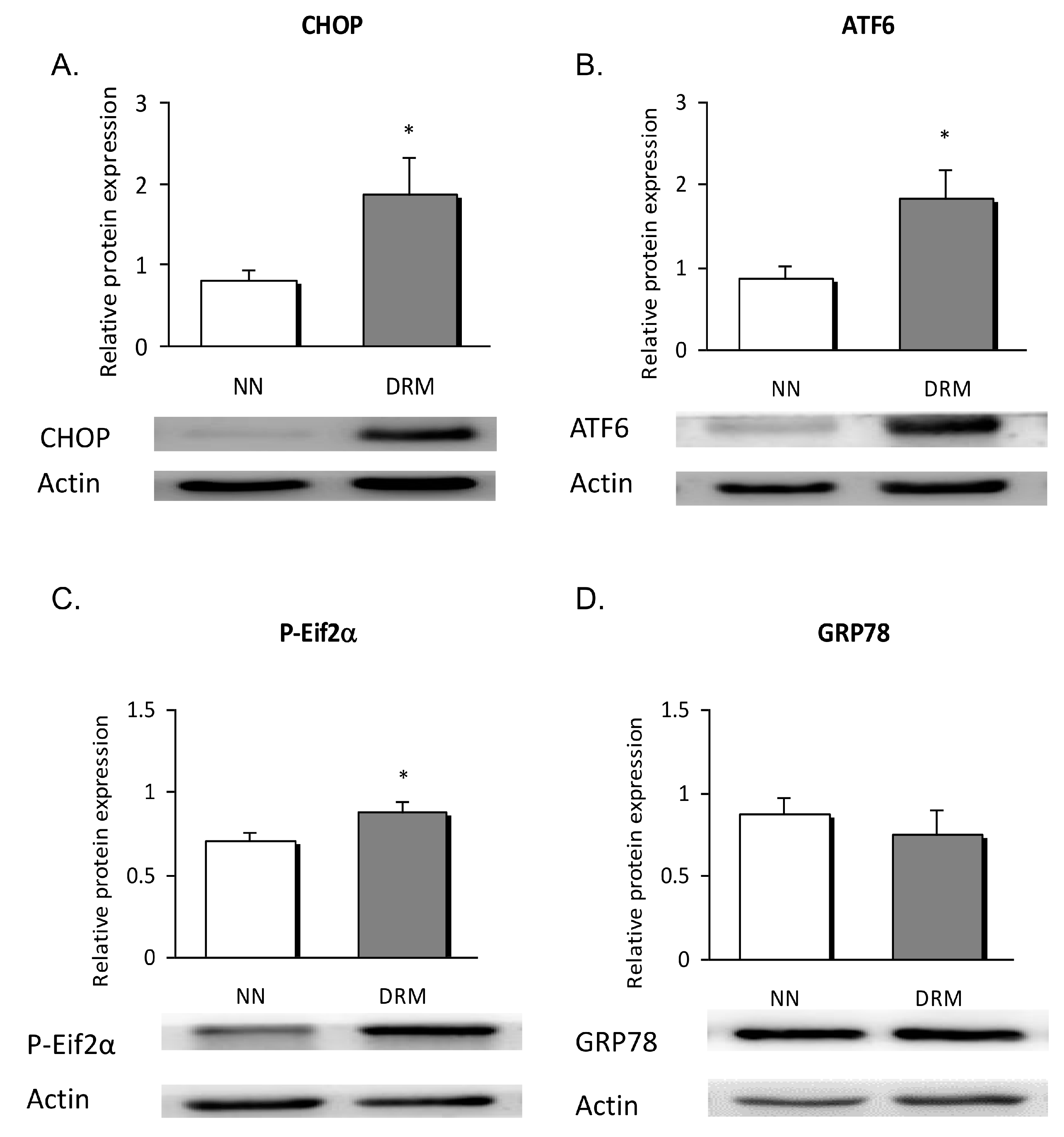
2.10. Western Blot
2.11. RT-PCR
2.12. Statistical Analysis
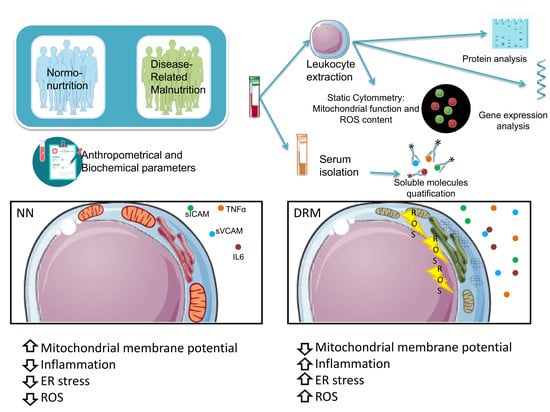
3. Results
3.1. Anthropometric, Metabolic, and Biochemical Parameters
3.2. Inflammatory Status
3.3. Mitochondrial Function
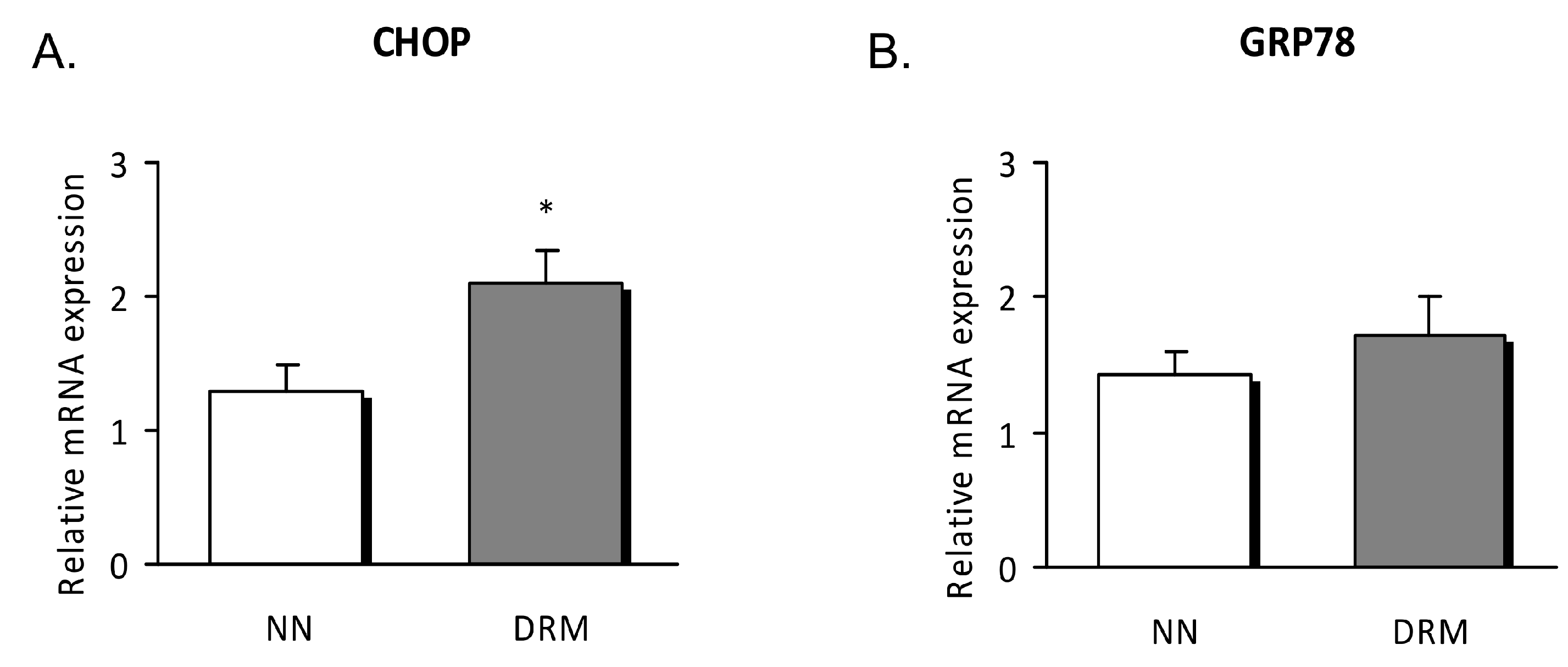
3.4. Endoplasmic Reticulum Stress Analysis
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMP | arm muscle perimeter |
| ASPEN | American Society of Parenteral and Enteral Nutrition |
| ATF6 | activating transcription factor 6 |
| CHOP | CCAAT/enhancer binding protein (C/EBP) homologous protein |
| DCFH-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
| DRM | disease-related malnutrition |
| ER | endoplasmic reticulum |
| GRP78 | 78-KDa glucose-regulated protein |
| hsCRP | high-sensitivity C-reactive protein |
| ICAM-1 | intercellular adhesion molecule-1 |
| IL6 | interleukin 6 |
| MUAC | mid-upper arm circumference |
| NN | normonutrition |
| P-eIF2α | phosphorylated eukaryotic translation initiation factor 2 subunit 1 alpha |
| ROS | reactive oxygen species |
| TMRM | tetramethylrhodamine |
| TNFα | tumor necrosis factor α |
| TST | triceps skinfold thickness |
| UPR | unfolded protein response |
| VCAM-1 | vascular cell adhesion molecule-1 |
References
- Cederholm, T.; Jensen, G.L. To Create a Consensus on Malnutrition Diagnostic Criteria. J. Parenter. Enter. Nutr. 2017, 41, 311–314. [Google Scholar] [CrossRef]
- Jensen, G.L.; Mirtallo, J.; Compher, C.; Dhaliwal, R.; Forbes, A.; Grijalba, R.F.; Hardy, G.; Kondrup, J.; Labadarios, D.; Nyulasi, I.; et al. Adult starvation and disease-related malnutrition: A proposal for etiology-based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. Clin. Nutr. 2010, 29, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Pichard, C.; Lochs, H.; Pirlich, M. Prognostic impact of disease-related malnutrition. Clin. Nutr. 2008, 27, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.K. Nutrition and the immune system: An introduction. Am. J. Clin. Nutr. 1997, 66, 460S–463S. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int. J. Obes. 2008, 32, S52–S54. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Lee, A.S. The mammalian endoplasmic reticulum stress response element consists of an evolutionarily conserved tripartite structure and interacts with a novel stress-inducible complex. Nucleic Acids Res. 1999, 27, 1437–1443. [Google Scholar] [CrossRef]
- Fu, X.L.; Gao, D.S. Endoplasmic reticulum proteins quality control and the unfolded protein response: The regulative mechanism of organisms against stress injuries. Biofactors 2014, 40, 569–585. [Google Scholar] [CrossRef]
- Biczo, G.; Vegh, E.T.; Shalbueva, N.; Mareninova, O.A.; Elperin, J.; Lotshaw, E.; Gretler, S.; Lugea, A.; Malla, S.R.; Dawson, D.; et al. Mitochondrial Dysfunction; Through Impaired Autophagy; Leads to Endoplasmic Reticulum Stress; Deregulated Lipid Metabolism; and Pancreatitis in Animal Models. Gastroenterology 2018, 154, 689–703. [Google Scholar] [CrossRef]
- White, J.V.; Guenter, P.; Jensen, G.; Malone, A.; Schofield, M.; Academy Malnutrition Work Group; A.S.P.E.N. Malnutrition Task Force; A.S.P.E.N. Board of Directors. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: Characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J. Parenter. Enter. Nutr. 2012, 36, 275–283. [Google Scholar]
- Bharadwaj, S.; Ginoya, S.; Tandon, P.; Gohel, T.D.; Guirguis, J.; Vallabh, H.; Jevenn, A.; Hanouneh, I. Malnutrition: Laboratory markers vs nutritional assessment. Gastroenterol. Rep. 2016, 4, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Hernández, J.; Planas Vila, M.; León-Sanz, M.; García de Lorenzo, A.; Celaya-Pérez, S.; García-Lorda, P.; Araujo, K.; Sarto Guerri, B. Prevalence and costs of malnutrition in hospitalized patients; the PREDyCES Study. Nutr. Hosp. 2012, 27, 1049–1059. [Google Scholar] [PubMed]
- Milà Villarroel, R.; Formiga, F.; Duran Alert, P.; Abellana Sangrà, R. Prevalence of malnutrition in Spanish elders: Systematic review. Med. Clin. 2012, 139, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Castro-Vega, I.; Veses Martin, S.; Cantero Llorca, J.; Barrios Marta, C.; Monzó Albiach, N.; Bañuls Morant, C.; Hernandez-Mijares, A. Prevalence of nutritional risk and malnutrition established in outpatient; institutionalized and hospitalized populations in a health department. Nutr. Hosp. 2017, 34, 889–898. [Google Scholar] [PubMed]
- Marcason, W. Should Albumin and Prealbumin Be Used as Indicators for Malnutrition? J. Acad. Nutr. Diet. 2017, 117, 1144. [Google Scholar] [CrossRef] [PubMed]
- Hernández Mijares, A.; Royo Taberner, R.; Martínez Triguero, M.L.; Graña Fandos, J.; López García, A.; Morales Suárez-Varela, M.M. Malnutrition prevalence in institutionalized elderly people in Valencia Community; Spain. Med. Clin. 2001, 117, 289–294. [Google Scholar] [CrossRef]
- Humphreys, J.; de la Maza, P.; Hirsch, S.; Barrera, G.; Gattas, V.; Bunout, D. Muscle strength as a predictor of loss of functional status in hospitalized patients. Nutrition 2002, 18, 616–620. [Google Scholar] [CrossRef]
- Norman, K.; Schutz, T.; Kemps, M. The Subjective Global Assessment reliably identifies malnutrition-related muscle dysfunction. Clin. Nutr. 2005, 24, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Alvares-da-Silva, M.R.; Reverbel da Silveira, T. Comparison between handgrip strength; subjective global assessment; and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition 2005, 21, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Epifânio, A.P.S.; Balbino, K.P.; Ribeiro, S.M.R.; Franceschini, S.C.C.; Hermsdorff, H.H.M. Clinical-nutritional; inflammatory and oxidative stress predictors in hemodialysis mortality: A review. Nutr. Hosp. 2018, 35, 461–468. [Google Scholar] [PubMed]
- Kim, S.; McClave, S.A.; Martindale, R.G.; Miller, K.R.; Hurt, R.T. Hypoalbuminemia and Clinical Outcomes: What is the Mechanism behind the Relationship? Am. Surg. 2017, 83, 1220–1227. [Google Scholar] [PubMed]
- McMillan, D.C.; Maguire, D.; Talwar, D. Relationship between nutritional status and the systemic inflammatory response: Micronutrients. Proc. Nutr. Soc. 2018, 78, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ghone, R.A.; Suryakar, A.; Kullhall, P.M.; Bhagat, S.; Ramchanda, K.; Padalka, R.; Karnik, A.C.; Hundekar, P.S.; Sangle, D.A. A study of oxidative stress biomarkers and efect of oral antioxidant supplementation in severe acute malnutrition. J. Clin. Diagn. Res. 2013, 7, 2146–2148. [Google Scholar] [PubMed]
- Valla, F.V.; Bost, M.; Roche, S.; Pitance, M.; Cuerq, C.; Ridout, J.; Ecochard, R.; Ginhoux, T.; Bellon, A.; Ford-Chessel, C.; et al. Multiple Micronutrient Plasma Level Changes Are Related to Oxidative Stress Intensity in Critically Ill Children. Pediatr. Crit. Care Med. 2018, 19, e455–e463. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.; Apostolova, N.; Herance, J.R.; Rovira-Llopis, S.; Hernandez-Mijares, A.; Victor, V.M. Perspectives and potential applications of mitochondria targeted antioxidants in cardiometabolic diseases and type 2 diabetes. Med. Res. Rev. 2014, 34, 160–189. [Google Scholar] [CrossRef] [PubMed]
- Allagnat, F.; Fukaya, M.; Nogueira, T.C.; Delaroche, D.; Welsh, N.; Marselli, L.; Marchetti, P.; Haefliger, J.A.; Eizirik, D.L.; Cardozo, A.K. C/EBP homologous protein contributes to cytokine-induced pro-inflammatory responses and apoptosis in β-cells. Cell Death Differ. 2012, 19, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
| NN | DRM | |
|---|---|---|
| Neurological disease | 18.80% | 16.30% |
| Pneumological disease | 31.00% | 20.90% |
| Digestive disease | 23.10% | 20.90% |
| Endocrine disease | 5.10% | 7.00% |
| Cardiovascular disease | 10.70% | 7.00% |
| Viral infection | 6.80% | 12.80% |
| Haematologic disease | 0.50% | 1.20% |
| Neoplasms | 1.50% | 11.60% |
| Other | 2.50% | 2.30% |
| NN (n = 37) | DRM (n = 45) | p-Value | |
|---|---|---|---|
| Age (years) | 57.9 ± 19.4 | 65.7 ± 15.9 | 0.15 |
| Weight (kg) | 65.4 ± 14 | 55.6 ± 10.6 | 0.021 |
| Weight loss (%) | 2.9 ± 3 | 26.2 ± 5.5 | <0.001 |
| BMI (kg/m2) | 25.9 ± 16.3 | 21.2 ± 2.8 | <0.001 |
| Triceps skin thickness (mm) | 19.3 ± 6.9 | 13.6 ± 4.9 | <0.001 |
| Mid-upper arm circumference (cm) | 28.8 ± 4.7 | 24.7 ± 2.8 | <0.001 |
| Arm muscular circumference (cm) | 22.8 ± 3.4 | 20.2 ± 3.1 | <0.001 |
| Calf circumference (cm) | 36.3 ± 4.1 | 31 ± 2.86 | <0.001 |
| Albumin (g/dL) | 4.2 ± 0.3 | 3.9 ± 0.6 | <0.001 |
| Prealbumin (mg/dL) | 24.2 ± 5 | 23.3 ± 6.7 | 0.470 |
| Transferrin (mg/dL) | 267.3 ± 64.8 | 236 ± 63.6 | 0.031 |
| Total cholesterol (mg/dL) | 185.9 ± 32.2 | 184.8 ± 43.9 | 0.865 |
| LDLc (mg/dL) | 110.5 ± 29.1 | 112.3 ± 35.8 | 0.805 |
| HDLc (mg/dL) | 50.8 ± 14.9 | 50.3 ± 15.9 | 0.885 |
| Triglycerides (mg/dL) | 98.0 (83.8;126.0) | 92.5(66.3;143.3) | 0.330 |
| hsCRP (mg/L) | 2.8 ± 2.8 | 4.4 ± 3.5 | 0.038 |
| Lymphocytes (109/L) | 2.1 ± 0.9 | 2.0 ± 0.8 | 0.710 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bañuls, C.; de Marañón, A.M.; Castro-Vega, I.; López-Doménech, S.; Escribano-López, I.; Salom, C.; Veses, S.; Hernández-Mijares, A. Role of Endoplasmic Reticulum and Oxidative Stress Parameters in the Pathophysiology of Disease-Related Malnutrition in Leukocytes of an Outpatient Population. Nutrients 2019, 11, 1838. https://doi.org/10.3390/nu11081838
Bañuls C, de Marañón AM, Castro-Vega I, López-Doménech S, Escribano-López I, Salom C, Veses S, Hernández-Mijares A. Role of Endoplasmic Reticulum and Oxidative Stress Parameters in the Pathophysiology of Disease-Related Malnutrition in Leukocytes of an Outpatient Population. Nutrients. 2019; 11(8):1838. https://doi.org/10.3390/nu11081838
Chicago/Turabian StyleBañuls, Celia, Aranzazu M. de Marañón, Iciar Castro-Vega, Sandra López-Doménech, Irene Escribano-López, Christian Salom, Silvia Veses, and Antonio Hernández-Mijares. 2019. "Role of Endoplasmic Reticulum and Oxidative Stress Parameters in the Pathophysiology of Disease-Related Malnutrition in Leukocytes of an Outpatient Population" Nutrients 11, no. 8: 1838. https://doi.org/10.3390/nu11081838
APA StyleBañuls, C., de Marañón, A. M., Castro-Vega, I., López-Doménech, S., Escribano-López, I., Salom, C., Veses, S., & Hernández-Mijares, A. (2019). Role of Endoplasmic Reticulum and Oxidative Stress Parameters in the Pathophysiology of Disease-Related Malnutrition in Leukocytes of an Outpatient Population. Nutrients, 11(8), 1838. https://doi.org/10.3390/nu11081838