The Lipid and Glyceride Profiles of Infant Formula Differ by Manufacturer, Region and Date Sold
Abstract
1. Introduction
2. Materials and Methods
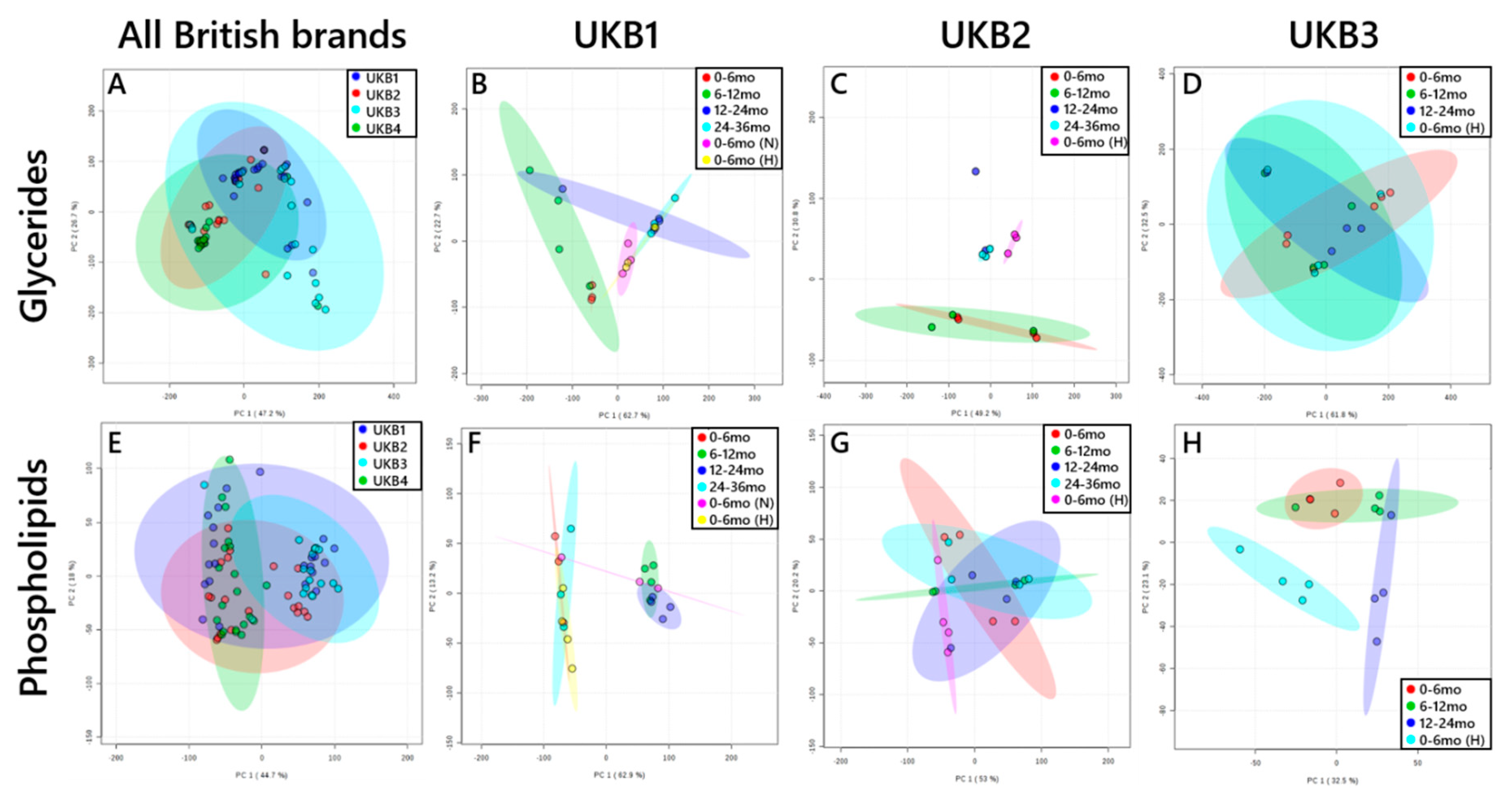
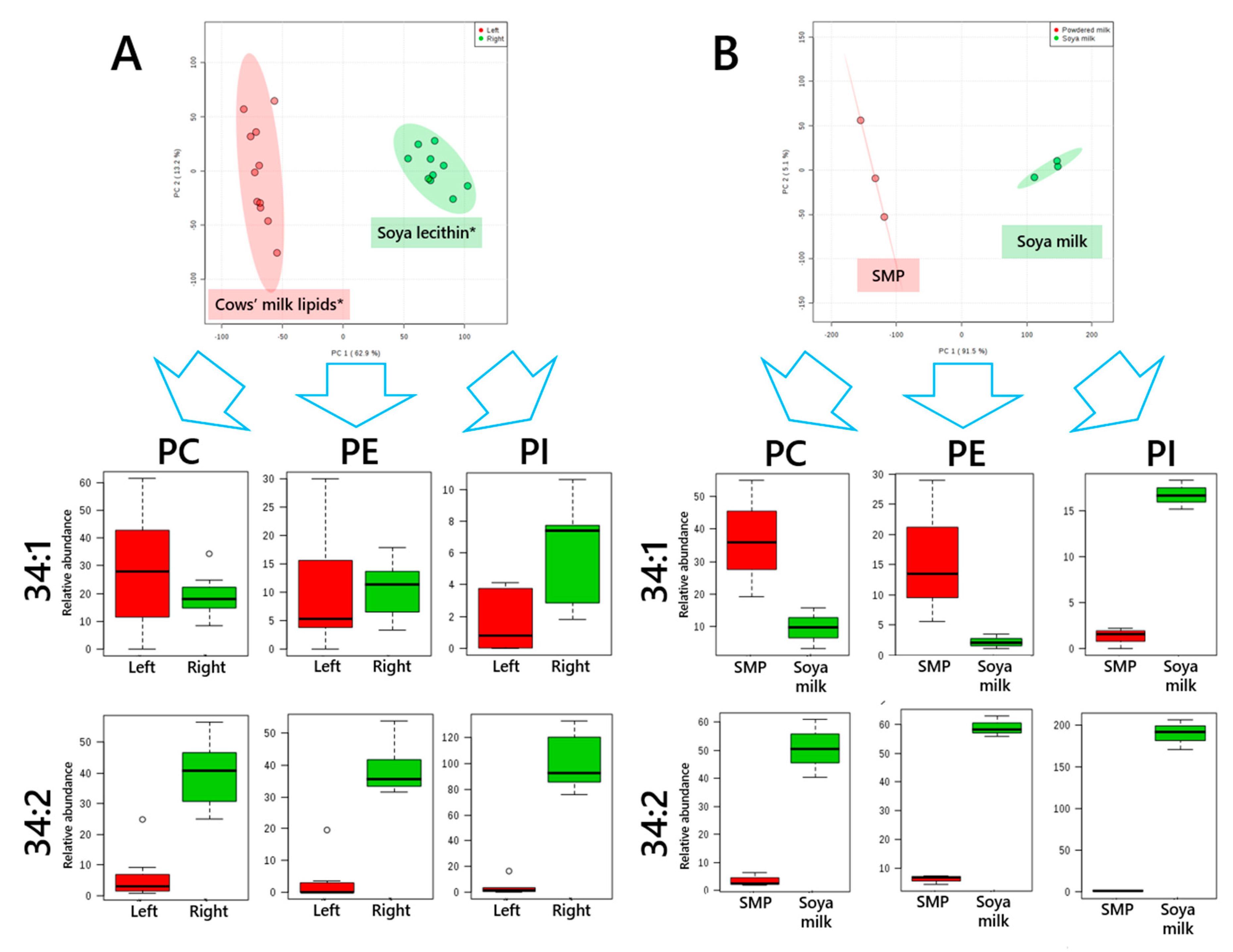
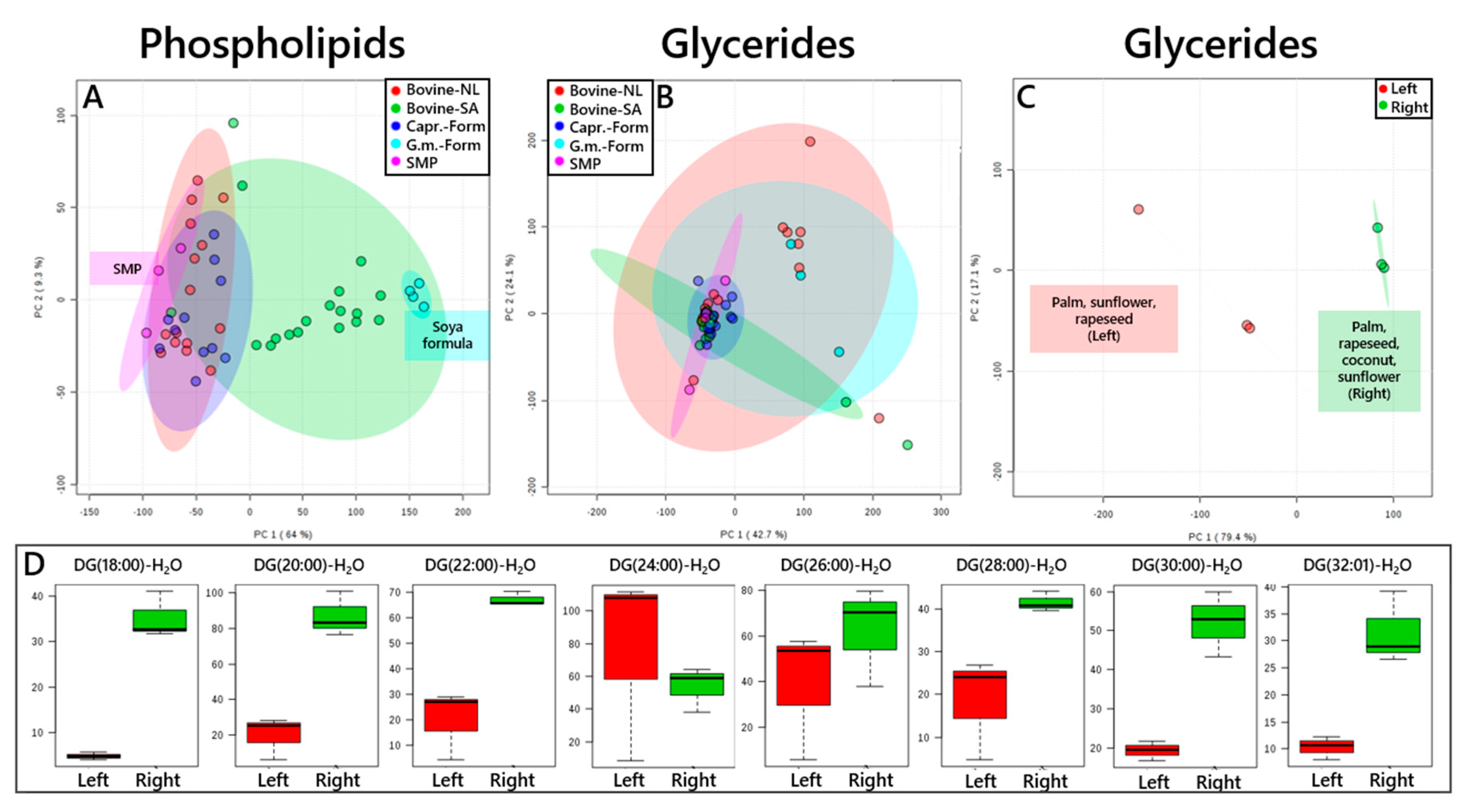
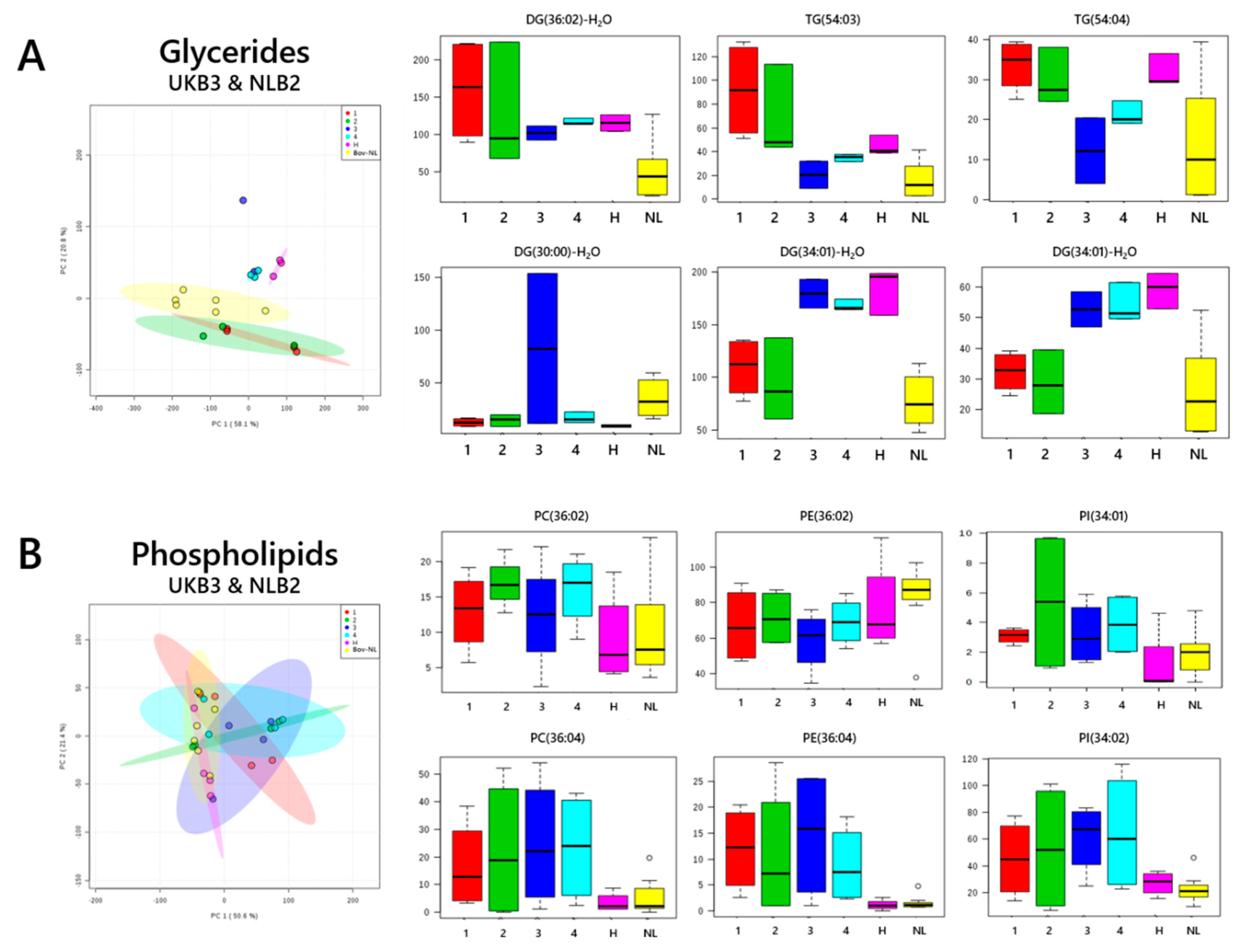
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Uauy, R.; Castillo, C. Lipid requirements of infants: Implications for nutrient composition of fortified complementary foods. J. Nutr. 2003, 133, 2962–2972. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, A.C.J.; van der Meulen, J.H.P.; Osmond, C.; Barker, D.J.P.; Bleker, O.P. Infant feeding and adult glucose tolerance, lipid profile, blood pressure, and obesity. Arch. Dis. Child. 2000, 82, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Hardy, S.C.; Kleinman, R.E. Fat and cholesterol in the diet of infants and young children: Implications for growth, development, and long-term health. J. Pediatr. 1994, 125, S69–S77. [Google Scholar] [CrossRef]
- Furse, S.; Billing, G.; Snowden, S.G.; Smith, J.; Goldberg, G.; Koulman, A. Evidence that maternal lipids influence their infant’s lipid profile through breast milk. Metabolomics 2019. under review. [Google Scholar]
- Furse, S.; Snowden, S.G.; Laurentya, O.; Prentice, P.; Ong, K.; Hughes, I.A.; Acerini, C.L.; Dunger, D.B.; Koulman, A. Evidence that feeding post partum and exposures in utero modulate shape lipid metabolism in infancy. Metabolomics. 2019. under review. [Google Scholar]
- Prentice, P.; Koulman, A.; Matthews, L.; Acerini, C.L.; Ong, K.K.; Dunger, D.B. Lipidomic analyses, breast- and formula-feeding and growth in infants. J. Pediatr. 2015, 166, 276–281. [Google Scholar] [CrossRef]
- Acharjee, A.; Prentice, P.; Acerini, C.; Smith, J.; Hughes, I.A.; Ong, K.; Griffin, J.L.; Dunger, D.; Koulman, A. The translation of lipid profiles to nutritional biomarkers in the study of infant metabolism. Metabolomics 2017, 13, 25. [Google Scholar] [CrossRef]
- Koulman, A.; Prentice, P.; Wong, M.C.Y.; Matthews, L.; Bond, N.J.; Eiden, M.; Griffin, J.L.; Dunger, D.B. The development and validation of a fast and robust dried blood spot based lipid profiling method to study infant metabolism. Metabolomics 2014, 10, 1018–1025. [Google Scholar] [CrossRef]
- Ghebremeskel, K.; Leighfield, M.; Leaf, A.; Costeloe, K.; Crawford, M. Fatty acid composition of plasma and red cell phospholipids of preterm babies fed on breast milk and formulae. Eur. J. Pediatr. 1995, 154, 46–52. [Google Scholar] [CrossRef]
- Sabel, K.G.; Lundqvist-Persson, C.; Bona, E.; Petzold, M.; Strandvik, B. Fatty acid patterns early after premature birth, simultaneously analysed in mothers’ food, breast milk and serum phospholipids of mothers and infants. Lipids Health Dis. 2009, 8, 20. [Google Scholar] [CrossRef]
- VanderJagt, D.J.; Arndt, C.D.; Okolo, S.N.; Huang, Y.S.; Chuang, L.T.; Glew, R.H. Fatty acid composition of the milk lipids of fulani women and the serum phospholipids of their exclusively breast-fed infants. Early Hum. Dev. 2000, 60, 73–87. [Google Scholar] [CrossRef]
- Harit, D.; Faridi, M.M.A.; Aggarwal, A.; Sharma, S.B. Lipid profile of term infants on exclusive breastfeeding and mixed feeding: A comparative study. Eur. J. Clin. Nutr. 2007, 62, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.M.; Requejo, A.M.; Navia, B.; Quintas, M.E.; Andrés, P.; López-Sobaler, M.; Perea, J.M. The consumption of milk products in a group of pre-school children: Influence on serum lipid profile. Nutr. Res. 2000, 20, 779–790. [Google Scholar] [CrossRef]
- Steinmetz, K.A.; Childs, M.T.; Stimson, C.; Kushi, L.H.; McGovern, P.G.; Potter, J.D.; Yamanaka, W.K. Effect of consumption of whole milk and skim milk on blood lipid profiles in healthy men. Am. J. Clin. Nutr. 1994, 59, 612–618. [Google Scholar] [CrossRef]
- Nagaya, T.; Yoshida, H.; Hayashi, T.; Takahashi, H.; Kawai, M.; Matsuda, Y. Serum lipid profile in relation to milk consumption in a japanese population. J. Am. Coll. Nutr. 1996, 15, 625–629. [Google Scholar] [CrossRef]
- Tricon, S.; Burdge, G.C.; Jones, E.L.; Russell, J.J.; El-Khazen, S.; Moretti, E.; Hall, W.L.; Gerry, A.B.; Leake, D.S.; Grimble, R.F.; et al. Effects of dairy products naturally enriched with cis-9,trans-11 conjugated linoleic acid on the blood lipid profile in healthy middle-aged men. Am. J. Clin. Nutr. 2006, 83, 744–753. [Google Scholar] [CrossRef]
- Mott, G.E.; Jackson, E.M.; DeLallo, L.; Lewis, D.S.; McMahan, C.A. Differences in cholesterol metabolism in juvenile baboons are programmed by breast- versus formula-feeding. J. Lipid Res. 1995, 36, 299–307. [Google Scholar]
- Mott, G.E.; Jackson, E.M.; Klein, M.L.; Shan, H.; Pang, J.; Wilson, W.K.; McMahan, C.A. Programming of initial steps in bile acid synthesis by breast-feeding vs. Formula-feeding in the baboon. Lipids 2003, 38, 1213–1220. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; França, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Rollins, N.C.; Bhandari, N.; Hajeebhoy, N.; Horton, S.; Lutter, C.K.; Martines, J.C.; Piwoz, E.G.; Richter, L.M.; Victora, C.G. Why invest, and what it will take to improve breastfeeding practices? Lancet 2016, 387, 491–504. [Google Scholar] [CrossRef]
- Barreiro, R.; Regal, P.; López-Racamonde, O.; Cepeda, A.; Fente, C.A. Comparison of the fatty acid profile of spanish infant formulas and galician women breast milk. J. Physiol. Biochem. 2018, 74, 127–138. [Google Scholar] [CrossRef]
- Mendonça, M.A.; Araújo, W.M.C.; Borgo, L.A.; Alencar, E.D.R. Lipid profile of different infant formulas for infants. PLoS ONE 2017, 12, e0177812. [Google Scholar] [CrossRef] [PubMed]
- Oleynik, A.S.; Eliseeva, T.A.; Vanderhoek, J. Comparative lipid profiles of milk bank breast milk and infant formulas. Open Nutr. J. 2013, 7, 26–31. [Google Scholar] [CrossRef][Green Version]
- Gianni, M.L.; Roggero, P.; Baudry, C.; Fressange-Mazda, C.; Galli, C.; Agostoni, C.; le Ruyet, P.; Mosca, F. An infant formula containing dairy lipids increased red blood cell membrane omega 3 fatty acids in 4 month-old healthy newborns: A randomized controlled trial. BMC Pediatr. 2018, 18, 53. [Google Scholar] [CrossRef]
- Uhl, O.; Fleddermann, M.; Hellmuth, C.; Demmelmair, H.; Koletzko, B. Phospholipid species in newborn and 4 month old infants after consumption of different formulas or breast milk. PLoS ONE 2016, 11, e0162040. [Google Scholar] [CrossRef]
- Furse, S.; Jakubec, M.; Rise, F.; Williams, H.E.; Rees, C.E.D.; Halskau, O. Evidence that listeria innocua modulates its membrane’s stored curvature elastic stress, but not fluidity, through the cell cycle. Sci. Rep. 2017, 7, 8012. [Google Scholar] [CrossRef]
- Vítová, M.; Lanta, V.; Rise, F.; Jakubec, M.; Halskau, Ø.; Furse, S. The phospholipid profile of desmodesmus quadricauda is a function of its cell cycle. Unpublished work. 2019. [Google Scholar]
- Furse, S.; Egmond, M.R.; Killian, J.A. Isolation of lipids from biological samples. Mol. Membr. Biol. 2015, 32, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Furse, S.; Eriksen, K.G.; Moore, S.E.; Koulman, A. Identification of candidate molecular biomarkers for growth faltering in infants at 12w. Unpublished work. 2018. [Google Scholar]
- Harshfield, E.L.; Koulman, A.; Ziemek, D.; Marney, L.; Fauman, E.B.; Paul, D.S.; Stacey, D.; Rasheed, A.; Lee, J.J.; Shah, N.; et al. An unbiased lipid phenotyping approach to study the genetic determinants of lipids and their association with coronary heart disease risk factors. J. Proteome Res. 2019, 18. [Google Scholar] [CrossRef]
- Deeth, H.C. Lipoprotein lipase and lipolysis in milk. Int. Dairy J. 2006, 16, 555–562. [Google Scholar] [CrossRef]
- Neville, M.C.; Waxman, L.J.; Jensen, D.; Eckel, R.H. Lipoprotein lipase in human milk: Compartmentalization and effect of fasting, insulin, and glucose. J. Lipid Res. 1991, 32, 251–257. [Google Scholar]
- Bengtsson-Olivecrona, G.; Olivecrona, T. [32] phospholipase activity of milk lipoprotein lipase. In Methods in Enzymology; Academic Press: London, UK, 1991; Volume 197, pp. 345–356. [Google Scholar]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. Metaboanalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed]
- Orsavova, J.; Misurcova, L.; Vavra Ambrozova, J.; Vicha, R.; Mlcek, J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Furse, S.; Liddell, S.; Ortori, C.A.; Williams, H.; Neylon, D.C.; Scott, D.J.; Barrett, D.A.; Gray, D.A. The lipidome and proteome of oil bodies from helianthus annuus (common sunflower). J. Chem. Biol. 2013, 6, 63–76. [Google Scholar] [CrossRef]
- Blaas, N.; Schüürmann, C.; Bartke, N.; Stahl, B.; Humpf, H.U. Structural profiling and quantification of sphingomyelin in human breast milk by hplc-ms/ms. J. Agric. Food Chem. 2011, 59, 6018–6024. [Google Scholar] [CrossRef]
- Fong, B.; Ma, L.; Norris, C. Analysis of phospholipids in infant formulas using high performance liquid chromatography–tandem mass spectrometry. J. Agric. Food Chem. 2013, 61, 858–865. [Google Scholar] [CrossRef]
- Murgia, S.; Mele, S.; Monduzzi, M. Quantitative characterization of phospholipids in milk fat via p-31 nmr using a monophasic solvent mixture. Lipids 2003, 38, 585–591. [Google Scholar] [CrossRef]
- Gale, C.; Parkinson, J.R.; Logan, K.M.; Hyde, M.J.; Santhakumaran, S.; Modi, N. Effect of breastfeeding compared with formula feeding on infant body composition: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zhou, J.; Yin, Y.; Jing, W.; Luo, B.; Wang, J. Effects of breast-feeding compared with formula-feeding on preterm infant body composition: A systematic review and meta-analysis. Br. J. Nutr. 2016, 116, 132–141. [Google Scholar] [CrossRef]
- Bernardo, H.; Cesar, V. Long-Term Effects of Breastfeeding: A Systematic Review; WHO: Geneva, Switzerland, 2013.
- Blackmore, H.L.; Niu, Y.; Fernandez-Twinn, D.S.; Tarry-Adkins, J.L.; Giussani, D.A.; Ozanne, S.E. Maternal diet-induced obesity programs cardiovascular dysfunction in adult male mouse offspring independent of current body weight. Endocrinology 2014, 155, 3970–3980. [Google Scholar] [CrossRef] [PubMed]
- Loche, E.; Blackmore, H.L.; Carpenter, A.A.; Beeson, J.H.; Pinnock, A.; Ashmore, T.J.; Aiken, C.E.; de Almeida-Faria, J.; Schoonejans, J.M.; Giussani, D.A.; et al. Maternal diet-induced obesity programmes cardiac dysfunction in male mice independently of post-weaning diet. Cardiovasc. Res. 2018, 114, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Twinn, D.S.; Gascoin, G.; Musial, B.; Carr, S.; Duque-Guimaraes, D.; Blackmore, H.L.; Alfaradhi, M.Z.; Loche, E.; Sferruzzi-Perri, A.N.; Fowden, A.L.; et al. Exercise rescues obese mothers’ insulin sensitivity, placental hypoxia and male offspring insulin sensitivity. Sci. Rep. 2017, 7, 44650. [Google Scholar] [CrossRef]
- Samuelsson, A.M.; Matthews, P.A.; Argenton, M.; Christie, M.R.; McConnell, J.M.; Jansen, E.H.J.M.; Piersma, A.H.; Ozanne, S.E.; Fernandez-Twinn, D.; Remacle, C.; et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance. Hypertension 2008, 51, 383–392. [Google Scholar] [CrossRef]
- Shelley, P.; Martin-Gronert, M.S.; Rowlerson, A.; Poston, L.; Heales, S.J.R.; Hargreaves, I.P.; McConnell, J.M.; Ozanne, S.E.; Fernandez-Twinn, D.S. Altered skeletal muscle insulin signaling and mitochondrial complex ii-iii linked activity in adult offspring of obese mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R675–R681. [Google Scholar] [CrossRef]
- Watkins, A.J.; Dias, I.; Tsuro, H.; Allen, D.; Emes, R.D.; Moreton, J.; Wilson, R.; Ingram, R.J.M.; Sinclair, K.D. Paternal diet programs offspring health through sperm- and seminal plasma-specific pathways in mice. Proc. Natl. Acad. Sci. USA 2018, 115, 10064–10069. [Google Scholar] [CrossRef]
- Watkins, A.J.; Sirovica, S.; Stokes, B.; Isaacs, M.; Addison, O.; Martin, R.A. Paternal low protein diet programs preimplantation embryo gene expression, fetal growth and skeletal development in mice. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1371–1381. [Google Scholar] [CrossRef]
- Garelnabi, M.; Ainsworth, G.; Mahini, H.; Jamil, N.; Ochin, C. Dietary oxidized linoleic acid modulates plasma lipids beyond triglycerides metabolism. J. Lipids 2017, 2017, 1645828. [Google Scholar] [CrossRef]
- Nagy, L.; Tontonoz, P.; Alvarez, J.G.A.; Chen, H.; Evans, R.M. Oxidized ldl regulates macrophage gene expression through ligand activation of pparγ. Cell 1998, 93, 229–240. [Google Scholar] [CrossRef]
- Itoh, T.; Yamamoto, K. Peroxisome proliferator activated receptor γ and oxidized docosahexaenoic acids as new class of ligand. Naunyn Schmiedeberg’s Arch. Pharmacol. 2008, 377, 541–547. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furse, S.; Koulman, A. The Lipid and Glyceride Profiles of Infant Formula Differ by Manufacturer, Region and Date Sold. Nutrients 2019, 11, 1122. https://doi.org/10.3390/nu11051122
Furse S, Koulman A. The Lipid and Glyceride Profiles of Infant Formula Differ by Manufacturer, Region and Date Sold. Nutrients. 2019; 11(5):1122. https://doi.org/10.3390/nu11051122
Chicago/Turabian StyleFurse, Samuel, and Albert Koulman. 2019. "The Lipid and Glyceride Profiles of Infant Formula Differ by Manufacturer, Region and Date Sold" Nutrients 11, no. 5: 1122. https://doi.org/10.3390/nu11051122
APA StyleFurse, S., & Koulman, A. (2019). The Lipid and Glyceride Profiles of Infant Formula Differ by Manufacturer, Region and Date Sold. Nutrients, 11(5), 1122. https://doi.org/10.3390/nu11051122





