Liquiritigenin Reduces Blood Glucose Level and Bone Adverse Effects in Hyperglycemic Adult Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethic Statement
2.2. Animals
2.3. Chemicals
2.4. Adult Treatments and Samples Collection
2.5. Bone Matrix Vital Staining
2.6. Histological TRAP and ALP Scale Assays
2.7. Biochemical TRAP and ALP Scale Assays
2.8. Gene Expression Analysis
2.9. Statistics
3. Results
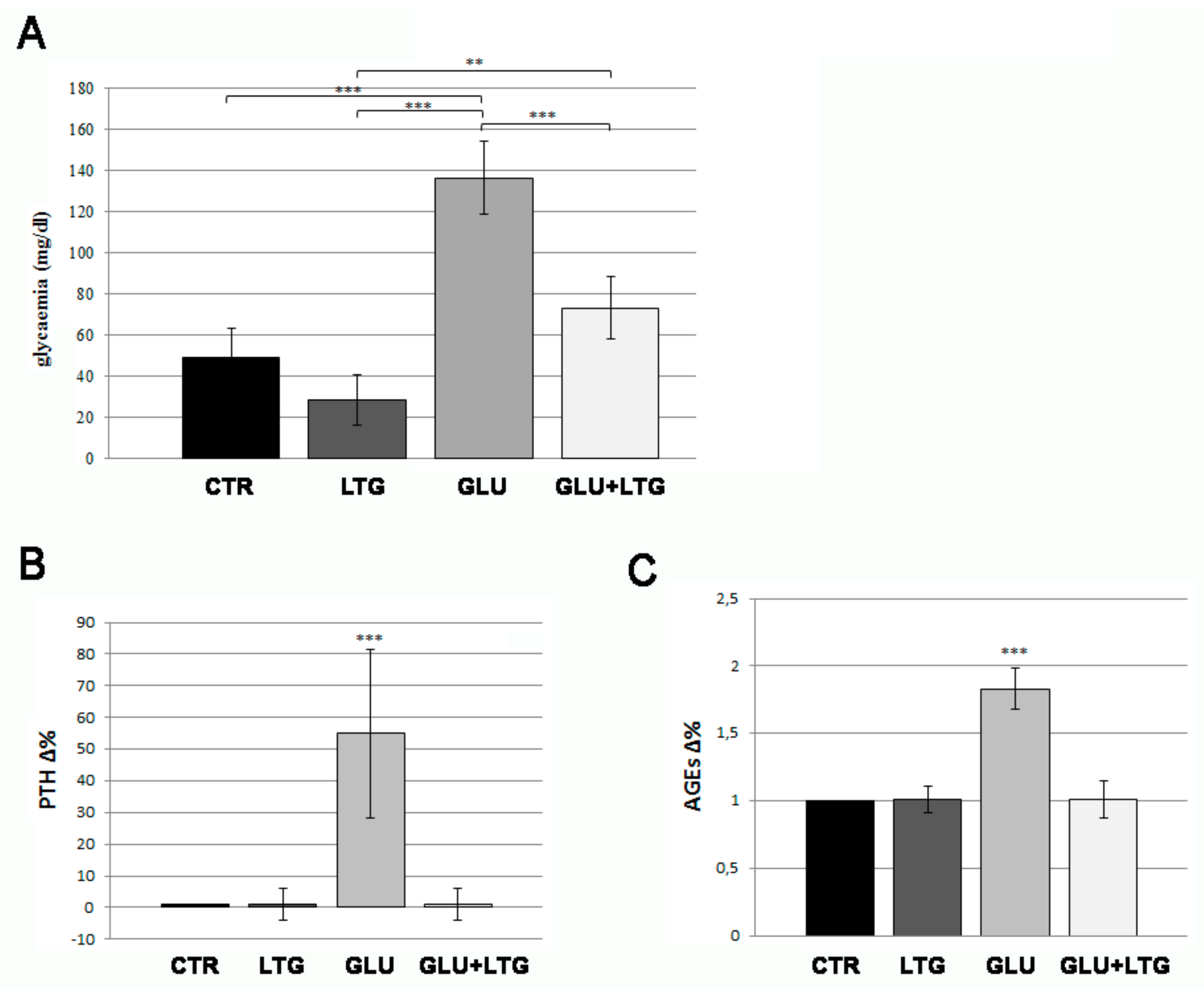
3.1. Liquiritigenin Decreases Blood Alterations in Hyperglycemic Zebrafish Model
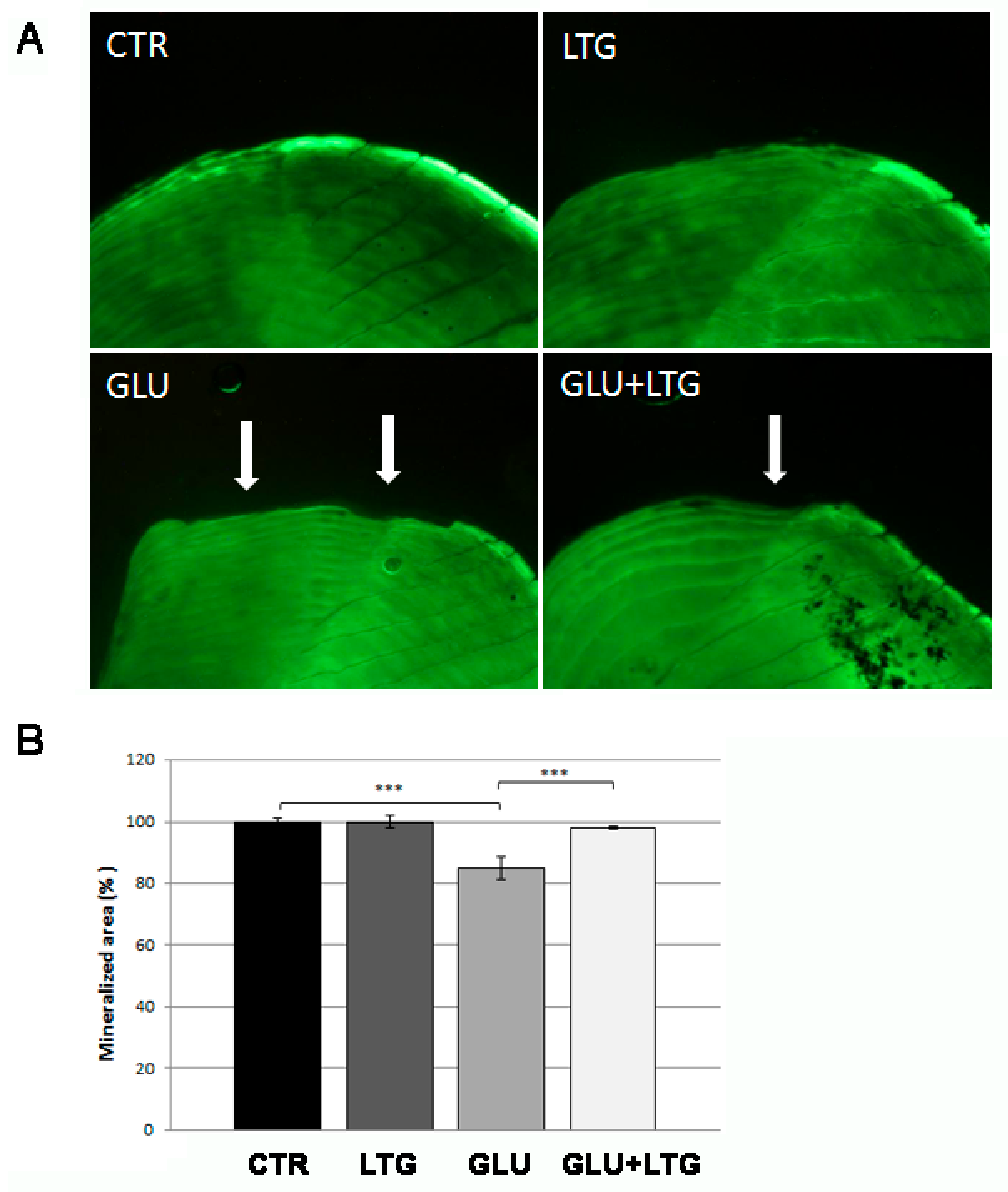
3.2. Liquiritigenin Treatment Reduces Bone Loss in Glucose-Treated Fish
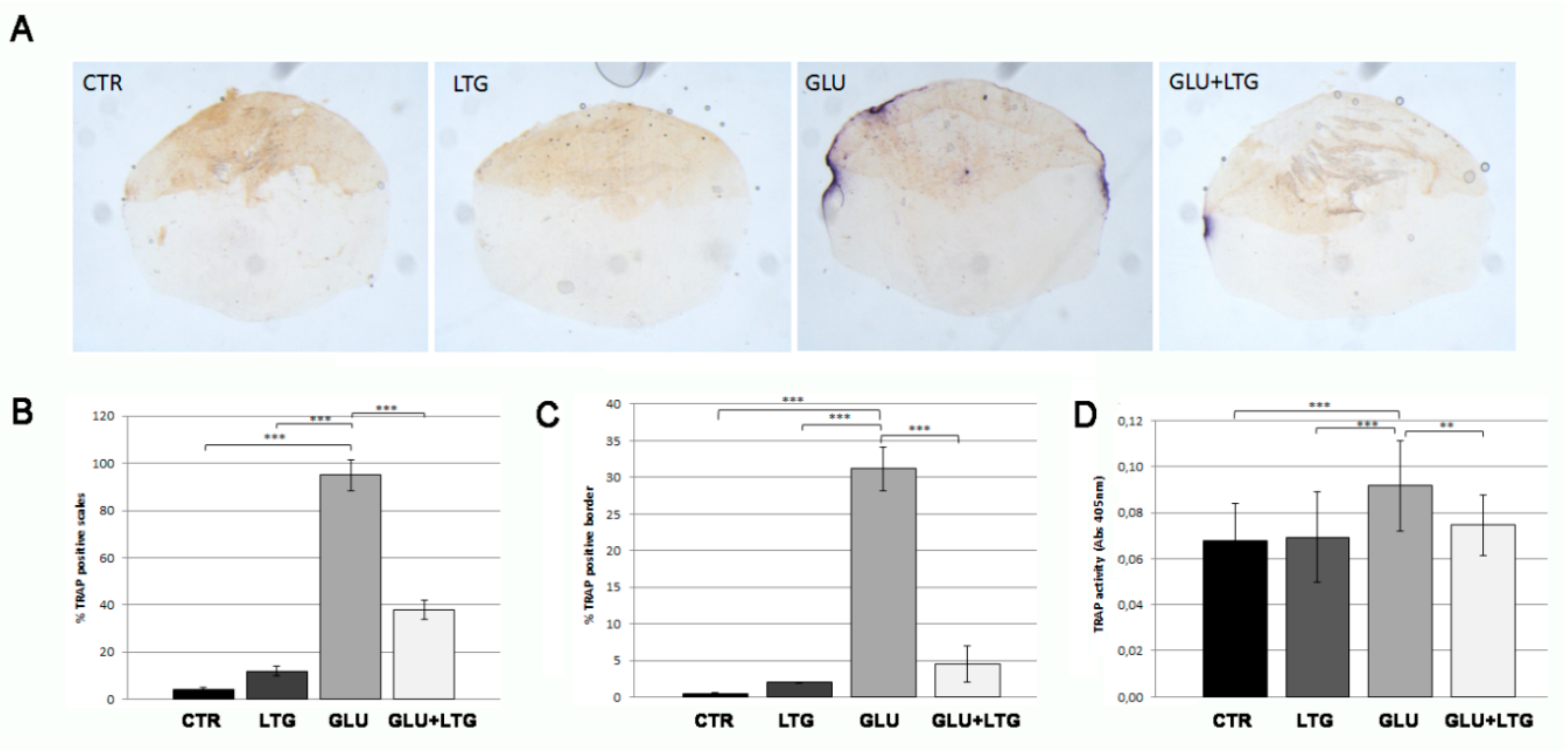
3.3. Liquiritigenin Prevents Osteoclast Activation in Glucose-Treated Fish
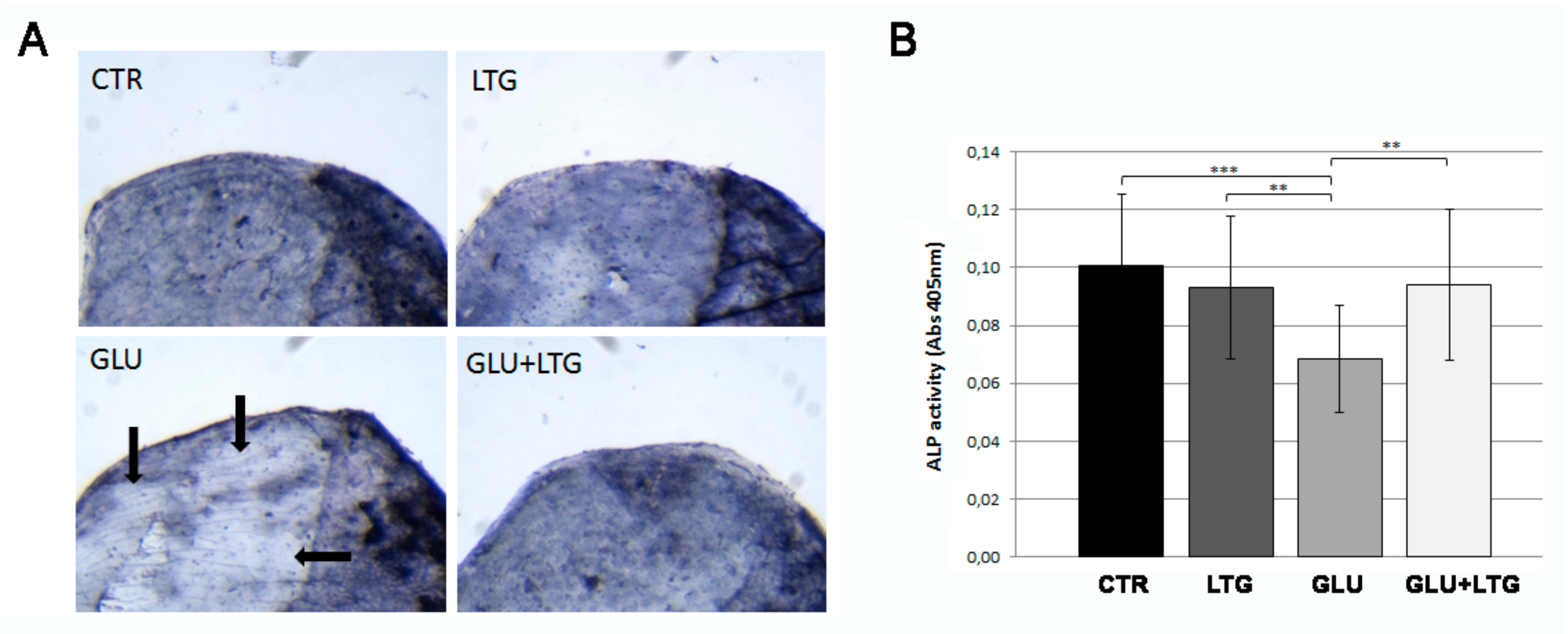
3.4. Liquiritigenin Treatment Prevents the Reduction of Alkaline Phosphatase Activity in Hyperglycemic Fish
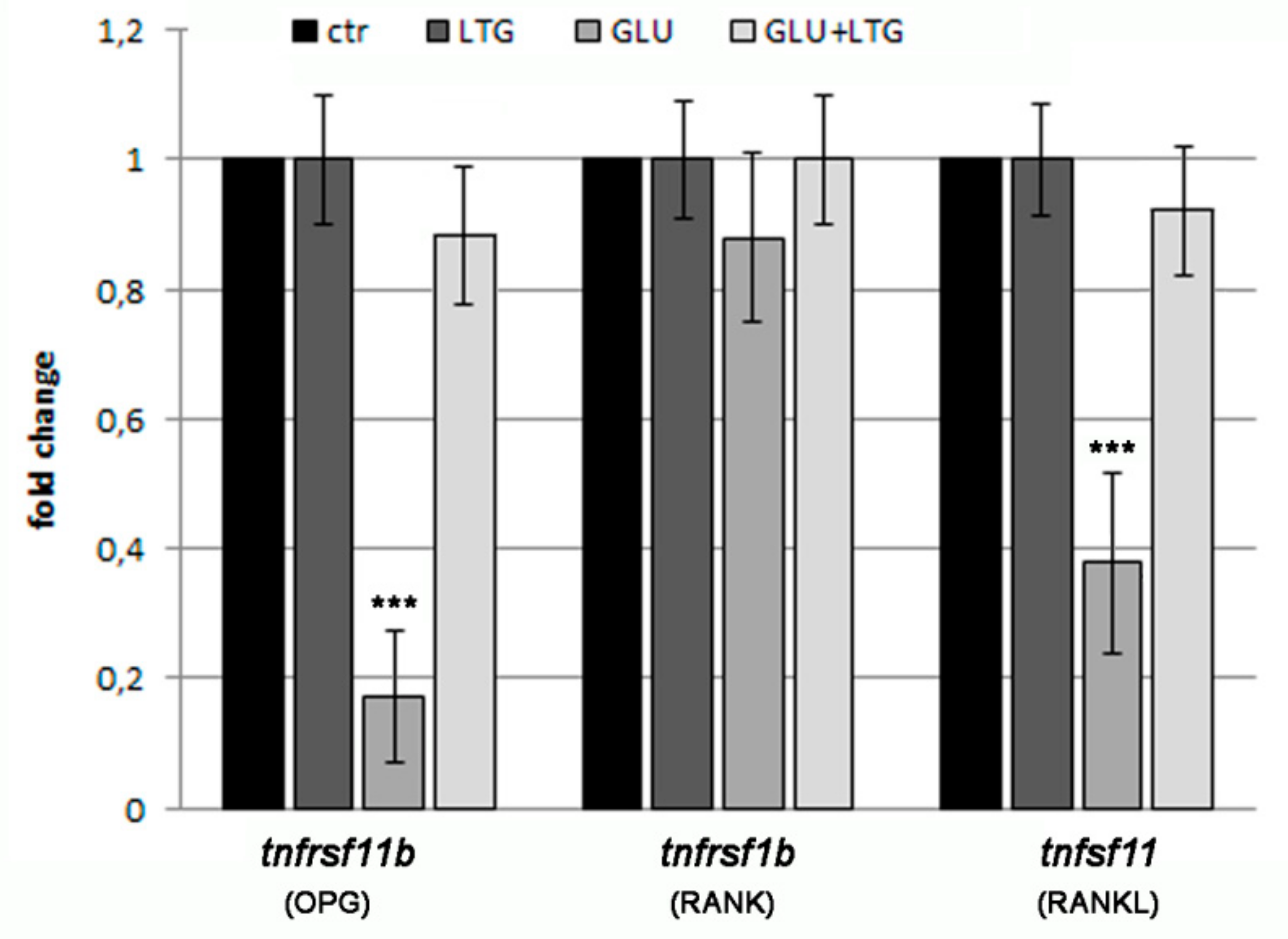
3.5. Liquiritigenin Treatment Prevents the Alteration of Bone Regulatory Genes in Hyperglycemic Fish
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wongdee, K.; Charoenphandhu, N. Osteoporosis in diabetes mellitus: Possible cellular and molecular mechanisms. World J. Diabetes 2011, 2, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Gaur, R.; Yadav, K.S.; Verma, R.K.; Yadav, N.P.; Bhakuni, R.S. In vivo anti-diabetic activity of derivatives of isoliquiritigenin and liquiritigenin. Phytomedicine 2014, 21, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Bae, G.D.; Park, E.Y.; Baek, D.J.; Jun, H.S.; Oh, Y.S. Liquiritigenin palmitate-induced beta-cell apoptosis via estrogen receptor-mediated AKT activation. Biomed. Pharmacother. 2018, 101, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Shi, J.; Li, H. Liquiritigenin attenuates high glucose-induced mesangial matrix accumulation, oxidative stress, and inflammation by suppression of the NF-kB and NLRP3 inflammasome pathways. Biomed. Pharmacother. 2018, 106, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Simmler, C.; Hajirahimkhan, A.; Lankin, D.C.; Bolton, J.L.; Jones, T.; Soejarto, D.D.; Chen, S.; Pauli, G.F. Dynamic residual complexity of the isoliquiritigenin-liquiritigenin interconversion during bioassay. J. Agric. Food Chem. 2013, 61, 2146–2157. [Google Scholar] [CrossRef] [PubMed]
- Kheniser, K.G.; Polanco Santos, C.M.; Kashyap, S.R. The effects of diabetes therapy on bone: A clinical perspective. J. Diabetes Complicat. 2018, 32, 713–719. [Google Scholar] [CrossRef]
- Wongdee, K.; Charoenphandhu, N. Update on type 2 diabetes-related osteoporosis. World J. Diabetes 2015, 685, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Napoli, N.; Strollo, R.; Paladini, A.; Briganti, S.I.; Pozzilli, P.; Epstein, S. The alliance of mesenchymal stem cells, bone, and diabetes. Int. J. Endocrinol. 2014, 2014, 26. [Google Scholar] [CrossRef]
- Saito, M.; Marumo, K. Collagen cross-links as a determinant of bone quality: A possible explanation for bone fragility in aging, osteoporosis, and diabete mellitus. Osteoporos. Int. 2010, 21, 195–214. [Google Scholar] [CrossRef]
- Choi, E.M. Liquiritigenin isolated from Glycyrrhiza uralensis stimulates osteoblas function in osteoblastic MC3T3-E1 cells. Int. Immunopharmacol. 2012, 12, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. A guide for the laboratory use of zebrafish (Danio rerio). In The Zebrafish Book, 5th ed.; University of Oregon Press: Eugene, OR, USA, 2007. [Google Scholar]
- Carnovali, M.; Luzi, L.; Banfi, G.; Mariotti, M. Chronic hyperglycemia affects bone metabolism in adult zebrafish scale model. Endocrine 2016, 54, 808–817. [Google Scholar] [CrossRef]
- Eames, S.C.; Philipson, L.H.; Prince, V.E.; Kinkel, M.D. Blood sugar measurement in zebrafish reveals dynamic of glucose homeostasis. Zebrafish 2010, 7, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Capiotti, K.M.; Antonioli, R., Jr.; Wilges, K.L.; Reis Bogo, M.; Bonan, C.D.; Souza Da Silva, R. Persistent impaired glucose metabolism in a zebrafish hyperglycemia model. Comp. Biochem. Physiol. B 2014, 171, 58–65. [Google Scholar] [CrossRef]
- Pasqualetti, S.; Banfi, G.; Mariotti, M. Osteoblast and osteoclast behavior in zebrafish cultured scales. Cell Tissue Res. 2012, 350, 69–75. [Google Scholar] [CrossRef]
- Perrson, P.; Takagi, Y.; Björnsson, B.T. Tartrate resistant acid phosphatases as a marker for scale resorption in rainbow trout, Oncorhynchus mykiss: Effects of estradiol-17β treatment and refeeding. Fish Physiol. Biochem. 1995, 14, 329–339. [Google Scholar] [CrossRef]
- Kitamura, K.; Takahira, K.; Inari, M.; Satoh, Y.; Hayakawa, K.; Tabuchi, Y.; Ogai, K.; Nishiuchi, T.; Kondo, T.; Mikuni-Takagaki, Y.; et al. Zebrafish scales respond differently to in vitro dynamic and static acceleration: Analysis of interaction between osteoblasts and osteoclasts. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 166, 74–80. [Google Scholar] [CrossRef]
- Ramalingam, M.; Kim, H.; Lee, Y.; Lee, Y.I. Phytochemical and pharmacological role of liquiritigenin and isoliquiritigenin from radix glycyrrhizae in human health and disease models. Front Aging Neurosci. 2018, 10, 348. [Google Scholar] [CrossRef]
- Suh, K.S.; Rhee, S.Y.; Kim, Y.S.; Choi, E.M. Protective effect of liquiritigenin against methylglyoxal cytotoxicity in osteoblastic MC3T3-E1 cells. Food Funct. 2014, 5, 1432–1440. [Google Scholar] [CrossRef]
- Xie, X.W. Liquiritigenin attenuates cardiac injury induced by high fructose-feeding through fibrosis and inflammation suppression. Biomed. Pharmacother. 2017, 86, 694–704. [Google Scholar] [CrossRef]
- Fishman, S.L.; Sonmez, H.; Basman, C.; Singh, V.; Poretsky, L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: A review. Mol. Med. 2018, 24, 59. [Google Scholar] [CrossRef] [PubMed]
- Lamb, L.S.; Alfonso, H.; Norman, P.E.; Davis, T.M.E.; Forbes, J.; Müench, G.; Irrgang, F.; Almeida, O.P.; Golledge, J.; Hankey, G.J.; et al. Advanced glycation end products and esRAGE are associated with bone turnover and incidence of hip fracture in older men. J. Clin. Endocrinol. Metab. 2018, 103, 4224–4231. [Google Scholar] [CrossRef]
- Kanazawa, I.; Sugimoto, T. Diabetes mellitus-induced bone fragility. Intern. Med. 2018, 57, 2773–2785. [Google Scholar] [CrossRef]
- Jud, P.; Sourij, H. Therapeutic options to reduce advanced glycation end products in patients with diabetes mellitus: A review. Diabetes Res. Clin. Pract. 2019, 148, 54–63. [Google Scholar] [CrossRef]
- Fatayerji, D.; Eastell, R. Age-related changes in bone turnover in men. J. Bone Miner. Res. 1999, 14, 1203–1210. [Google Scholar] [CrossRef]
- Li, W.; Yuan, L.; Tong, G.; He, Y.; Meng, Y.; Hao, S.; Chen, J.; Guo, J.; Bringhurst, R.; Yang, D. Phospholipase C signaling activated by parathyroid hormone mediates the rapid osteoclastogenesis in the fracture healing of orchiectomized mice. BMC Musculoskelet. Disord. 2018, 19, 311. [Google Scholar] [CrossRef]
- Zohreh, R. Parathyroid hormone, glucose metabolism and diabetes mellitus. J. Parathyr. Dis. 2014, 2, 55–56. [Google Scholar]
- Choi, E.M.; Suh, K.S.; Lee, Y.S. Liquiritigenin restores osteoblast damage through regulating oxidative stress and mitochondrial dysfunction. Phytother. Res. 2014, 28, 880–886. [Google Scholar] [CrossRef]
- Uchino, K.; Okamoto, K.; Sakai, E.; Yoneshima, E.; Iwatake, M.; Fukuma, Y.; Nishishita, K.; Tsukuba, T. Dual effects of liquiritigenin on the proliferation of bone cells: Promotion of osteoblast differentiation and inhibition of osteoclast differentiation. Phytother. Res. 2015, 29, 1714–1721. [Google Scholar] [CrossRef]
- Sanguineti, R.; Puddu, A.; Mach, F.; Montecucco, F.; Viviani, G.L. Advanced glycation end products play adverse proinflammatory activities in osteoporosis. Mediat. Inflamm. 2014, 2014, 9–975872. [Google Scholar] [CrossRef]
- Yang, X.; Mostafa, A.J.; Appleford, M.; Sun, L.W.; Wang, X. Bone formation is affected by matrix advanced glycation end products (AGEs) In Vivo. Calcif. Tissue Int. 2016, 99, 373–383. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnovali, M.; Luzi, L.; Terruzzi, I.; Banfi, G.; Mariotti, M. Liquiritigenin Reduces Blood Glucose Level and Bone Adverse Effects in Hyperglycemic Adult Zebrafish. Nutrients 2019, 11, 1042. https://doi.org/10.3390/nu11051042
Carnovali M, Luzi L, Terruzzi I, Banfi G, Mariotti M. Liquiritigenin Reduces Blood Glucose Level and Bone Adverse Effects in Hyperglycemic Adult Zebrafish. Nutrients. 2019; 11(5):1042. https://doi.org/10.3390/nu11051042
Chicago/Turabian StyleCarnovali, Marta, Livio Luzi, Ileana Terruzzi, Giuseppe Banfi, and Massimo Mariotti. 2019. "Liquiritigenin Reduces Blood Glucose Level and Bone Adverse Effects in Hyperglycemic Adult Zebrafish" Nutrients 11, no. 5: 1042. https://doi.org/10.3390/nu11051042
APA StyleCarnovali, M., Luzi, L., Terruzzi, I., Banfi, G., & Mariotti, M. (2019). Liquiritigenin Reduces Blood Glucose Level and Bone Adverse Effects in Hyperglycemic Adult Zebrafish. Nutrients, 11(5), 1042. https://doi.org/10.3390/nu11051042



