Dietary Supplements of Shiikuwasha Extract Attenuates Osteoarthritis Progression in Meniscal/ligamentous Injury and Obese Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Shiikuwasha Extract Preparation (SE)
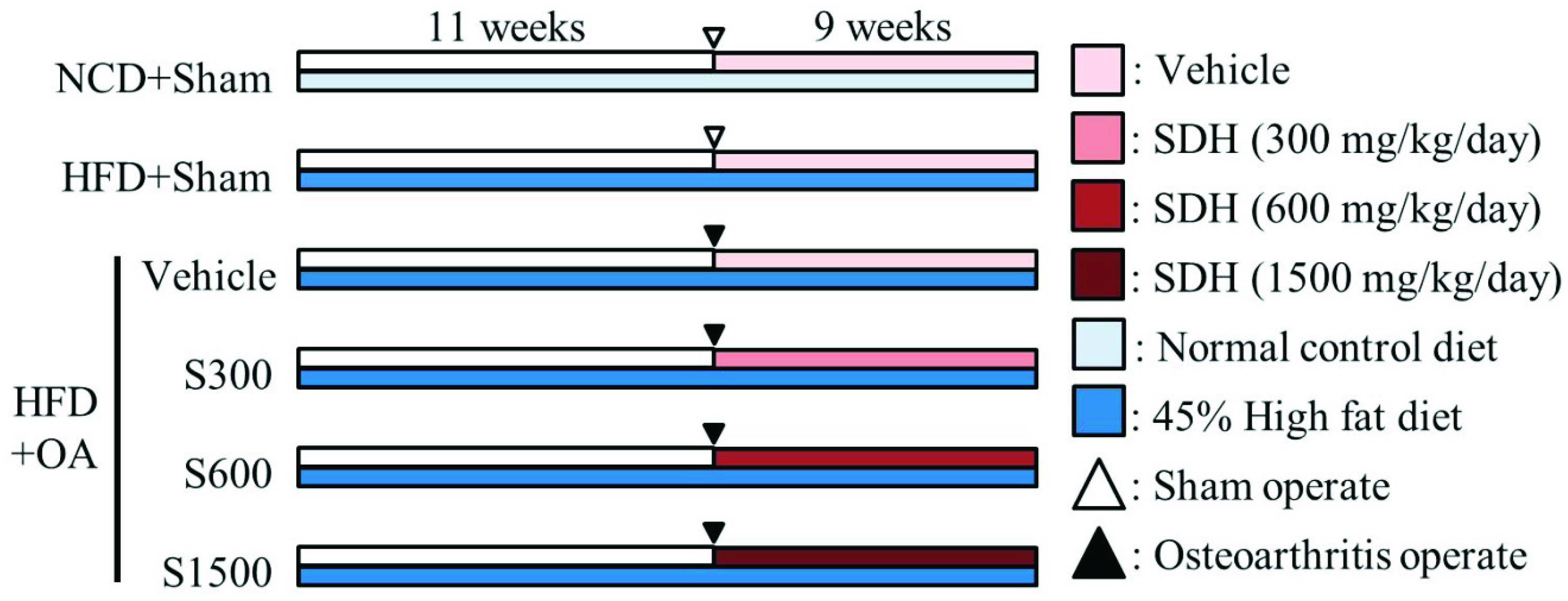
2.2. Experimental Design and Shiikuwasha Extract Administration
2.3. ACLT/MMx
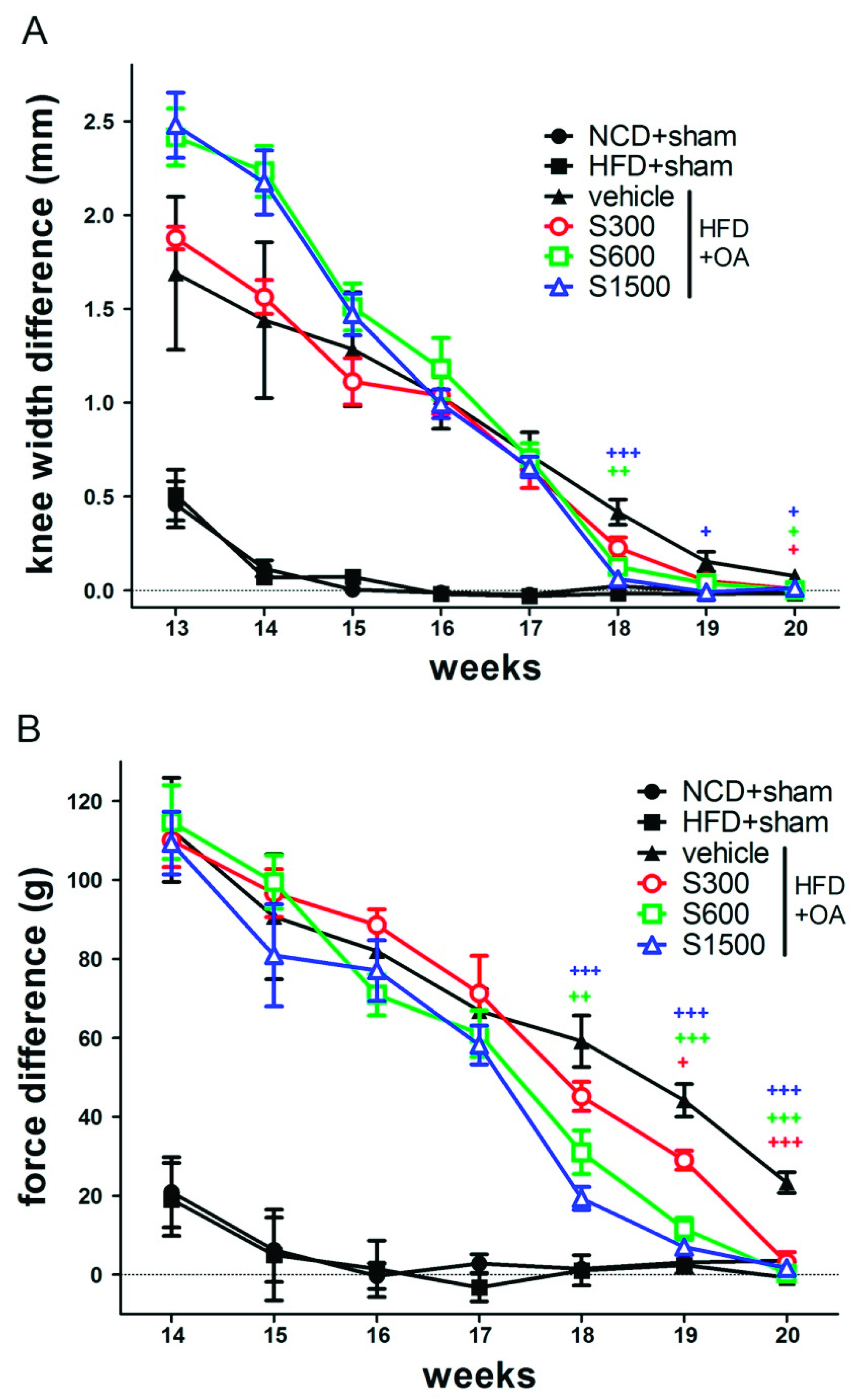
2.4. Incapacitance Test (Weight-Bearing Test)
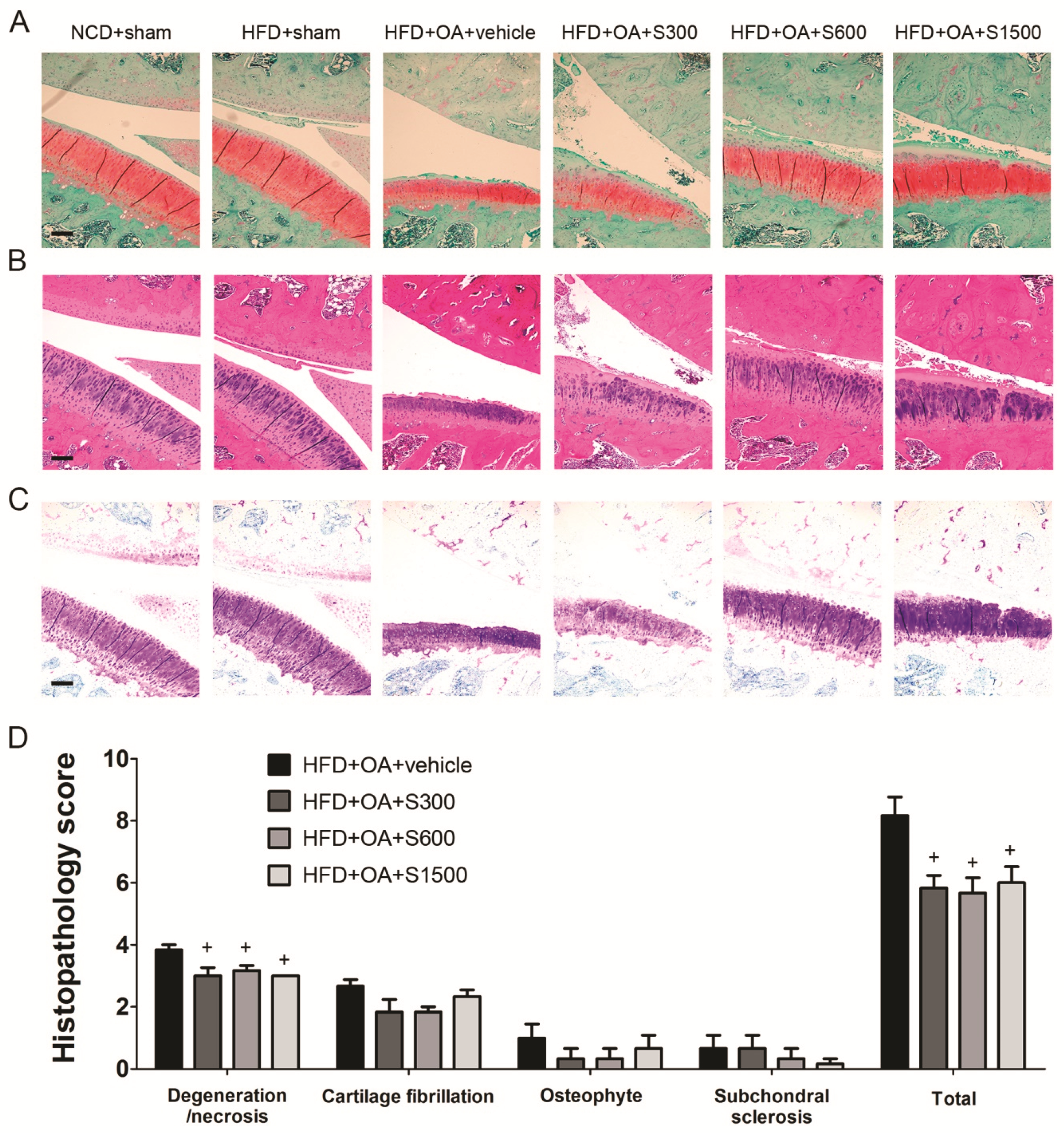
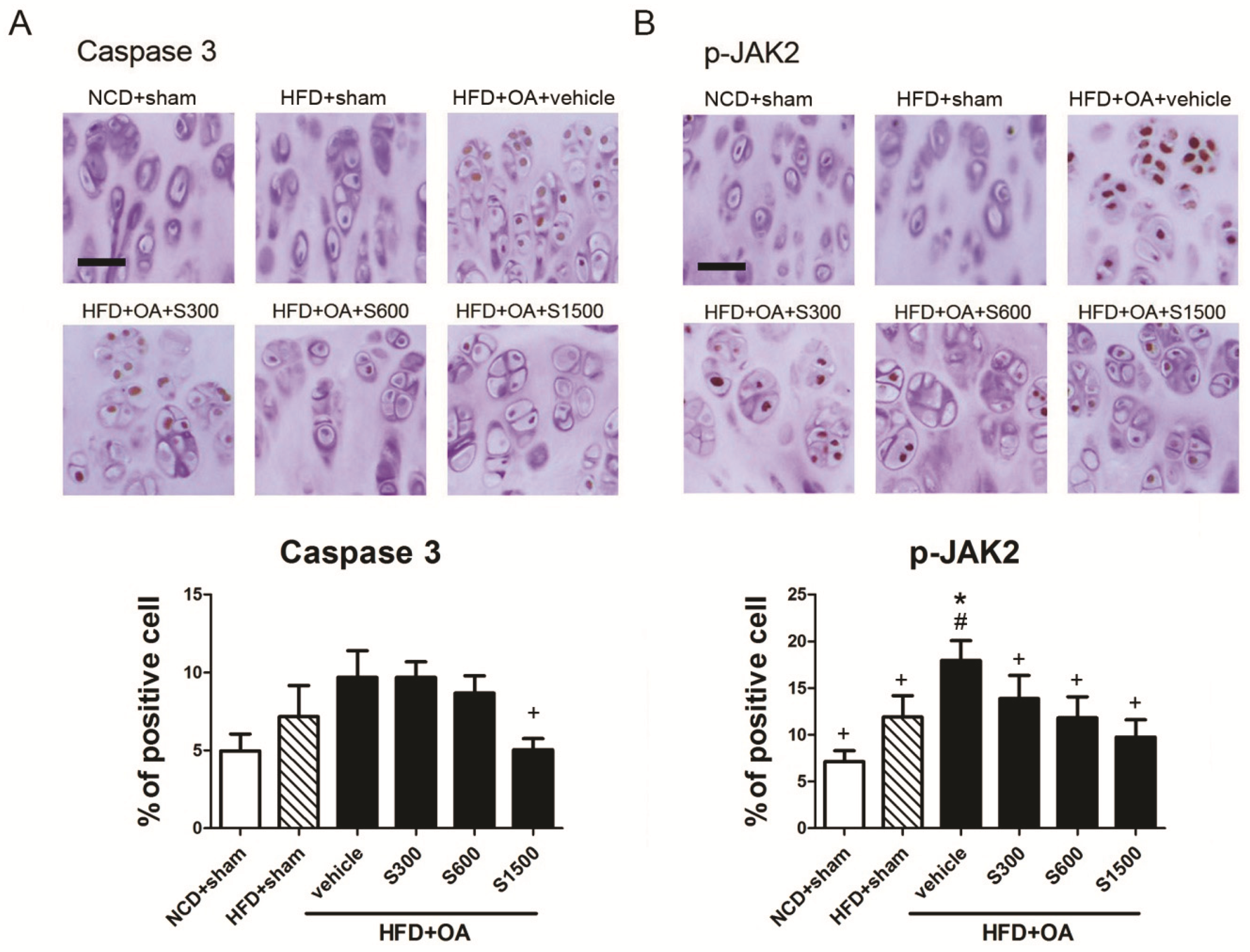
2.5. Histopathological Analysis of Joints
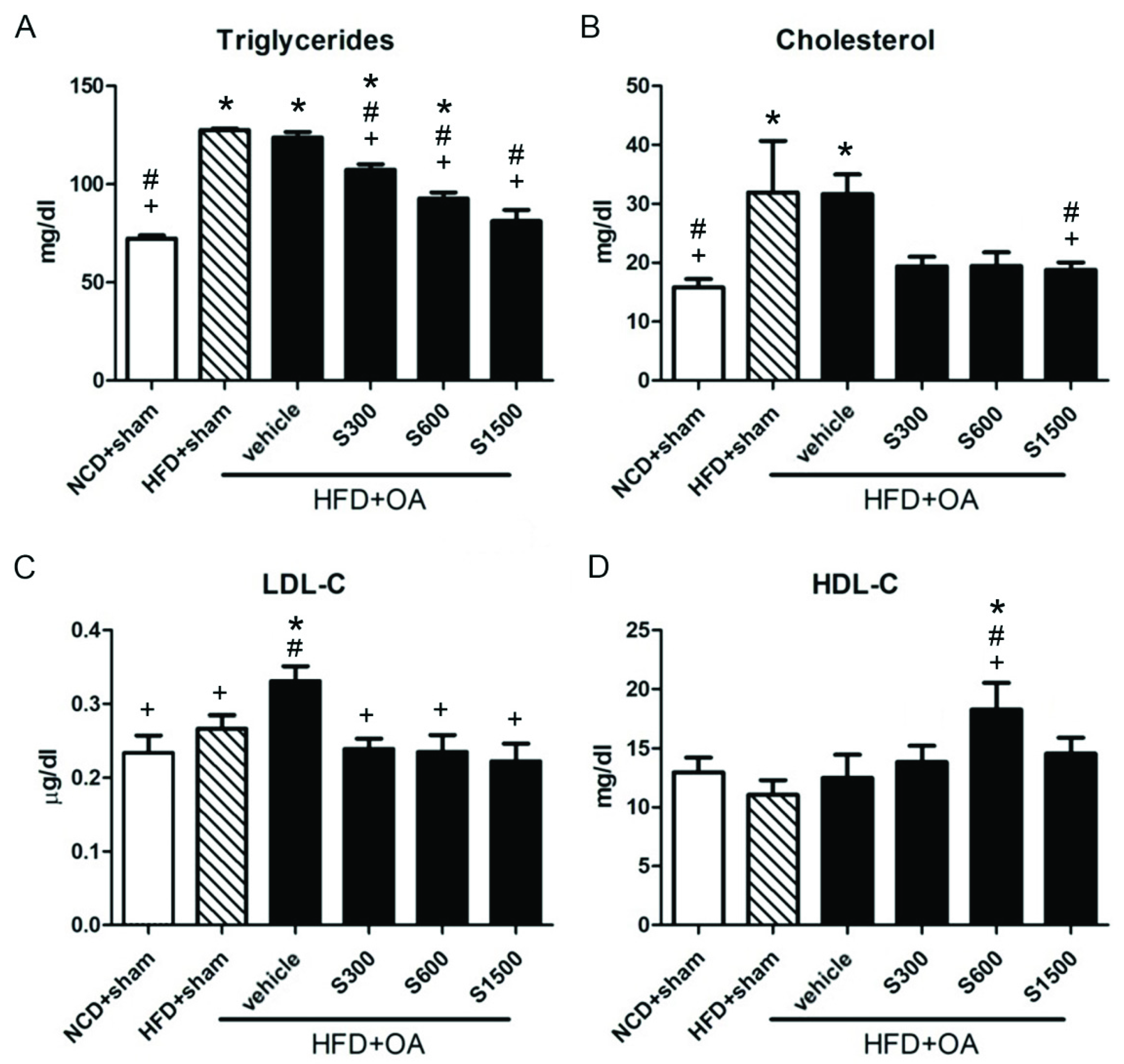
2.6. Lipid Profile, Liver Function, Kidney Function, and TNF-α Measurement
2.7. Lipid Peroxidation Analysis
2.8. Determination of Antioxidant Enzymes
2.9. Statistical Analyses
3. Results
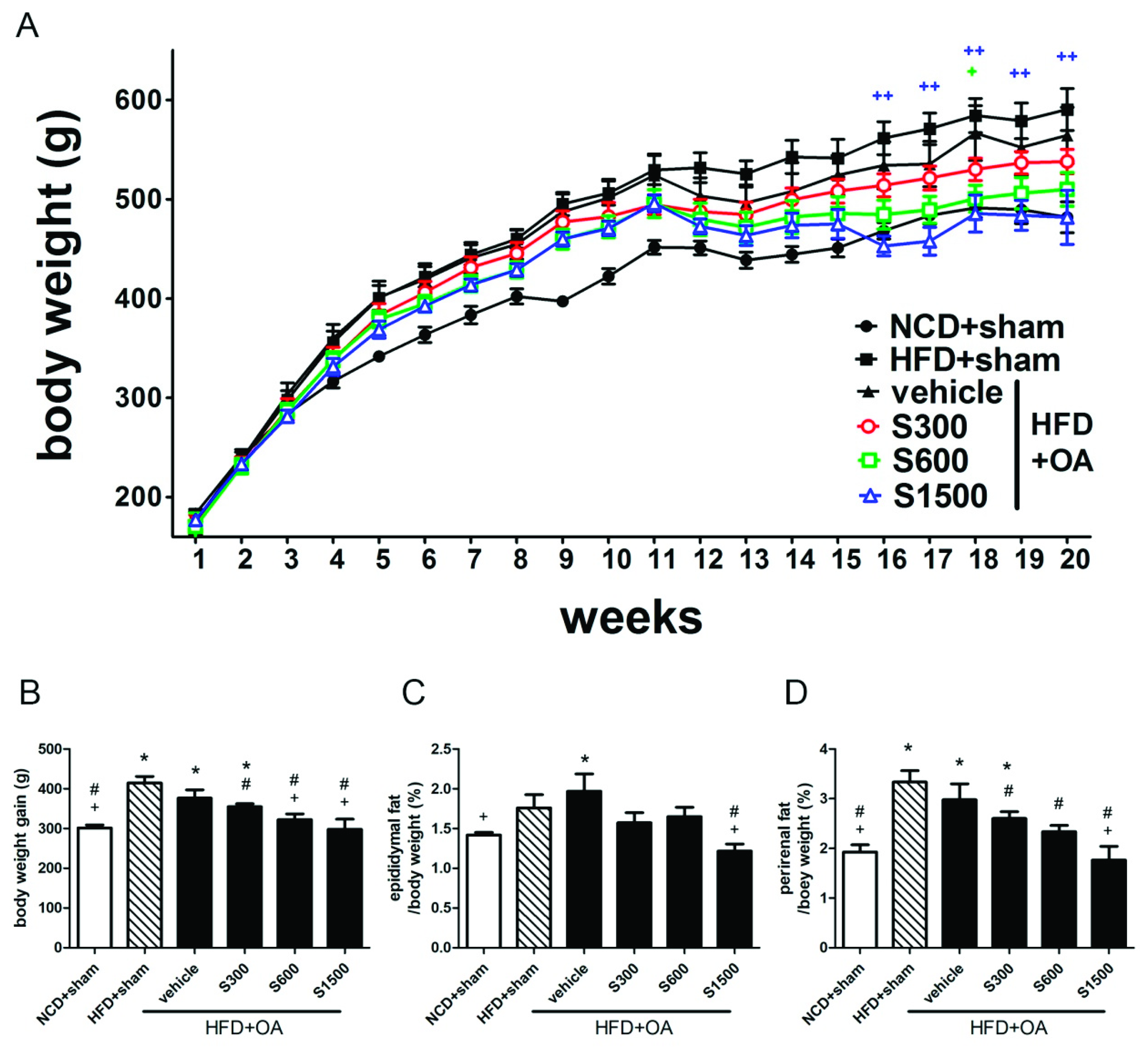
3.1. Effect of SE on Reduction of Body Weight and Fat Pad
3.2. Effect of Shiikuwasha Extract on Antioxidative Properties and Anti-Inflammatory
3.3. Effect of Shiikuwasha Extract on Attenuating Osteoarthritis Caused Pain and Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Felson, D.T.; Lawrence, R.C.; Dieppe, P.A.; Hirsch, R.; Helmick, C.G.; Jordan, J.M.; Kington, R.S.; Lane, N.E.; Nevitt, M.C.; Zhang, Y.; et al. Osteoarthritis: New insights. Part 1: The disease and its risk factors. Ann. Intern. Med. 2000, 133, 635–646. [Google Scholar] [CrossRef]
- Maiese, K. Picking a bone with wisp1 (ccn4): New strategies against degenerative joint disease. J. Transl. Sci. 2016, 1, 83. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.-M.; Rannou, F.; Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Litwic, A.; Edwards, M.H.; Dennison, E.M.; Cooper, C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013, 105, 185–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wassilew, G.I.; Lehnigk, U.; Duda, G.N.; Taylor, W.R.; Matziolis, G.; Dynybil, C. The expression of proinflammatory cytokines and matrix metalloproteinases in the synovial membranes of patients with osteoarthritis compared with traumatic knee disorders. Arthrosc. J. Arthrosc. Relat. Surg. 2010, 26, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.C.; Martel-Pelletier, J.; Pelletier, J.P. The role of cytokines in osteoarthritis pathophysiology. Biorheology 2002, 39, 237–246. [Google Scholar] [PubMed]
- Altindag, O.; Erel, O.; Aksoy, N.; Selek, S.; Celik, H.; Karaoglanoglu, M. Increased oxidative stress and its relation with collagen metabolism in knee osteoarthritis. Rheumatol. Int. 2007, 27, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Tiku, M.L.; Shah, R.; Allison, G.T. Evidence linking chondrocyte lipid peroxidation to cartilage matrix protein degradation. Possible role in cartilage aging and the pathogenesis of osteoarthritis. J. Biol. Chem. 2000, 275, 20069–20076. [Google Scholar] [CrossRef]
- Hadipour-Jahromy, M.; Mozaffari-Kermani, R. Chondroprotective effects of pomegranate juice on monoiodoacetate-induced osteoarthritis of the knee joint of mice. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2010, 24, 182–185. [Google Scholar] [CrossRef]
- Zhang, W.; Ouyang, H.; Dass, C.R.; Xu, J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016, 4, 15040. [Google Scholar] [CrossRef] [PubMed]
- Ameye, L.G.; Chee, W.S. Osteoarthritis and nutrition. From nutraceuticals to functional foods: A systematic review of the scientific evidence. Arthritis Res. Ther. 2006, 8, R127. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Soeken, K.; Ernst, E. Herbal medicines for the treatment of osteoarthritis: A systematic review. Rheumatology 2001, 40, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-L.; Smith, B.J.; Lo, D.-F.; Chyu, M.-C.; Dunn, D.M.; Chen, C.-H.; Kwun, I.-S. Dietary polyphenols and mechanisms of osteoarthritis. J. Nutr. Biochem. 2012, 23, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Manthey, J.A.; Guthrie, N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J. Agric. Food Chem. 2002, 50, 5837–5843. [Google Scholar] [CrossRef] [PubMed]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid composition of fruit tissues of citrus species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Gao, W.; Zeng, S.-L.; Li, P.; Liu, E.-H. Chemical structures, bioactivities and molecular mechanisms of citrus polymethoxyflavones. J. Funct. Foods 2018, 40, 498–509. [Google Scholar] [CrossRef]
- Saokham, P.; Loftsson, T. Γ-cyclodextrin. Int. J. Pharm. 2017, 516, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Sakata, Y.; Okamoto, T.; Oshio, K.; Nakamura, H.; Iwamoto, H.; Namba, K.; Takeda, Y.; Yoshizawa, F. Dietary supplementation with shiikuwasha extract attenuates dexamethasone-induced skeletal muscle atrophy in aged rats. SpringerPlus 2016, 5, 816. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.-H.; Lin, S.-H.; Lai, C.-F.; Lin, Y.-C.; Kong, Z.-L.; Wong, C.-S. Shea nut oil triterpene concentrate attenuates knee osteoarthritis development in rats: Evidence from knee joint histology. PLoS ONE 2016, 11, e0162022. [Google Scholar] [CrossRef]
- Shackelford, C.; Long, G.; Wolf, J.; Okerberg, C.; Herbert, R. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol. Pathol. 2002, 30, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Placer, Z.A.; Cushman, L.L.; Johnson, B.C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem. 1966, 16, 359–364. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Pottie, P.; Presle, N.; Terlain, B.; Netter, P.; Mainard, D.; Berenbaum, F. Obesity and osteoarthritis: More complex than predicted! Ann. Rheum. Dis. 2006, 65, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Messier, S.P.; Gutekunst, D.J.; Davis, C.; DeVita, P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheumatol. 2005, 52, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Puett, D.W.; Griffin, M.R. Published trials of nonmedicinal and noninvasive therapies for hip and knee osteoarthritis. Ann. Intern. Med. 1994, 121, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Mascarenhas, R.; Saltzman, B.M.; Rollins, M.; Bach, B.R.; MacDonald, P. The relationship between anterior cruciate ligament injury and osteoarthritis of the knee. Adv. Orthop. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Cha, B.-Y.; Saito, K.; Choi, S.-S.; Wang, X.X.; Choi, B.-K.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.-T. Effects of a citrus depressa hayata (shiikuwasa) extract on obesity in high-fat diet-induced obese mice. Phytomedicine 2011, 18, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Brzoska, M.; Moniuszko-Jakoniuk, J.; Piłat-Marcinkiewicz, B.; Sawicki, B. Liver and kidney function and histology in rats exposed to cadmium and ethanol. Alcohol Alcohol. 2003, 38, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Ostalowska, A.; Birkner, E.; Wiecha, M.; Kasperczyk, S.; Kasperczyk, A.; Kapolka, D.; Zon-Giebel, A. Lipid peroxidation and antioxidant enzymes in synovial fluid of patients with primary and secondary osteoarthritis of the knee joint. Osteoarthr. Cartil. 2006, 14, 139–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, J.L.; Gabrielides, C.; Davidson, R.K.; Swingler, T.E.; Clark, I.M.; Wallis, G.A.; Boot-Handford, R.P.; Kirkwood, T.B.; Taylor, R.W.; Young, D.A. Superoxide dismutase downregulation in osteoarthritis progression and end-stage disease. Ann. Rheum. Dis. 2010, 69, 1502–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henrotin, Y.; Bruckner, P.; Pujol, J.-P. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthr. Cartil. 2003, 11, 747–755. [Google Scholar] [CrossRef] [Green Version]
- Ishiwa, J.; Sato, T.; Mimaki, Y.; Sashida, Y.; Yano, M.; Ito, A. A citrus flavonoid, nobiletin, suppresses production and gene expression of matrix metalloproteinase 9/gelatinase b in rabbit synovial fibroblasts. J. Rheumatol. 2000, 27, 20–25. [Google Scholar] [PubMed]
- Liu, J.-W.; Hao, C.-W.; Wang, Z.-H.; Jin, D. Nobiletin inhibits expression of inflammatory mediators and regulates jnk/erk/p38 mapk and pi3k/akt pathways in human osteoarthritic chondrocytes. Trop. J. Pharm. Res. 2016, 15, 535–545. [Google Scholar] [CrossRef]
- Gu, Y.-T.; Chen, J.; Meng, Z.-L.; Ge, W.-Y.; Bian, Y.-Y.; Cheng, S.-W.; Xing, C.-K.; Yao, J.-L.; Fu, J.; Peng, L. Research progress on osteoarthritis treatment mechanisms. Biomed. Pharmacother. 2017, 93, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.M.; Shen, C.; Li, H.; Fan, Q.; Ding, J.; Jin, F.C.; Sha, L. Leptin induces the apoptosis of chondrocytes in an in vitro model of osteoarthritis via the jak2-stat3 signaling pathway. Mol. Med. Rep. 2016, 13, 3684–3690. [Google Scholar] [CrossRef]
- Xu, X.; Lv, H.; Li, X.; Su, H.; ZHang, X.; Yang, J. Danshen attenuates cartilage injuries in osteoarthritis in vivo and in vitro by activating jak2/stat3 and akt pathways. Exp. Anim. 2018, 67, 127–137. [Google Scholar] [CrossRef]

| ALT (U/L) | AST (U/L) | Urea (mmol/L) | Creatinine (mg/dl) | |
|---|---|---|---|---|
| NCD+sham | 21.80 ± 6.05 | 31.75 ± 3.89 | 15.06 ± 0.07 | 0.68 ± 0.05 |
| HFD+sham | 19.56 ± 10.06 | 33.41 ± 3.95 | 14.98 ± 0.08 | 0.74 ± 0.02 |
| HFD+OA+vehicle | 20.92 ± 3.03 | 33.47 ± 4.56 | 15.28 ± 0.04 | 0.77 ± 0.01 |
| HFD+OA+S300 | 21.53 ± 3.76 | 34.31 ± 7.01 | 15.03 ± 0.30 | 0.68 ± 0.02 |
| HFD+OA+S600 | 19.03 ± 3.21 | 35.01 ± 7.39 | 15.24 ± 0.08 | 0.73 ± 0.03 |
| HFD+OA+S1500 | 20.66 ± 6.00 | 31.49 ± 2.94 | 14.82 ± 0.53 | 0.72 ± 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, Y.-W.; Lai, Y.-J.; Kong, Z.-L. Dietary Supplements of Shiikuwasha Extract Attenuates Osteoarthritis Progression in Meniscal/ligamentous Injury and Obese Rats. Nutrients 2019, 11, 1312. https://doi.org/10.3390/nu11061312
Yen Y-W, Lai Y-J, Kong Z-L. Dietary Supplements of Shiikuwasha Extract Attenuates Osteoarthritis Progression in Meniscal/ligamentous Injury and Obese Rats. Nutrients. 2019; 11(6):1312. https://doi.org/10.3390/nu11061312
Chicago/Turabian StyleYen, Yu-Wen, Ying-Jiun Lai, and Zwe-Ling Kong. 2019. "Dietary Supplements of Shiikuwasha Extract Attenuates Osteoarthritis Progression in Meniscal/ligamentous Injury and Obese Rats" Nutrients 11, no. 6: 1312. https://doi.org/10.3390/nu11061312
APA StyleYen, Y.-W., Lai, Y.-J., & Kong, Z.-L. (2019). Dietary Supplements of Shiikuwasha Extract Attenuates Osteoarthritis Progression in Meniscal/ligamentous Injury and Obese Rats. Nutrients, 11(6), 1312. https://doi.org/10.3390/nu11061312






