The Concentration of Omega-3 Fatty Acids in Human Milk Is Related to Their Habitual but Not Current Intake
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Session Design
2.2. Human Milk Collection
2.3. Lipid Concentration and Fatty Acid Analysis of Human Milk
2.3.1. Fat Extraction
2.3.2. Gas Chromatography-Mass Spectrometry Analysis
2.4. Fatty Acids Dietary Intake and Intake Frequency of Food Products
2.5. Statistical Analysis
3. Results
3.1. Maternal Characteristics
3.2. Fatty Acids Concentrations in Human Milk
3.3. Fatty Acids Dietary Intake and Its Association with Concentration in Human Milk
3.4. Association between Intake Frequency of Food Products and DHA Concentrations in Human Milk
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Innis, S.M. Impact of maternal diet on human milk composition and neurological development of infants. Am. J. Clin. Nutr. 2014, 99, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Rodriguez-Palmero, M.; Demmelmair, H.; Fidler, N.; Jensen, R.; Sauerwald, T. Physiological aspects of human milk lipids. Early Hum. Dev. 2001, 65, 3–18. [Google Scholar] [CrossRef]
- Birch, E.E.; Castaneda, Y.S.; Wheaton, D.H.; Birch, D.G.; Uauy, R.D.; Hoffman, D.R. Visual maturation of term infants fed long-chain polyunsaturated fatty acid-supplemented or control formula for 12 mo. Am. J. Clin. Nutr. 2005, 81, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.J.H.; Rideout, T. Lipids, sterols, and their metabolites. In Modern Nutrition in Health and Disease, 11st ed.; Ross, A.C., Caballero, B., Cousins, R.J., Tucker, K.L., Ziegler, T.R., Eds.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2014. [Google Scholar]
- Innis, S.M. Human milk: Maternal dietary lipids and infant development. Proc. Nutr. Soc. 2007, 66, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.J.; Butt, C.M.; Mohajeri, M.H. Docosahexaenoic acid and cognition throughout the lifespan. Nutrients 2016, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M. Metabolic programming of long-term outcomes due to fatty acid nutrition in early life. Matern. Child Nutr. 2011, 7, 112–123. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Scientific Opinion on Nutrient Requirements and Dietary Intakes of Infants and Young Children in the European Union. 2013. Available online: https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2013.3408 (accessed on 1 October 2018).
- Goyens, P.L.; Spilker, M.E.; Zock, P.L.; Katan, M.B.; Mensink, R.P. Conversion of alpha-linolenic acid in humans is influenced by the absolute amounts of alpha-linolenic acid and linoleic acid in the diet and not by their ratio. Am. J. Clin. Nutr. 2006, 84, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Pawlosky, R.J.; Hibbeln, J.R.; Novotny, J.A.; Salem, N. Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. J. Lipid Res. 2001, 42, 1257–1265. [Google Scholar] [PubMed]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Greiner, R.C.; Winter, J.; Nathanielsz, P.W.; Brenna, J.T. Brain docosahexaenoate accretion in fetal baboons: Bioequivalence of dietary alpha-linolenic and docosahexaenoic acids. Pediatr. Res. 1997, 42, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Lien, E.; Agostoni, C.; Bohles, H.; Campoy, C.; Cetin, I.; Decsi, T.; Dudenhausen, J.W.; Dupont, C.; Forsyth, S.; et al. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: Review of current knowledge and consensus recommendations. J. Perinat. Med. 2008, 36, 5–14. [Google Scholar] [CrossRef]
- Brenna, J.T.; Lapillonne, A. Background paper on fat and fatty acid requirements during pregnancy and lactation. Ann. Nutr. Metab. 2009, 55, 97–122. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Horvath, A.; Rybak, A.; Socha, P. Breastfeeding. A Position Paper by the Polish Society for Paediatric Gastroenterology, Hepatology and Nutrition. Med. Standards/Ped. 2016, 13, 9–24. [Google Scholar]
- Oken, E.; Kleinman, K.P.; Olsen, S.F.; Rich-Edwards, J.W.; Gillman, M.W. Associations of seafood and elongated n-3 fatty acid intake with fetal growth and length of gestation: Results from a US pregnancy cohort. Am. J. Epidemiol. 2004, 160, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Friesen, R.W.; Innis, S.M. Dietary arachidonic acid to EPA and DHA balance is increased among Canadian pregnant women with low fish intake. J. Nutr. 2009, 139, 2344–2350. [Google Scholar] [CrossRef] [PubMed]
- Denomme, J.; Stark, K.D.; Holub, B.J. Directly quantitated dietary (n-3) fatty acid intakes of pregnant Canadian women are lower than current dietary recommendations. J. Nutr. 2005, 135, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Sioen, I.; Devroe, J.; Inghels, D.; Terwecoren, R.; De Henauw, S. The influence of n-3 PUFA supplements and n-3 PUFA enriched foods on the n-3 LC PUFA intake of Flemish women. Lipids 2010, 45, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Coletto, D.; Morrison, J. Seafood Survey: Public Opinion on Aquaculture and a National Aquaculture Act. 2011. Available online: http://www.aquaculture.ca/ files/CAIA-PUBLIC-REPORT-May-2011.pdf (accessed on 2 October 2018).
- Oken, E.; Kleinman, K.P.; Berland, W.E.; Simon, S.R.; Rich-Edwards, J.W.; Gillman, M.W. Decline in fish consumption among pregnant women after a national mercury advisory. Obstet. Gynecol. 2003, 102, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Amaral, Y.N.; Marano, D.; Silva, L.M.; Guimarães, A.C.; Moreira, M.E. Are There Changes in the Fatty Acid Profile of Breast Milk with Supplementation of Omega-3 Sources? A Systematic Review. Rev. Bras. Ginecol. Obstet. 2017, 39, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Kim, M.K.; Hwang, S.H.; Ahn, Y.; Shim, J.E.; Kim, D.H. Relative validities of 3-day food records and the food frequency questionnaire. Nutr. Res. Pract. 2010, 4, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.J.; Li, H.T.; Yu, L.X.; Xu, G.S.; Ge, H.; Wang, L.L.; Zhang, Y.L.; Zhou, Y.B.; Li, Y.; Bai, M.X.; et al. A correlation study of DHA dietary intake and plasma, erythrocyte and breast milk DHA concentrations in lactating women from coastland, Lakeland, and inland areas of China. Nutrients 2016, 8, 312. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Nissensohn, M.; Øverby, N.C.; Fekete, K. Dietary methods and biomarkers of omega 3 fatty acids: A systematic review. Br. J. Nutr. 2012, 107, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Euro Who. Available online: http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/ahealthy-lifestyle/body-mass-index-bmi (accessed on 14 May 2019).
- Bzikowska-Jura, A.; Czerwonogrodzka-Senczyna, A.; Olędzka, G.; Szostak-Węgierek, D.; Weker, H.; Wesołowska, A. Maternal Nutrition and Body Composition During Breastfeeding: Association with Human Milk Composition. Nutrients 2018, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Szponar, L.; Wolnicka, K.; Rychlik, E. Album of Photographs of Food Products and Dishes; National Food and Nutrition Institute: Warsaw, Poland, 2011. [Google Scholar]
- Brenna, J.T.; Varamini, B.; Jensen, R.G.; Diersen-Schade, D.A.; Boettcher, J.A.; Arterburn, L.M. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am. J. Clin. Nutr. 2007, 85, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Nochera, C.L.; Goossen, L.H.; Brutus, A.R.; Cristales, M.; Eastman, B. Consumption of DHA + EPA by low-income women during pregnancy and lactation. Nutr. Clin. Pract. 2011, 26, 445–450. [Google Scholar] [CrossRef]
- Hibbeln, J.R.; Davis, J.M.; Steer, C.; Emmett, P.; Rogers, I.; Williams, C.; Golding, J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): An observational cohort study. Lancet 2007, 369, 578–585. [Google Scholar] [CrossRef]
- Sontrop, J.; Avison, W.R.; Evers, S.E.; Speechley, K.N.; Campbell, M.K. Depressive symptoms during pregnancy in relation to fish consumption and intake of n-3 polyunsaturated fatty acids. Paediatr. Perinat. Epidemiol. 2008, 22, 389–399. [Google Scholar] [CrossRef]
- Rodriguez-Bernal, C.L.; Ramon, R.; Quiles, J.; Murcia, M.; Navarrete-Munoz, E.M.; Vioque, J.; Ballester, F.; Rebagliato, M. Dietary intake in pregnant women in a Spanish Mediterranean area: As good as it is supposed to be? Public Health Nutr. 2013, 16, 1379–1389. [Google Scholar] [CrossRef]
- Jia, X.; Pakseresht, M.; Wattar, N.; Wildgrube, J.; Sontag, S.; Andrews, M.; Begum Subhan, F.; McCargar, F.; Field, C. Women who take n-3 long-chain polyunsaturated fatty acid supplements during pregnancy and lactation meet the recommended intake. Appl. Physiol. Nutr. Metab. 2015, 40, 474–481. [Google Scholar] [CrossRef]
- Aumeistere, L.; Ciprovica, I.; Zavadska, D.; Volkovs, V. Fish intake reflects DHA level in breast milk among lactating women in Latvia. Int. Breastfeed. J. 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Antonakou, A.; Skenderi, K.P.; Chiou, A.; Anastasiou, C.A.; Bakoula, C.; Matalas, A.L. Breast milk fat concentration and fatty acid pattern during the first six months in exclusively breastfeeding Greek women. Eur. J. Nutr. 2013, 52, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Makrides, M.; Gibson, R.A.; McPhee, A.J.; Collins, C.T.; Davis, P.G.; Doyle, L.W.; Simmer, K.; Colditz, P.B.; Morris, S.; Smithers, L.G.; et al. Neurodevelopmental outcomes of preterm infants fed high-dose docosahexaenoic acid: A randomized controlled trial. JAMA 2009, 301, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Agostoni, C.; Lammardo, A.M.; Bonvissuto, M.; Giovannini, M.; Galli, C.; Riva, E. Polyunsaturated fatty acids in maternal plasma and in breast milk. Prostaglandins Leukot. Essent. Fat. Acids 2002, 66, 535–540. [Google Scholar] [CrossRef]
- Marangoni, F.; Agostoni, C.; Lammardo, A.M.; Giovannini, M.; Galli, C.; Riva, E. Polyunsaturated fatty acid concentrations in human hindmilk are stable throughout 12-months of lactation and provide a sustained intake to the infant during exclusive breastfeeding: An Italian study. Br. J. Nutr. 2000, 84, 103–109. [Google Scholar] [PubMed]
- Rueda, R.; Ramirez, M.; Garcia-Salmeron, J.L.; Maldonado, J.; Gil, A. Gestational age and origin of human milk influence total lipid and fatty acid contents. Ann. Nutr. Metab. 1998, 42, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Sala-Vila, A.; Campoy, C.; Castellote, A.I.; Garrido, F.J.; Rivero, M.; Rodríguez-Palmero, M.; López-Sabater, M.C. Influence of dietary source of docosahexaenoic and arachidonic acids on their incorporation into membrane phospholipids of red blood cells in term infants. Prostaglandins Leukot. Essent. Fat. Acids 2006, 74, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Olafsdottir, A.S.; Thorsdottir, I.; Wagner, K.H.; Elmadfa, I. Polyunsaturated fatty acids in the diet and breast milk of lactating icelandic women with traditional fish and cod liver oil consumption. Ann. Nutr. Metab. 2006, 50, 270–276. [Google Scholar] [CrossRef]
- Kovacs, A.F.S.; Marosvolgyi, T.; Burus, I.; Decsi, T. Fatty acids in early human milk after preterm and full-term delivery. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 454–459. [Google Scholar] [CrossRef]
- Jackson, K.H.; Harris, W.S. Should there be a target level of docosahexaenoic acid in breast milk? Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 92–96. [Google Scholar] [CrossRef]
- Gibson, R.A.; Neumann, M.A.; Makrides, M. Effect of increasing breast milk docosahexaenoic acid on plasma and erythrocyte phospholipid fatty acids and neural indices of exclusively breast fed infants. Eur. J. Clin. Nutr. 1997, 51, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Presa-Owens, S.D.; Lopez-Subater, M.C.; Rivero-Urgell, M. Fatty acid composition of human milk in Spain. J. Pediatr. Gastroenterol. Nutr. 1996, 22, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Thiel, I.; Abiodun, P.O. The fatty acid composition of human milk in Europe and Africa. J. Pediatr. 1992, 120, 62–70. [Google Scholar] [CrossRef]
- Wu, T.C.; Lau, B.H.; Chen, P.H.; Wu, L.T.; Tang, R.B. Fatty acid composition of Taiwanese Human Milk. J. Chin. Med Assoc. 2010, 73, 581–588. [Google Scholar] [CrossRef]
- ISSFAL. Recommendations for Intake of Polyunsaturated Fatty Acids in Healthy Adults. 2004. Available online: http://www.issfal.org/ (accessed on 31 May 2019).
- Lassek, W.D.; Gaulin, S.J.C. Maternal milk DHA concentration predicts cognitive performance in a sample of 28 nations. Matern. Child Nutr. 2015, 11, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.Y.; Armand, M.; Peyre, H.; Garcia, C.; Forhan, A.; De Agostini, M.; Charles, M.A.; Heude, B. Breastfeeding, polyunsaturated fatty acid levels in colostrum and child intelligence quotient at age 5–6 years. J. Pediatr. 2017, 183, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, M.; Hamulka, J.; Wesolowska, A. Carotenoid Content in Breastmilk in the 3rd and 6th Month of Lactation and Its Associations with Maternal Dietary Intake and Anthropometric Characteristics. Nutrients 2019, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Lassek, W.D.; Gaulin, S.J. Linoleic and docosahexaenoic acids in human milk have opposite relationships with cognitive test performance in a sample of 28 countries. Prostaglandins Leukot. Essent. Fat. Acids 2014, 91, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.H.L.; Silva, M.T.C.; Brandão, S.C.C.; Gomes, J.C.; Peternelli, L.A.; Franceschini, S.C.C. Fatty acid composition of mature breast milk in Brazilian women. Food Chem. 2005, 93, 297–303. [Google Scholar] [CrossRef]
- Nishimura, R.Y.; de Castro, G.S.F.; Junior, A.A.J.; Sartorelli, D.S. Breast milk fatty acid composition of women living far from the coastal area in Brazil. J. Pediatr. 2013, 89, 263–268. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Lauritzen, L.; Jorgensen, M.H.; Hansen, H.S.; Michaelsen, K.F. Fluctuations in human milk long-chain PUFA levels in relation to dietary fish intake. Lipids 2002, 37, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Juber, B.A.; Jackson, K.H.; Johnson, K.B.; Harris, W.S.; Baack, M.L. Breast milk DHA levels may increase after informing women: A community-based cohort study from South Dakota USA. Int. Breastfeed. J. 2016, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Strobel, C.; Jahreis, G.; Kuhnt, K. Survey of n-3 and n-6 polyunsaturated fatty acids in fish and fish products. Lipids Health Dis. 2012, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Quinn, E.A.; Kuzawa, C.W. A dose-response relationship between fish consumption and human milk DHA concentration among Filipino women in Cebu City. Philippines Acta Paediatr. 2012, 101, 439–445. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture (USDA). Available online: https://ndb.nal.usda.gov/ndb/search/list (accessed on 30 October 2018).
- Kunachowicz, H.; Nadolna, I.; Przygoda, B.; Iwanow, K. Food Composition Tables; PZWL: Warsaw, Poland, 2005. [Google Scholar]
- Torres, A.G.; Ney, J.G.; Meneses, F.; Trugo, N.M. Polyunsaturated fatty acids and conjugated linoleic acid isomers in breast milk are associated with plasma non-esterified and erythrocyte membrane fatty acid composition in lactating women. Br. J. Nutr. 2006, 95, 517–524. [Google Scholar] [CrossRef]
- Haggarty, P. Fatty acid supply to the human fetus. Annu. Rev. Nutr. 2010, 30, 237–255. [Google Scholar] [CrossRef]
- Fidler, N.; Sauerwald, T.; Pohl, A.; Demmelmair, H.; Koletzko, B. Docosahexaenoic acid transfer into human milk after dietary supplementation: A randomized clinical trial. J. Lipid Res. 2000, 41, 1376–1383. [Google Scholar]
- Valenzuela, R.; Videla, L.A. The importance of the long-chain polyunsaturated fatty acid n-6/n-3 ratio in development of non-alcoholic fatty liver associated with obesity. Food Funct. 2011, 2, 644–648. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Tian, H.; Lu, T.; Yu, M.; Xu, W.; Liu, G.; Xie, L. DHA intake interacts with ELOVL2 and ELOVL5 genetic variants to influence polyunsaturated fatty acids in human milk. J. Lipid. Res. 2019, 60, 1043–1049. [Google Scholar] [CrossRef]
- Moltó-Puigmartí, C.; Plat, J.; Mensink, R.P.; Müller, A.; Jansen, E.; Zeegers, M.P.; Thijs, C. FADS1 FADS2 gene variants modify the association between fish intake and the docosahexaenoic acid proportions in human milk. Am. J. Clin. Nutr. 2010, 91, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Bravi, F.; Wiens, F.; Decarli, A.; Dal Pont, A.; Agostoni, C.; Ferraroni, M. Impact of maternal nutrition on breast-milk composition: A systematic review. Am. J. Clin. Nutr. 2016, 104, 646–662. [Google Scholar] [CrossRef] [PubMed]
- Van, R.M.; Hunter, D. Biochemical Indicators of Dietary Intake. In Nutritional Epidemiology, 3rd ed.; Oxford University Press: Oxford, UK, 2013; pp. 150–212. ISBN -13: 978-0199754038. [Google Scholar]
| Characteristic | Mean ± SD | Range |
|---|---|---|
| Age (years) | 30.9 ± 6.5 | 27–44 |
| Height (cm) | 1.66 ± 0.1 | 1.54–1.8 |
| Pre-pregnancy weight (kg) | 62.2 ± 11.8 | 44–90 |
| Pre-pregnancy body mass index (kg/m2) | 22.6 ± 3.4 | 18.6–30.9 |
| Weight gain during pregnancy (kg) | 15.1 ± 4.8 | 7–30 |
| Weight at first month postpartum (kg) | 65.5 ± 13.2 | 45.6–95 |
| Body mass index at first month postpartum (kg/m2) | 23.6 ± 3.8 | 18.5–32.1 |
| Fatty Acids | Mean ± SD | Median (Interquartile Range) |
|---|---|---|
| Saturated fatty acids (SFA) | 41.9 ± 4.9 | 42.3 (38.0–45.7) |
| C4:0 (butanoic acid) | 0.0 ± 0.0 | 0.0 (0.0–0.0) |
| C6:0 (caproic acid) | 0.0 ± 0.0 | 0.0 (0.0–0.0) |
| C8:0 (caprylic acid) | 0.1 ± 0.0 | 0.1 (0.1–0.1) |
| C10:0 (capric acid) | 1.1 ± 0.3 | 1.1 (1.0–1.4) |
| C12:0 (lauric acid) | 3.5 ± 1.1 | 3.3 (2.5–4.1) |
| C13:0 (tridecanoic acid) | 0.1 ± 0.0 | 0.1 (0.0–0.1) |
| C14:0 (myristic acid) | 9.5 ± 2.5 | 9.3 (7.9–11.1) |
| C15:0 (pentadecanoic acid) | 0.8 ± 0.2 | 0.7 (0.7–0.8) |
| C16:0 (palmitic acid) | 19.7 ± 2.5 | 19.9 (17.4–22.0) |
| C17:0 (margaric acid) | 0.5 ± 0.1 | 0.5 (0.4–0.6) |
| C18:0 (stearic acid) | 6.4 ± 1.5 | 6.0 (5.4–7.3) |
| C20:0 (arachidic acid) | 0.2 ± 0.1 | 0.2 (0.1–0.2) |
| C21:0 (henicosanoic acid) | 0.0 ± 0.0 | 0.0 (0.0–0.0) |
| C22:0 (behenic acid) | 0.1 ± 0.0 | 0.1 (0.1–0.1) |
| C23:0 (tetracosanoic acid) | 0.0 ± 0.0 | 0.0 (0.0–0.0) |
| Monounsaturated fatty acids (MUFA) | 39.6 ± 3.1 | 39.0 (38.0–42.0) |
| C14:1 (myristoleic acid) | 0.2 ± 0.1 | 0.2 (0.2–0.3) |
| C15:1 (pentadecenoic acid) | 0.0 ± 0.0 | 0.0 (0.0–0.0) |
| C16:1 trans | 0.4 ± 0.1 | 0.4 (0.3–0.4) |
| C16:1 cis | 2.6 ± 0.5 | 2.6 (2.3–2.9) |
| C17:1 (heptadecenoic acid) | 0.2 ± 0.0 | 0.2 (0.2–0.3) |
| C18:1 cis (oleic acid) | 35.4 ± 3.1 | 34.9 (33.6–37.6) |
| C18:1 trans (vaccenic acid) | 1.2 ± 0.5 | 1.2 (0.8–1.5) |
| C20:1 (gadoleic acid) | 0.8 ± 0.2 | 0.7 (0.7–0.9) |
| C22:1 (erucic acid) | 0.2 ± 0.1 | 0.1 (0.1–0.2) |
| C24:1 (lignoceric acid) | 0.2 ± 0.0 | 0.2 (0.1–0.2) |
| Polyunsaturated fatty acids (PUFA) | 15.1 ± 3.4 | 15.3 (12.7–16.8) |
| n-3 polyunsaturated | 2.7 ± 0.9 | 2.6 (2.1–3.1) |
| C18:3 (α-linolenic acid, ALA) | 1.5 ± 0.6 | 1.4 (1.0–1.8) |
| C20:3 (eicosatrienoic acid) | 0.1 ± 0.0 | 0.1 (0.0–0.1) |
| C20:5 (eicosapentaenoic acid, EPA) | 0.2 ± 0.1 | 0.2 (0.2–0.3) |
| C22:6 (docosahexaenoic acid, DHA) | 0.7 ± 0.3 | 0.7 (0.5–1.0) |
| n-6 polyunsaturated | 12.1 ± 2.7 | 12.1 (10.4–13.4) |
| C18:2 (linoleic acid, LA) | 11.1 ± 2.6 | 11.1 (9.5–12.3) |
| C18:3 (γ-linoleic acid) | 0.1 ± 0.0 | 0.1 (0.1–0.1) |
| C20:3 (dihomo-γ-linoleic acid) | 0.3 ± 0.1 | 0.3 (0.2–0.4) |
| C20:4 (arachidonic acid, ARA) | 0.5 ± 0.1 | 0.5 (0.4–0.6) |
| Ratio | ||
| n-6:n-3 | 4.6 ± 1.0 | 4.8 (4.1–5.1) |
| DHA:LA 2 | 0.1 ± 0.0 | 0.1 (0.0–0.1) |
| ARA 3:DHA 4 | 0.9 ± 0.4 | 0.7 (0.5–1.1) |
| LA:ALA 5 | 8.1 ± 2.4 | 7.8 (6.4–9.6) |
| Total fat concentration 6 | 3.49 ± 1.0 | 3.5 (3.0–4.2) |
| Fatty Acids | Mean ± SD (g) | Median (Interquartile Range) (g) |
|---|---|---|
| Saturated fatty acids (SFA) | 23.9 ± 10.3 | 20.9 (7.3–69.5) |
| C4:0 (butanoic acid) | 0.4 ± 0.3 | 0.5 (0–1.1) |
| C6:0 (caproic acid) | 0.3 ± 0.2 | 0.3 (0–0.8) |
| C8:0 (caprylic acid) | 0.2 ± 0.2 | 0.2 (0–1.0) |
| C10:0 (capric acid) | 0.5 ± 0.3 | 0.5 (0–1.6) |
| C12:0 (lauric acid) | 0.9 ± 0.9 | 0.7 (0.2–5.5) |
| C14:0 (myristic acid) | 2.8 ± 1.4 | 2.7 (0.7–7.9) |
| C15:0 (pentadecanoic acid) | 0.3 ± 0.2 | 0.3 (0–0.9) |
| C16:0 (palmitic acid) | 12.6 ± 5.2 | 11.6 (4.5–35.0) |
| C17:0 (margaric acid) | 0.2 ± 0.1 | 0.2 (0–0.7) |
| C18:0 (stearic acid) | 5.2 ± 2.8 | 5.1 (1.2–18.3) |
| C20:0 (arachidic acid | 0.1 ± 0.1 | 0.1 (0–0.3) |
| Monounsaturated fatty acids (MUFA) | 24.7 ± 8.9 | 24.8 (13.2–55.0) |
| C14:1 (myristoleic acid) | 0.2 ± 0.2 | 0.2 (0–0.8) |
| C15:1 (pentadecenoic acid) | 0.1 ± 0.1 | 0.1 (0–0.2) |
| C16:1 | 1.3 ± 0.4 | 1.2 (0.6–2.4) |
| C17:1 (heptadecenoic acid) | 0.1 ± 0.1 | 0.1 (0–0.5) |
| C18:1 (oleic and vaccenic acids) | 22.1 ± 8.3 | 21.7 (11.5–50.3) |
| C20:1 (gadoleic acid) | 0.3 ± 0.2 | 0.2 (0.1–0.9) |
| C22:1 (erucic acid) | 0.3 ± 0.3 | 0.1 (0–1.1) |
| Polyunsaturated fatty acids (PUFA) | 10.7 ± 4.0 13.5 ± 14.2 s | 11.2 (4.9–22.9) 11.3 (4.9–88.4) s |
| C18:2 (linoleic acid, LA) | 8.5 ± 3.4 | 8.5 (4.1–21.1) |
| C18:3 (α-linolenic acid, ALA) | 1.5 ± 0.8 | 1.4 (0.5–4.3) |
| C20:5 (eicosapentaenoic acid, EPA) | 0.1 ± 0.2 0.1 ± 0.2 s | 0 (0–0.5) 0.1 (0–0.5) s |
| C22:6 (docosahexaenoic acid, DHA) | 0.2 ± 0.3 0.6 ± 0.6 s | 0.1 (0–1.2) 0.4 (0–1.8) s |
| EPA (mg) | DHA (mg) | |
|---|---|---|
| Food | 104.4 ± 152.0 (18, 0–190) | 243.3 ± 333.5 (50, 37–411) |
| Supplement 1 | 29.7 ± 43.6 (0, 0–43) | 370.6 ± 465.1 (250, 8–549) |
| Food + supplement | 134.1 ± 153.9 (85, 1–215) | 614.0 ± 574.6 (354, 202–905) |
| Concentration in Human Milk | Dietary Intake, Sole or Together with Supplementation | |||||
|---|---|---|---|---|---|---|
| SFA | MUFA | PUFA | ALA | EPA | DHA | |
| SFA 1 | 0.26 | 0.16 | −0.20 | −0.13 | −0.18 −0.19 s | −0.10 −0.16 s |
| MUFA 2 | −0.14 | −0.04 | 0.14 | 0.26 | 0.21 | 0.19 |
| PUFA 3 | −0.20 | −0.14 | 0.20 | 0.01 | 0.08 | −0.01 |
| ALA 4 | −0.19 | −0.09 | 0.04 | 0.32 | 0.20 | −0.06 |
| EPA 5 | −0.16 | 0.05 | 0.05 | −0.11 | 0.20 0.17 s | −0.02 0.17 s |
| DHA 6 | −0.24 | −0.18 | −0.04 | −0.26 | 0.16 0.23 s | 0.04 0.24 s |
| Food | Never | Less than Once a Week | Once or Twice a Week | More than Twice a Week but Not Every Day | Every Day | Correlation 1 with Concentrations in Human Milk | ||
|---|---|---|---|---|---|---|---|---|
| DHA 2 | EPA 3 | ALA 4 | ||||||
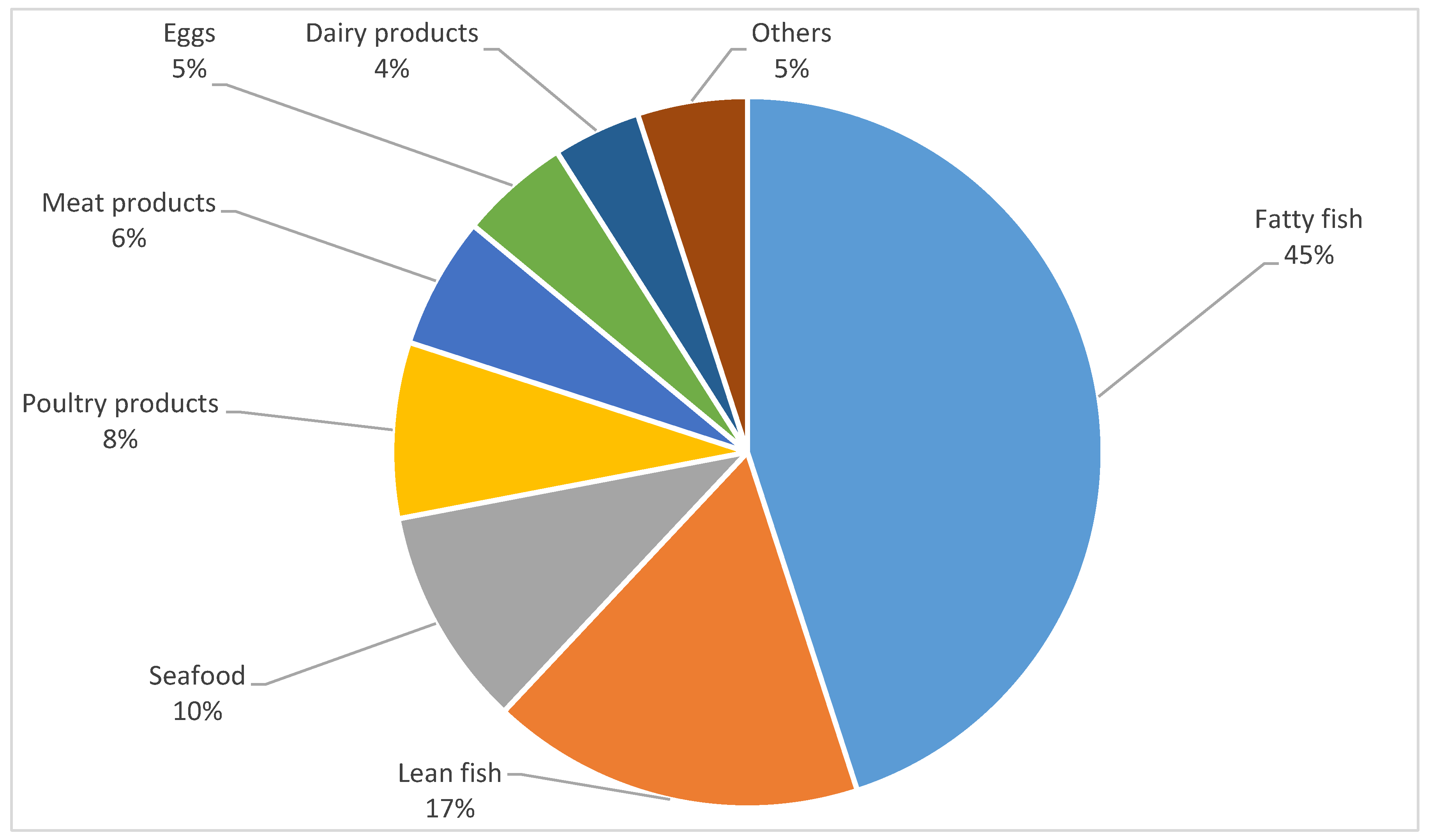
| Fatty fish (e.g., salmon, herring) | 12.50 | 31.25 | 46.88 | 9.38 | 0.00 | 0.25 * | 0.27 * | 0.28 * |
| Lean fish (e.g., cod, sole) | 21.88 | 31.25 | 43.75 | 3.13 | 0.00 | 0.14 | 0.08 | 0.21 |
| Seafood | 31.25 | 43.75 | 25.17 | 0.00 | 0.00 | 0.21 | 0.13 | 0.19 |
| Poultry and turkey | 3.13 | 3.13 | 43.75 | 40.63 | 9.38 | 0.09 | 0.13 | 0.08 |
| Pork | 6.25 | 40.63 | 37.50 | 15.63 | 0.00 | 0.02 | 0.29 * | 0.18 |
| Beef | 9.38 | 50.00 | 34.38 | 6.25 | 0.00 | −0.25 * | −0.14 | 0.11 |
| Meat products (e.g., sausages, sliced meats) | 6.25 | 12.50 | 37.50 | 18.75 | 25.00 | 0.24 | −0.11 | −0.10 |
| Eggs | 15.63 | 6.25 | 34.38 | 34.38 | 9.38 | −0.14 | −0.06 | −0.00 |
| Milk | 31.25 | 15.63 | 9.38 | 6.25 | 37.50 | 0.02 | 0.05 | 0.26 * |
| Fermented dairy products | 37.50 | 28.13 | 21.88 | 12.50 | 0.00 | 0.17 | 0.10 | 0.29 * |
| Cheese | 25.00 | 28.13 | 15.63 | 18.75 | 12.50 | −0.02 | −0.02 | 0.00 |
| Cottage cheese | 21.88 | 12.50 | 43.75 | 15.63 | 6.25 | 0.22 | 0.06 | 0.17 |
| Milk desserts | 46.88 | 28.13 | 9.38 | 15.63 | 0.00 | 0.11 | −0.12 | −0.18 |
| Butter | 15.63 | 9.38 | 12.50 | 18.75 | 43.75 | −0.14 | −0.11 | 0.10 |
| Canola oil | 18.75 | 28.13 | 21.88 | 25.00 | 6.25 | 0.09 | −0.0 | 0.13 |
| Olive oil | 12.50 | 18.75 | 25.00 | 43.75 | 0.00 | 0.08 | 0.02 | 0.03 |
| Linseed oil | 62.50 | 15.63 | 9.38 | 9.38 | 3.13 | 0.01 | 0.10 | 0.30 * |
| Coconut oil | 56.25 | 25.00 | 15.63 | 3.13 | 0.00 | −0.12 | −0.07 | 0.29 * |
| Nuts and seeds | 6.25 | 18.75 | 21.88 | 28.13 | 25.00 | −0.03 | 0.02 | 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bzikowska-Jura, A.; Czerwonogrodzka-Senczyna, A.; Jasińska-Melon, E.; Mojska, H.; Olędzka, G.; Wesołowska, A.; Szostak-Węgierek, D. The Concentration of Omega-3 Fatty Acids in Human Milk Is Related to Their Habitual but Not Current Intake. Nutrients 2019, 11, 1585. https://doi.org/10.3390/nu11071585
Bzikowska-Jura A, Czerwonogrodzka-Senczyna A, Jasińska-Melon E, Mojska H, Olędzka G, Wesołowska A, Szostak-Węgierek D. The Concentration of Omega-3 Fatty Acids in Human Milk Is Related to Their Habitual but Not Current Intake. Nutrients. 2019; 11(7):1585. https://doi.org/10.3390/nu11071585
Chicago/Turabian StyleBzikowska-Jura, Agnieszka, Aneta Czerwonogrodzka-Senczyna, Edyta Jasińska-Melon, Hanna Mojska, Gabriela Olędzka, Aleksandra Wesołowska, and Dorota Szostak-Węgierek. 2019. "The Concentration of Omega-3 Fatty Acids in Human Milk Is Related to Their Habitual but Not Current Intake" Nutrients 11, no. 7: 1585. https://doi.org/10.3390/nu11071585
APA StyleBzikowska-Jura, A., Czerwonogrodzka-Senczyna, A., Jasińska-Melon, E., Mojska, H., Olędzka, G., Wesołowska, A., & Szostak-Węgierek, D. (2019). The Concentration of Omega-3 Fatty Acids in Human Milk Is Related to Their Habitual but Not Current Intake. Nutrients, 11(7), 1585. https://doi.org/10.3390/nu11071585







