Prevalence and Consequences of Preoperative Weight Loss in Gynecologic Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Assessment of Weight and Definition of Significant Weight Loss
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patients
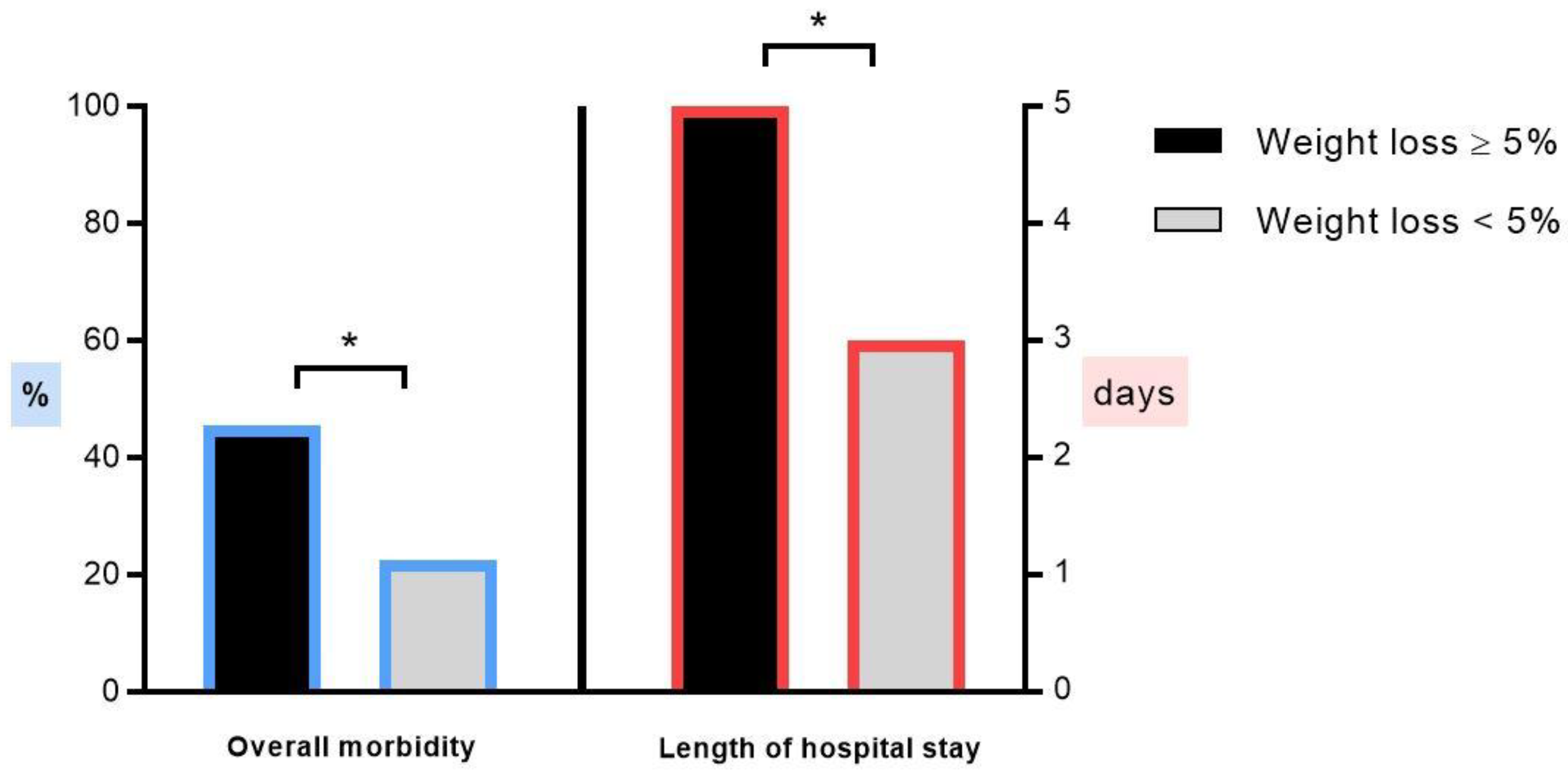
3.2. Outcome
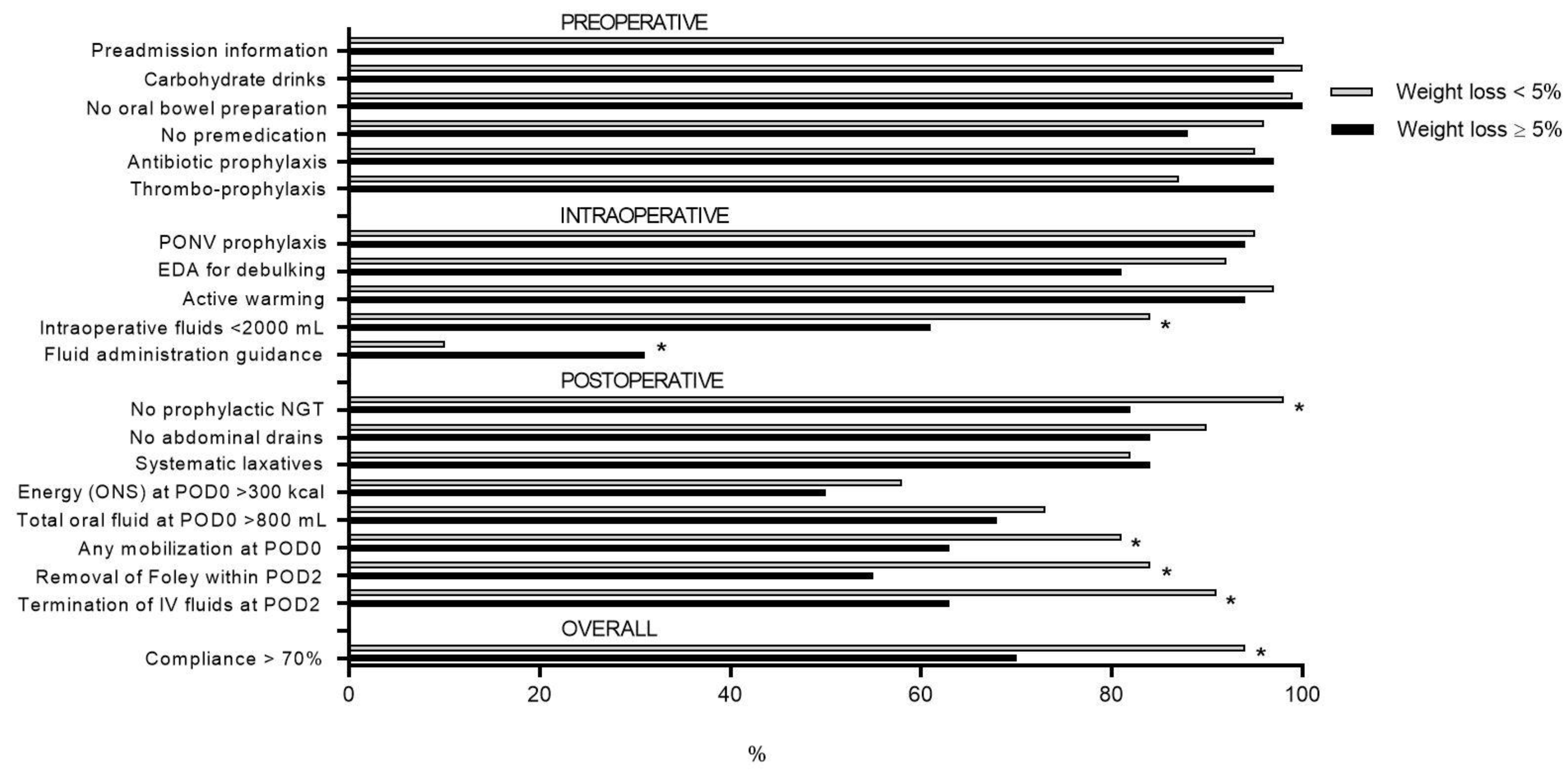
3.3. ERAS Compliance
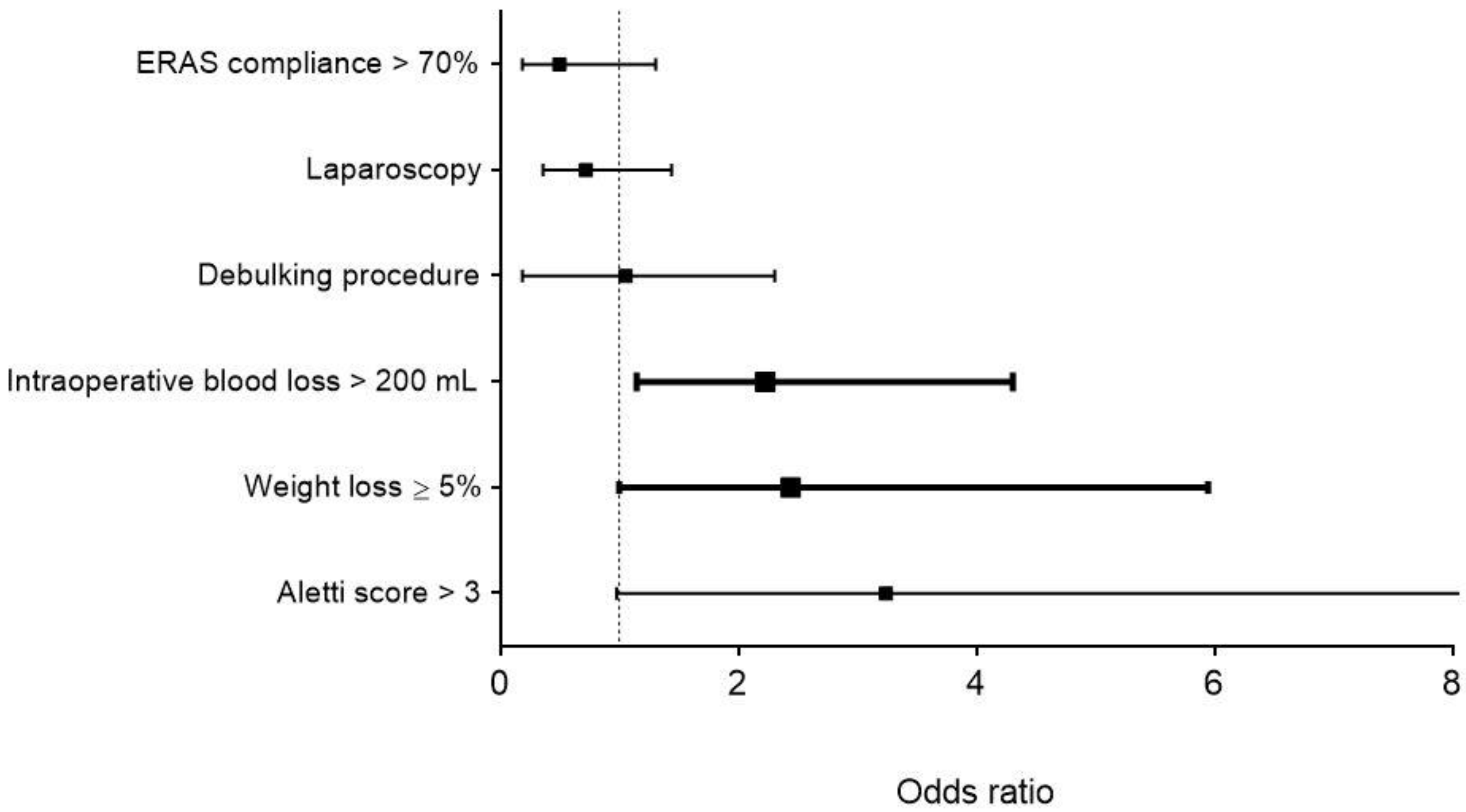
3.4. Risk Factors for Postoperative Complications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hubner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS®) society recommendations: 2018. World J. Surg. 2019, 43, 659–695. [Google Scholar] [CrossRef] [PubMed]
- Bolshinsky, V.; Li, M.H.; Ismail, H.; Burbury, K.; Riedel, B.; Heriot, A. Multimodal prehabilitation programs as a bundle of care in gastrointestinal cancer surgery: A systematic review. Dis. Colon Rectum 2018, 61, 124–138. [Google Scholar] [CrossRef]
- Huang, T.H.; Hsieh, C.C.; Kuo, L.M.; Chang, C.C.; Chen, C.H.; Chi, C.C.; Liu, C.H. Malnutrition associated with an increased risk of postoperative complications following hepatectomy in patients with hepatocellular carcinoma. HPB 2019. [Google Scholar] [CrossRef] [PubMed]
- Benton, K.; Thomson, I.; Isenring, E.; Mark Smithers, B.; Agarwal, E. An investigation into the nutritional status of patients receiving an Enhanced Recovery After Surgery (ERAS) protocol versus standard care following Oesophagectomy. Support. Care Cancer 2018, 26, 2057–2062. [Google Scholar] [CrossRef]
- Grass, F.; Pache, B.; Martin, D.; Hahnloser, D.; Demartines, N.; Hubner, M. Preoperative nutritional conditioning of crohn’s patients-systematic review of current evidence and practice. Nutrients 2017, 9. [Google Scholar] [CrossRef]
- Carli, F.; Silver, J.K.; Feldman, L.S.; McKee, A.; Gilman, S.; Gillis, C.; Scheede-Bergdahl, C.; Gamsa, A.; Stout, N.; Hirsch, B. Surgical prehabilitation in patients with cancer: State-of-the-Science and recommendations for future research from a panel of subject matter experts. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 49–64. [Google Scholar] [CrossRef]
- Nelson, G.; Altman, A.D.; Nick, A.; Meyer, L.A.; Ramirez, P.T.; Achtari, C.; Antrobus, J.; Huang, J.; Scott, M.; Wijk, L.; et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations—Part I. Gynecol. Oncol. 2016, 140, 313–322. [Google Scholar] [CrossRef]
- Nelson, G.; Altman, A.D.; Nick, A.; Meyer, L.A.; Ramirez, P.T.; Achtari, C.; Antrobus, J.; Huang, J.; Scott, M.; Wijk, L.; et al. Guidelines for postoperative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations—Part II. Gynecol. Oncol. 2016, 140, 323–332. [Google Scholar] [CrossRef]
- Pache, B.; Jurt, J.; Grass, F.; Hubner, M.; Demartines, N.; Mathevet, P.; Achtari, C. Compliance with enhanced recovery after surgery program in gynecology: Are all items of equal importance? Int. J. Gynecol. Cancer 2019. [Google Scholar] [CrossRef]
- Jurt, J.; Slieker, J.; Frauche, P.; Addor, V.; Sola, J.; Demartines, N.; Hubner, M. Enhanced recovery after surgery: Can we rely on the key factors or do we need the bel ensemble? World J. Surg. 2017. [Google Scholar] [CrossRef]
- Aletti, G.D.; Santillan, A.; Eisenhauer, E.L.; Hu, J.; Aletti, G.; Podratz, K.C.; Bristow, R.E.; Chi, D.S.; Cliby, W.A. A new frontier for quality of care in gynecologic oncology surgery: Multi-institutional assessment of short-term outcomes for ovarian cancer using a risk-adjusted model. Gynecol. Oncol. 2007, 107, 99–106. [Google Scholar] [CrossRef]
- Collins, N. Protein-energy malnutrition and involuntary weight loss: Nutritional and pharmacological strategies to enhance wound healing. Expert Opin. Pharmacother. 2003, 4, 1121–1140. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Butterworth, C.E. The skeleton in the hospital closet. 1974. Nutrition 1994, 10, 435–441. [Google Scholar]
- Correia, M.; Perman, M.I.; Waitzberg, D.L. Hospital malnutrition in Latin America: A systematic review. Clin. Nutr. 2017, 36, 958–967. [Google Scholar] [CrossRef]
- Obermair, A.; Simunovic, M.; Isenring, L.; Janda, M. Nutrition interventions in patients with gynecological cancers requiring surgery. Gynecol. Oncol. 2017, 145, 192–199. [Google Scholar] [CrossRef]
- Gupta, D.; Lis, C.G.; Vashi, P.G.; Lammersfeld, C.A. Impact of improved nutritional status on survival in ovarian cancer. Support. Care Cancer 2010, 18, 373–381. [Google Scholar] [CrossRef]
- Ljungqvist, O. Modulating postoperative insulin resistance by preoperative carbohydrate loading. Best Pract. Res. Clin. Anaesthesiol. 2009, 23, 401–409. [Google Scholar] [CrossRef]
- Fukuda, Y.; Yamamoto, K.; Hirao, M.; Nishikawa, K.; Maeda, S.; Haraguchi, N.; Miyake, M.; Hama, N.; Miyamoto, A.; Ikeda, M.; et al. Prevalence of Malnutrition Among Gastric Cancer Patients Undergoing Gastrectomy and Optimal Preoperative Nutritional Support for Preventing Surgical Site Infections. Ann. Surg. Oncol. 2015, 22 (Suppl. 3), S778–S785. [Google Scholar] [CrossRef]
- Gellrich, N.C.; Handschel, J.; Holtmann, H.; Kruskemper, G. Oral cancer malnutrition impacts weight and quality of life. Nutrients 2015, 7, 2145–2160. [Google Scholar] [CrossRef]
- Kyle, U.G.; Kossovsky, M.P.; Karsegard, V.L.; Pichard, C. Comparison of tools for nutritional assessment and screening at hospital admission: A population study. Clin. Nutr. 2006, 25, 409–417. [Google Scholar] [CrossRef]
- Schneider, S.M.; Veyres, P.; Pivot, X.; Soummer, A.M.; Jambou, P.; Filippi, J.; van Obberghen, E.; Hebuterne, X. Malnutrition is an independent factor associated with nosocomial infections. Br. J. Nutr. 2004, 92, 105–111. [Google Scholar] [CrossRef]
- Celik, J.B.; Gezginc, K.; Ozcelik, K.; Celik, C. The role of immunonutrition in gynecologic oncologic surgery. Eur. J. Gynaecol. Oncol. 2009, 30, 418–421. [Google Scholar]
- Grass, F.; Hubner, M.; Schafer, M.; Ballabeni, P.; Cerantola, Y.; Demartines, N.; Pralong, F.P.; Bertrand, P.C. Preoperative nutritional screening by the specialist instead of the nutritional risk score might prevent excess nutrition: A multivariate analysis of nutritional risk factors. Nutr. J. 2015, 14, 37. [Google Scholar] [CrossRef][Green Version]
- Challine, A.; Rives-Langes, C.; Danoussou, D.; Katsahian, S.; Ait Boudaoud, A.; Gaujoux, S.; Dousset, B.; Carette, C.; Lazzati, A.; Czernichow, S. Impact of oral immunonutrition on postoperative morbidity in digestive oncologic surgery: A nation-wide cohort study. Ann. Surg. 2019. [Google Scholar] [CrossRef]
- Probst, P.; Ohmann, S.; Klaiber, U.; Huttner, F.J.; Billeter, A.T.; Ulrich, A.; Buchler, M.W.; Diener, M.K. Meta-analysis of immunonutrition in major abdominal surgery. Br. J. Surg. 2017, 104, 1594–1608. [Google Scholar] [CrossRef]
- Laky, B.; Janda, M.; Kondalsamy-Chennakesavan, S.; Cleghorn, G.; Obermair, A. Pretreatment malnutrition and quality of life—Association with prolonged length of hospital stay among patients with gynecological cancer: A cohort study. BMC Cancer 2010, 10, 232. [Google Scholar] [CrossRef]
- Kathiresan, A.S.; Brookfield, K.F.; Schuman, S.I.; Lucci, J.A., 3rd. Malnutrition as a predictor of poor postoperative outcomes in gynecologic cancer patients. Arch. Gynecol. Obstet. 2011, 284, 445–451. [Google Scholar] [CrossRef]
- Nelson, G.; Bakkum-Gamez, J.; Kalogera, E.; Glaser, G.; Altman, A.; Meyer, L.A.; Taylor, J.S.; Iniesta, M.; Lasala, J.; Mena, G.; et al. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery After Surgery (ERAS) Society recommendations-2019 update. Int. J. Gynecol. Cancer 2019. [Google Scholar] [CrossRef] [PubMed]
| All Patients (n = 339) | Weight Loss ≥5% (n = 33) | Weight Loss <5% (n = 306) | p | |
|---|---|---|---|---|
| Age (years; mean ± SD) | 51 ± 12 | 53 ± 14 | 51 ± 12 | 0.491 |
| >70 years (%) | 31 (9) | 4 (12) | 27 (9) | 0.524 |
| ASA group (III–IV; %) | 23 (7) | 3 (9) | 20 (7) | 0.481 |
| BMI (kg/m2; mean ± SD) | 26 ± 6 | 26 ± 7 | 26 ± 6 | 0.798 |
| >25 kg/m2 | 174 (51) | 13 (39) | 161 (53) | 0.149 |
| Weight at surgery (kg; mean ± SD) | 70 ± 15 | 69 ± 17 | 70 ± 15 | 0.790 |
| 6 months preoperatively Weight loss over 6 months (kg; mean ± SD) | 70 ± 15 −0.4 ± 3 | 76 ± 18 −7 ± 4 | 70 ± 15 0 ± 2 | 0.070 <0.001 |
| Smoker (%) | 64 (19) | 4 (12) | 60 (20) | 0.358 |
| Diabetes (%) Malignancy (%) | 16 (5) 132 (39) | 0 22 (67) | 16 (5) 110 (36) | 0.383 0.001 |
| Immunosuppression (%) | 12 (4) | 5 (15) | 7 (2) | 0.003 |
| Minimal invasive approach (%) | 244 (72) | 17 (52) | 227 (74) | 0.008 |
| Debulking procedure (%) | 37 (8) | 9 (27) | 16 (5) | <0.001 |
| Duration of operation (min; mean ± SD) | 180 ± 80 | 240 ± 90 | 180 ± 80 | 0.001 |
| >180 min | 137 (40) | 23 (70) | 114 (37) | 0.001 |
| Modified Aletti score (>3; %) | 24 (7) | 7 (21) | 17 (6) | 0.005 |
| Intraoperative blood loss (mL; mean ± SD) | 270 ± 260 | 320 ± 270 | 270 ± 260 | 0.352 |
| >200 mL | 192 (57) | 20 (61) | 172 (56) | 0.628 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pache, B.; Grass, F.; Hübner, M.; Kefleyesus, A.; Mathevet, P.; Achtari, C. Prevalence and Consequences of Preoperative Weight Loss in Gynecologic Surgery. Nutrients 2019, 11, 1094. https://doi.org/10.3390/nu11051094
Pache B, Grass F, Hübner M, Kefleyesus A, Mathevet P, Achtari C. Prevalence and Consequences of Preoperative Weight Loss in Gynecologic Surgery. Nutrients. 2019; 11(5):1094. https://doi.org/10.3390/nu11051094
Chicago/Turabian StylePache, Basile, Fabian Grass, Martin Hübner, Amaniel Kefleyesus, Patrice Mathevet, and Chahin Achtari. 2019. "Prevalence and Consequences of Preoperative Weight Loss in Gynecologic Surgery" Nutrients 11, no. 5: 1094. https://doi.org/10.3390/nu11051094
APA StylePache, B., Grass, F., Hübner, M., Kefleyesus, A., Mathevet, P., & Achtari, C. (2019). Prevalence and Consequences of Preoperative Weight Loss in Gynecologic Surgery. Nutrients, 11(5), 1094. https://doi.org/10.3390/nu11051094








