Dihydrocapsaicin Inhibits Epithelial Cell Transformation through Targeting Amino Acid Signaling and c-Fos Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Cell Viability
2.4. Cell Transformation Assay
2.5. Luciferase Assays
2.6. Immunoblot Assay
2.7. AP-1 Transcription Activity Assay
2.8. Immunofluorescence Assay
2.9. Analysis of Cell Death
2.10. Colony Formation Assay
2.11. Statistical Analysis
3. Results
3.1. Dihydrocapsaicin Suppresses EGF- and TPA-Mediated Neoplastic Cell Transformation
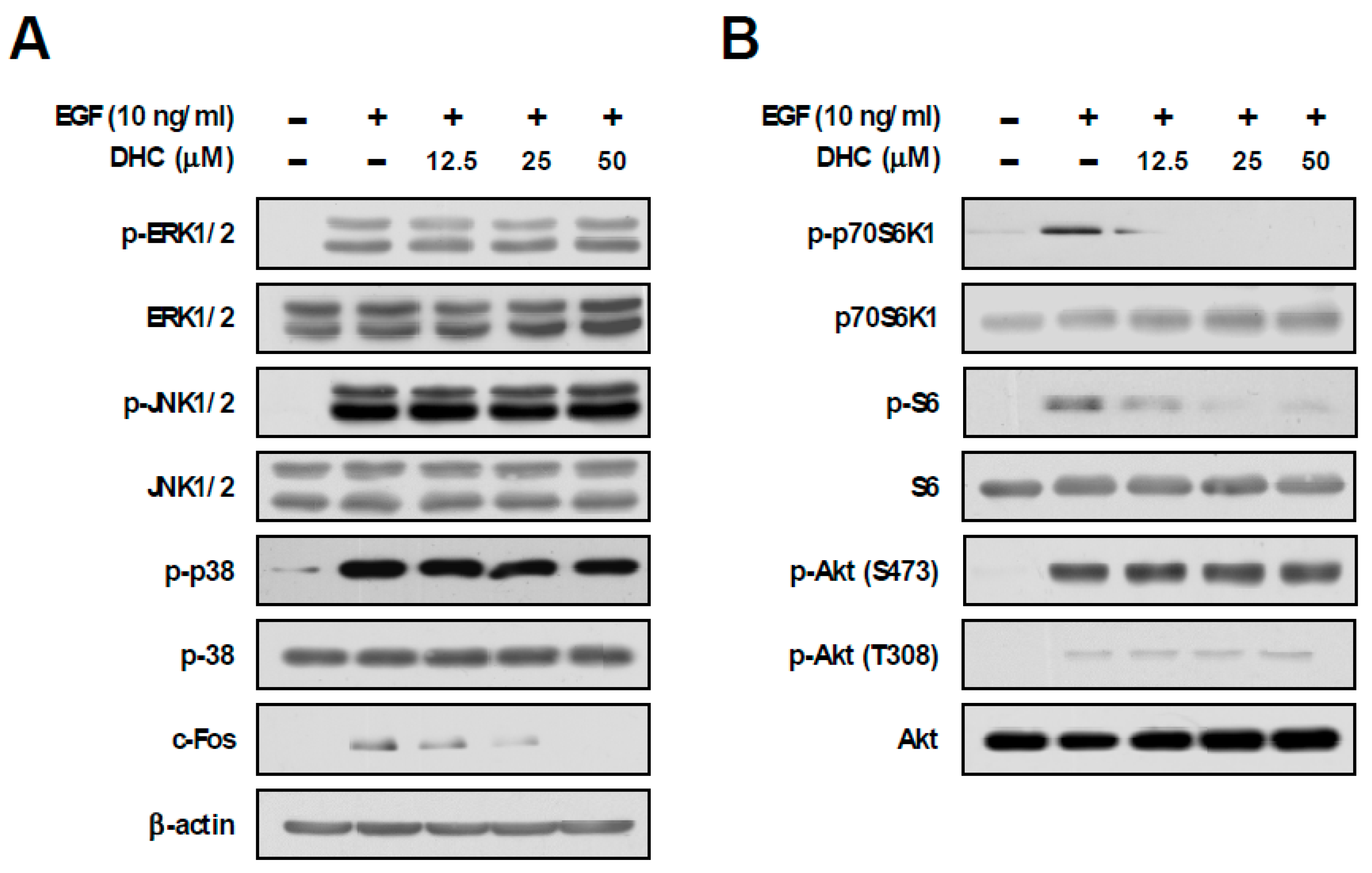
3.2. Dihydrocapsaicin Suppresses p70S6K1 Phosphorylation and c-Fos Expression
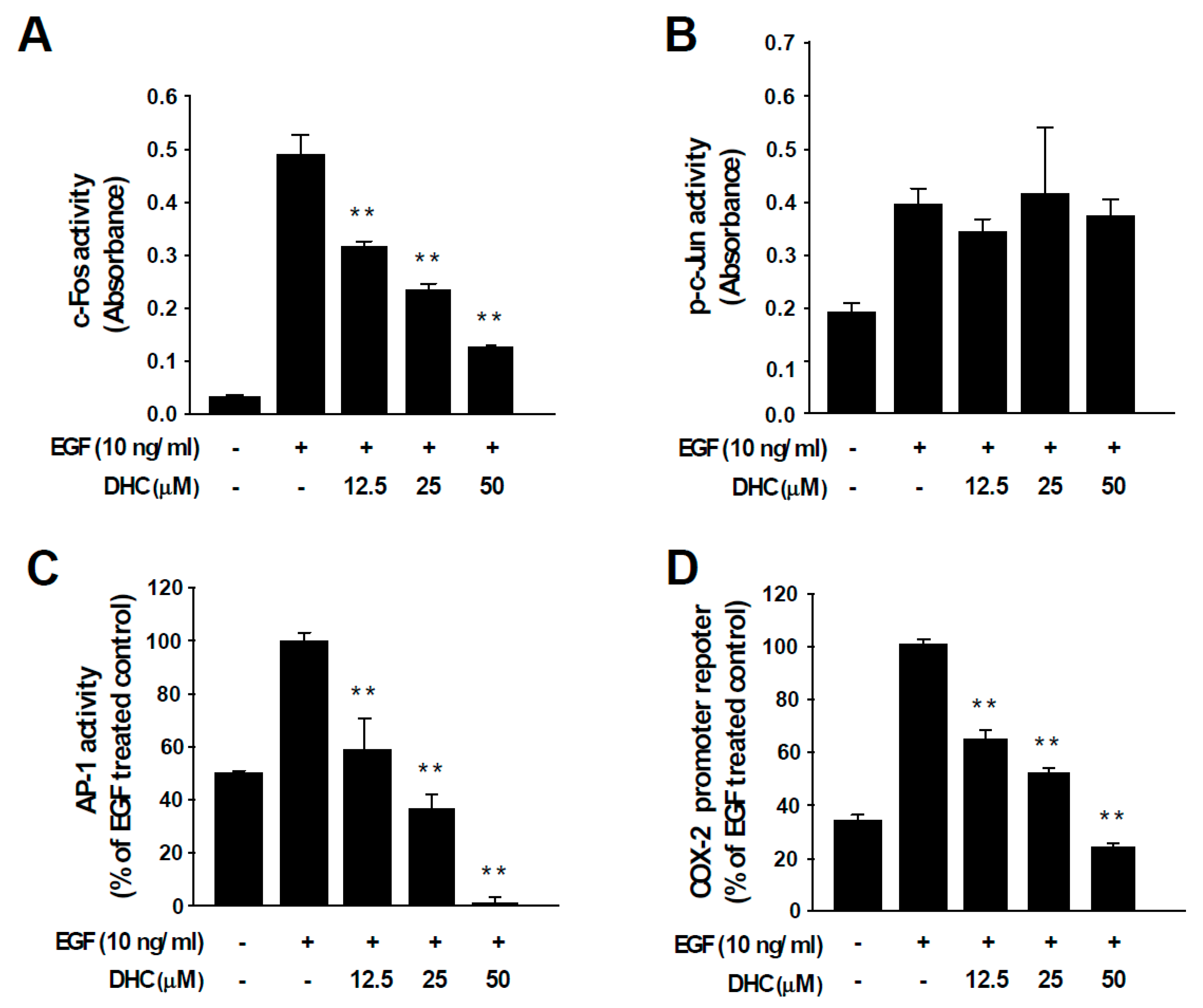
3.3. Dihydrocapsaicin Attenuates EGF-Induced c-Fos and AP-1 Activities and COX-2 Transcriptional Activity
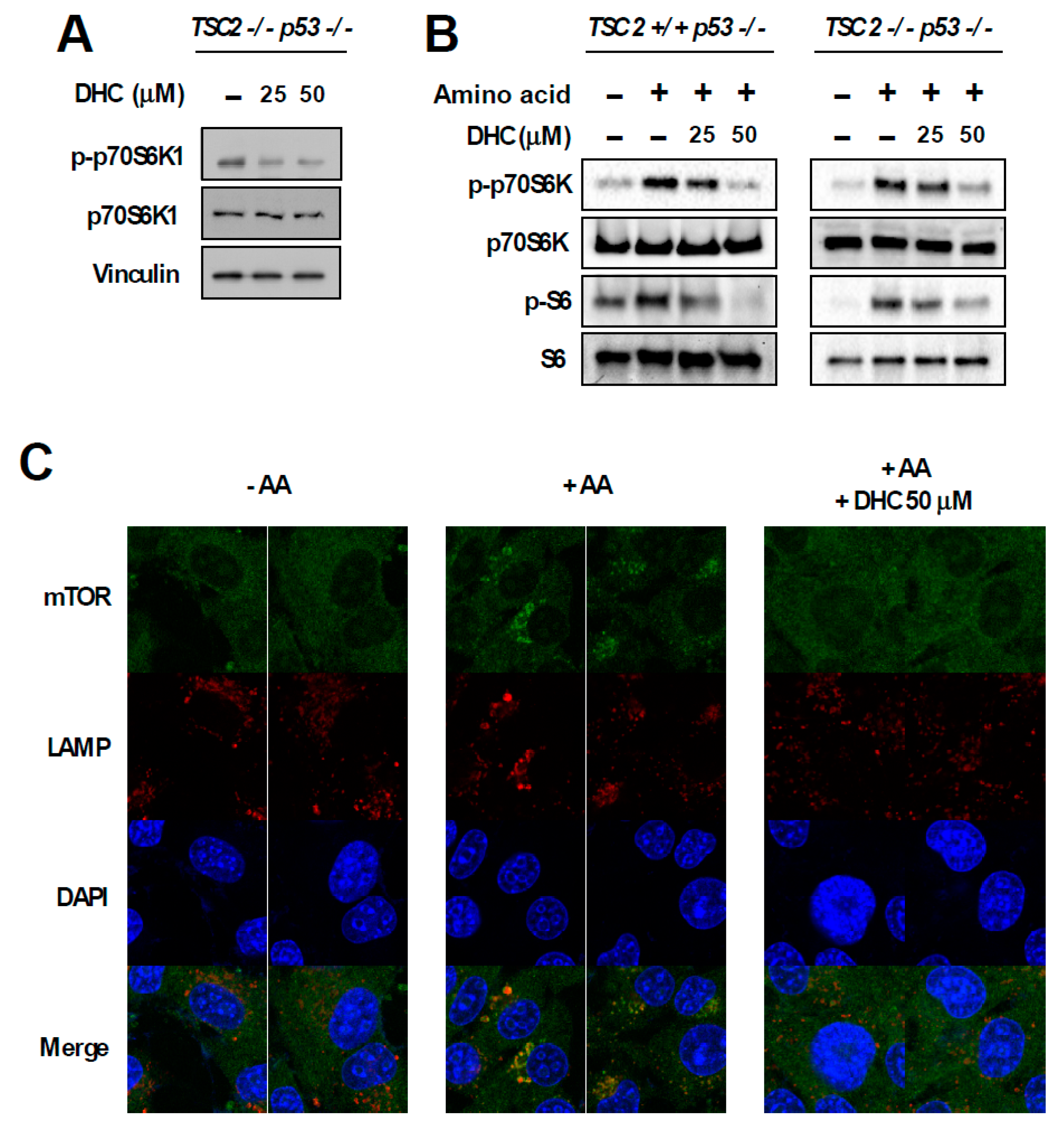
3.4. Dihydrocapsaicin Targets the Amino Acid Signaling Pathway
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Usman, M.G.; Rafii, M.Y.; Ismail, M.R.; Malek, M.A.; Latif, M.A. Capsaicin and dihydrocapsaicin determination in chili pepper genotypes using ultra-fast liquid chromatography. Molecules 2014, 19, 6474–6488. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.J.; Peng, J.; Li, Y.J. Recent advances in the study on capsaicinoids and capsinoids. Eur. J. Pharmacol. 2011, 650, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Arimboor, R.; Natarajan, R.B.; Menon, K.R.; Chandrasekhar, L.P.; Moorkoth, V. Red pepper (Capsicum annuum) carotenoids as a source of natural food colors: Analysis and stability-a review. J. Food Sci. Technol. 2015, 52, 1258–1271. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ortega, M.; Ortiz-Moreno, A.; Hernandez-Navarro, M.D.; Chamorro-Cevallos, G.; Dorantes-Alvarez, L.; Necoechea-Mondragon, H. Antioxidant, antinociceptive, and anti-inflammatory effects of carotenoids extracted from dried pepper (Capsicum annuum L.). J. Biomed. Biotechnol. 2012, 2012, 524019. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Wang, Y.; Yu, Y.; Li, Y.; Zhao, S.; Chen, Y.; Waqar, A.B.; Fan, J.; Liu, E. Expression of TRPV1 in rabbits and consuming hot pepper affects its body weight. Mol. Biol. Rep. 2012, 39, 7583–7589. [Google Scholar] [CrossRef] [PubMed]
- Bode, A.M.; Dong, Z. The two faces of capsaicin. Cancer Res. 2011, 71, 2809–2814. [Google Scholar] [CrossRef]
- Hwang, M.K.; Bode, A.M.; Byun, S.; Song, N.R.; Lee, H.J.; Lee, K.W.; Dong, Z. Cocarcinogenic effect of capsaicin involves activation of EGFR signaling but not TRPV1. Cancer Res. 2010, 70, 6859–6869. [Google Scholar] [CrossRef]
- Toth, B.; Gannett, P. Carcinogenicity of lifelong administration of capsaicin of hot pepper in mice. In Vivo 1992, 6, 59–63. [Google Scholar]
- Guertin, D.A.; Sabatini, D.M. Defining the role of mTOR in cancer. Cancer Cell 2007, 12, 9–22. [Google Scholar] [CrossRef]
- Alayev, A.; Holz, M.K. mTOR signaling for biological control and cancer. J. Cell. Physiol. 2013, 228, 1658–1664. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.P.; Colburn, N.H.; Young, M.R. AP-1 a target for cancer prevention. Curr. Cancer Drug Targets 2007, 7, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Piechaczyk, M.; Blanchard, J.M. c-fos proto-oncogene regulation and function. Crit. Rev. Oncol. Hematol. 1994, 17, 93–131. [Google Scholar] [CrossRef]
- Liu, Z.G.; Jiang, G.; Tang, J.; Wang, H.; Feng, G.; Chen, F.; Tu, Z.; Liu, G.; Zhao, Y.; Peng, M.J.; et al. c-Fos over-expression promotes radioresistance and predicts poor prognosis in malignant glioma. Oncotarget 2016, 7, 65946–65956. [Google Scholar] [CrossRef] [PubMed]
- Bland, K.I.; Konstadoulakis, M.M.; Vezeridis, M.P.; Wanebo, H.J. Oncogene protein co-expression. Value of Ha-ras, c-myc, c-fos, and p53 as prognostic discriminants for breast carcinoma. Ann. Surg. 1995, 221, 706–718, discussion 718-720. [Google Scholar] [CrossRef]
- Mahner, S.; Baasch, C.; Schwarz, J.; Hein, S.; Wolber, L.; Janicke, F.; Milde-Langosch, K. C-Fos expression is a molecular predictor of progression and survival in epithelial ovarian carcinoma. Br. J. Cancer 2008, 99, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Abarrategi, A.; Gambera, S.; Alfranca, A.; Rodriguez-Milla, M.A.; Perez-Tavarez, R.; Rouault-Pierre, K.; Waclawiczek, A.; Chakravarty, P.; Mulero, F.; Trigueros, C.; et al. c-Fos induces chondrogenic tumor formation in immortalized human mesenchymal progenitor cells. Sci. Rep. 2018, 8, 15615. [Google Scholar] [CrossRef]
- Saez, E.; Rutberg, S.E.; Mueller, E.; Oppenheim, H.; Smoluk, J.; Yuspa, S.H.; Spiegelman, B.M. c-fos is required for malignant progression of skin tumors. Cell 1995, 82, 721–732. [Google Scholar] [CrossRef]
- Muhammad, N.; Bhattacharya, S.; Steele, R.; Phillips, N.; Ray, R.B. Involvement of c-Fos in the Promotion of Cancer Stem-like Cell Properties in Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2017, 23, 3120–3128. [Google Scholar] [CrossRef]
- Shin, S.H.; Lee, S.R.; Lee, E.; Kim, K.H.; Byun, S. Caffeic Acid Phenethyl Ester from the Twigs of Cinnamomum cassia Inhibits Malignant Cell Transformation by Inducing c-Fos Degradation. J. Nat. Prod. 2017, 80, 2124–2130. [Google Scholar] [CrossRef]
- Dong, Z.; Birrer, M.J.; Watts, R.G.; Matrisian, L.M.; Colburn, N.H. Blocking of tumor promoter-induced AP-1 activity inhibits induced transformation in JB6 mouse epidermal cells. Proc. Natl. Acad. Sci. USA 1994, 91, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.; Lee, K.W.; Jung, S.K.; Lee, E.J.; Hwang, M.K.; Lim, S.H.; Bode, A.M.; Lee, H.J.; Dong, Z. Luteolin inhibits protein kinase C(epsilon) and c-Src activities and UVB-induced skin cancer. Cancer Res. 2010, 70, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Hoffman, G.R.; Poulogiannis, G.; Buel, G.R.; Jang, Y.J.; Lee, K.W.; Kim, B.Y.; Erikson, R.L.; Cantley, L.C.; Choo, A.Y.; et al. Metabolic stress controls mTORC1 lysosomal localization and dimerization by regulating the TTT-RUVBL1/2 complex. Mol. Cell 2013, 49, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.Y.; Grandis, J.R.; Shin, D.M. Targeting epidermal growth factor receptor: Novel therapeutics in the management of cancer. Expert Rev. Anticancer Ther. 2003, 3, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; He, Z.; Koyama, I.; Ma, W.Y.; Miyamoto, K.; Dong, Z. Involvement of the Akt/mTOR pathway on EGF-induced cell transformation. Mol. Carcinog. 2003, 38, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Yarden, Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur. J. Cancer 2001, 37 (Suppl. 4), 3–8. [Google Scholar] [CrossRef]
- Seshacharyulu, P.; Ponnusamy, M.P.; Haridas, D.; Jain, M.; Ganti, A.K.; Batra, S.K. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 15–31. [Google Scholar] [CrossRef]
- Lindsey, S.; Langhans, S.A. Epidermal growth factor signaling in transformed cells. Int. Rev. Cell Mol. Biol. 2015, 314, 1–41. [Google Scholar] [CrossRef]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef]
- Subbaramaiah, K.; Telang, N.; Ramonetti, J.T.; Araki, R.; DeVito, B.; Weksler, B.B.; Dannenberg, A.J. Transcription of cyclooxygenase-2 is enhanced in transformed mammary epithelial cells. Cancer Res. 1996, 56, 4424–4429. [Google Scholar] [PubMed]
- Zoncu, R.; Bar-Peled, L.; Efeyan, A.; Wang, S.; Sancak, Y.; Sabatini, D.M. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 2011, 334, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Sancak, Y.; Bar-Peled, L.; Zoncu, R.; Markhard, A.L.; Nada, S.; Sabatini, D.M. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 2010, 141, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Bar-Peled, L.; Sabatini, D.M. Regulation of mTORC1 by amino acids. Trends Cell Biol. 2014, 24, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xiang, G.H.; Tang, T.; Tang, Y.; Zhao, L.Y.; Liu, D.; Zhang, Y.R.; Tang, J.T.; Zhou, S.; Wu, D.H. Capsaicin and dihydrocapsaicin induce apoptosis in human glioma cells via ROS and Ca2+-mediated mitochondrial pathway. Mol. Med. Rep. 2016, 14, 4198–4208. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Kim, Y.S.; Lim, S.C.; Hou, Y.F.; Chang, I.Y.; You, H.J. Dihydrocapsaicin (DHC), a saturated structural analog of capsaicin, induces autophagy in human cancer cells in a catalase-regulated manner. Autophagy 2008, 4, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Jung, Y.K.; Oh, S.H. Selective induction of catalase-mediated autophagy by dihydrocapsaicin in lung cell lines. Free Radic. Biol. Med. 2010, 49, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.; Lim, S.; Mun, J.Y.; Kim, K.H.; Ramadhar, T.R.; Farrand, L.; Shin, S.H.; Thimmegowda, N.R.; Lee, H.J.; Frank, D.A.; et al. Identification of a Dual Inhibitor of Janus Kinase 2 (JAK2) and p70 Ribosomal S6 Kinase1 (S6K1) Pathways. J. Biol. Chem. 2015, 290, 23553–23562. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Lee, K.W.; Byun, S.; Jung, S.K.; Song, N.; Lim, S.H.; Heo, Y.S.; Kim, J.E.; Kang, N.J.; Kim, B.Y.; et al. 7,3’,4’-Trihydroxyisoflavone, a metabolite of the soy isoflavone daidzein, suppresses ultraviolet B-induced skin cancer by targeting Cot and MKK4. J. Biol. Chem. 2011, 286, 14246–14256. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.J.; Dai, M.; Pellegrino, G.; Wagner, B.K.; Stern, A.M.; Shamji, A.F.; Schreiber, S.L. Synthesis, cellular evaluation, and mechanism of action of piperlongumine analogs. Proc. Natl. Acad. Sci. USA 2012, 109, 15115–15120. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.D.; Curran, T.; Verma, I.M. c-fos protein can induce cellular transformation: A novel mechanism of activation of a cellular oncogene. Cell 1984, 36, 51–60. [Google Scholar]
- Memmott, R.M.; Dennis, P.A. The role of the Akt/mTOR pathway in tobacco carcinogen-induced lung tumorigenesis. Clin. Cancer Res. 2010, 16, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Sully, K.; Akinduro, O.; Philpott, M.P.; Naeem, A.S.; Harwood, C.A.; Reeve, V.E.; O’Shaughnessy, R.F.; Byrne, C. The mTOR inhibitor rapamycin opposes carcinogenic changes to epidermal Akt1/PKBalpha isoform signaling. Oncogene 2013, 32, 3254–3262. [Google Scholar] [CrossRef] [PubMed]
- Callejas-Valera, J.L.; Iglesias-Bartolome, R.; Amornphimoltham, P.; Palacios-Garcia, J.; Martin, D.; Califano, J.A.; Molinolo, A.A.; Gutkind, J.S. mTOR inhibition prevents rapid-onset of carcinogen-induced malignancies in a novel inducible HPV-16 E6/E7 mouse model. Carcinogenesis 2016, 37, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Czerninski, R.; Amornphimoltham, P.; Patel, V.; Molinolo, A.A.; Gutkind, J.S. Targeting mammalian target of rapamycin by rapamycin prevents tumor progression in an oral-specific chemical carcinogenesis model. Cancer Prev. Res. 2009, 2, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, A.C.; Liu, Y.; Edlind, M.P.; Ingolia, N.T.; Janes, M.R.; Sher, A.; Shi, E.Y.; Stumpf, C.R.; Christensen, C.; Bonham, M.J.; et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature 2012, 485, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Hoxhaj, G.; Hughes-Hallett, J.; Timson, R.C.; Ilagan, E.; Yuan, M.; Asara, J.M.; Ben-Sahra, I.; Manning, B.D. The mTORC1 Signaling Network Senses Changes in Cellular Purine Nucleotide Levels. Cell Rep. 2017, 21, 1331–1346. [Google Scholar] [CrossRef]
- Li, L.; Kim, E.; Yuan, H.; Inoki, K.; Goraksha-Hicks, P.; Schiesher, R.L.; Neufeld, T.P.; Guan, K.L. Regulation of mTORC1 by the Rab and Arf GTPases. J. Biol. Chem. 2010, 285, 19705–19709. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.; Lee, S.H. Anticancer Properties of Capsaicin against Human Cancer. Anticancer Res. 2016, 36, 837–843. [Google Scholar]
- Nagabhushan, M.; Bhide, S.V. Mutagenicity of chili extract and capsaicin in short-term tests. Environ. Mutagen. 1985, 7, 881–888. [Google Scholar] [CrossRef]
- Duelund, L.; Mouritsen, O.G. Contents of capsaicinoids in chillies grown in Denmark. Food Chem. 2017, 221, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum-Titze, P.; Hiepler, C.; Mueller-Seitz, E.; Petz, M. Pungency in paprika (Capsicum annuum). 1. Decrease of capsaicinoid content following cellular disruption. J. Agric. Food Chem. 2002, 50, 1260–1263. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.S.; Kim, Y.A.; Jang, Y.J.; Oh, Y.; Byun, S. Dihydrocapsaicin Inhibits Epithelial Cell Transformation through Targeting Amino Acid Signaling and c-Fos Expression. Nutrients 2019, 11, 1269. https://doi.org/10.3390/nu11061269
Lee JS, Kim YA, Jang YJ, Oh Y, Byun S. Dihydrocapsaicin Inhibits Epithelial Cell Transformation through Targeting Amino Acid Signaling and c-Fos Expression. Nutrients. 2019; 11(6):1269. https://doi.org/10.3390/nu11061269
Chicago/Turabian StyleLee, Ji Su, Yeong A. Kim, Young Jin Jang, Yongtaek Oh, and Sanguine Byun. 2019. "Dihydrocapsaicin Inhibits Epithelial Cell Transformation through Targeting Amino Acid Signaling and c-Fos Expression" Nutrients 11, no. 6: 1269. https://doi.org/10.3390/nu11061269
APA StyleLee, J. S., Kim, Y. A., Jang, Y. J., Oh, Y., & Byun, S. (2019). Dihydrocapsaicin Inhibits Epithelial Cell Transformation through Targeting Amino Acid Signaling and c-Fos Expression. Nutrients, 11(6), 1269. https://doi.org/10.3390/nu11061269




