Cynanchum wilfordii Etanolic Extract Controls Blood Cholesterol: A Double-blind, Randomized, Placebo-Controlled, Parallel Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of CW Extract
2.2. Participants
2.3. Study Design
2.4. Sample Collection
2.5. Statistical Analysis
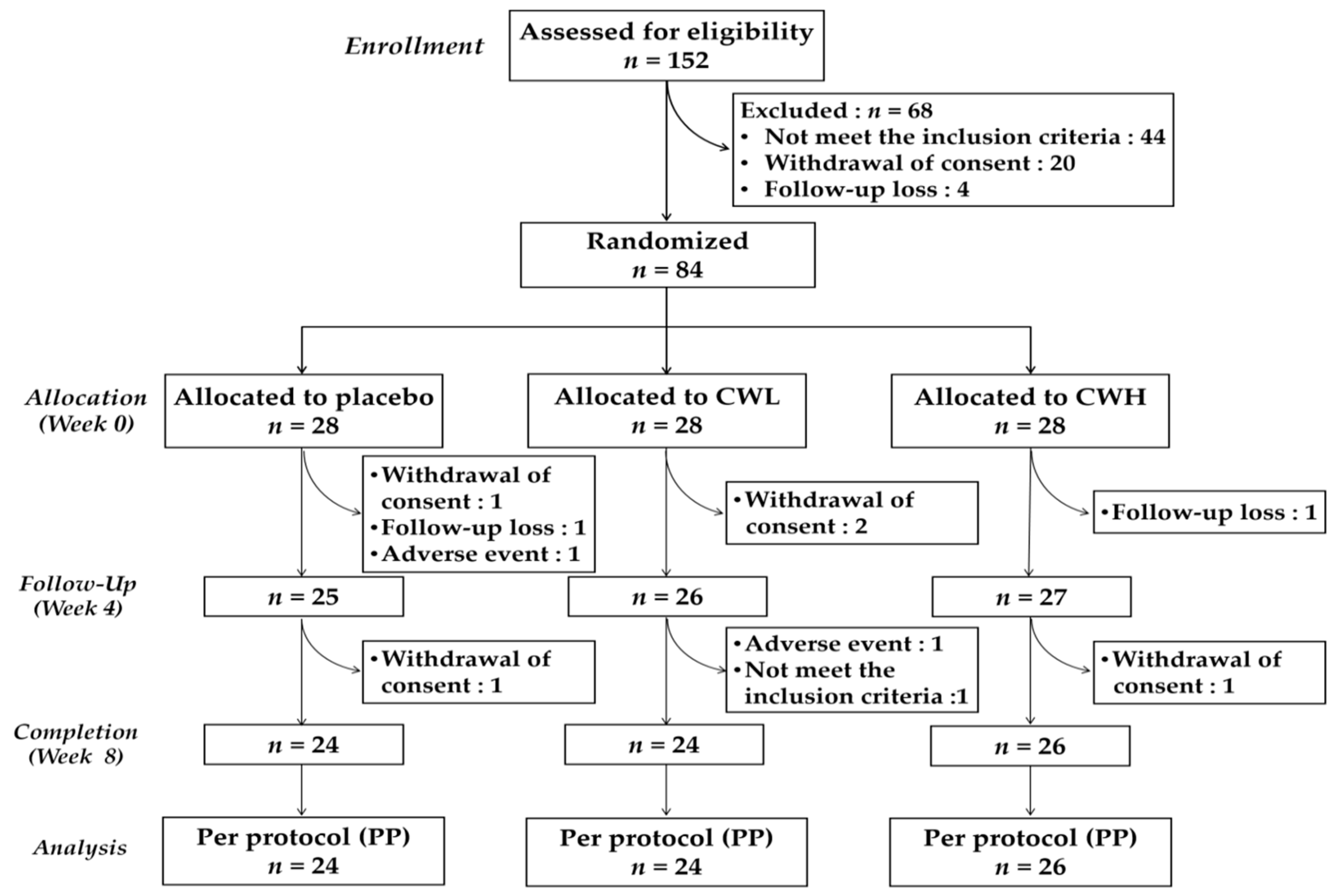
3. Results
3.1. Characteristics of Subjects
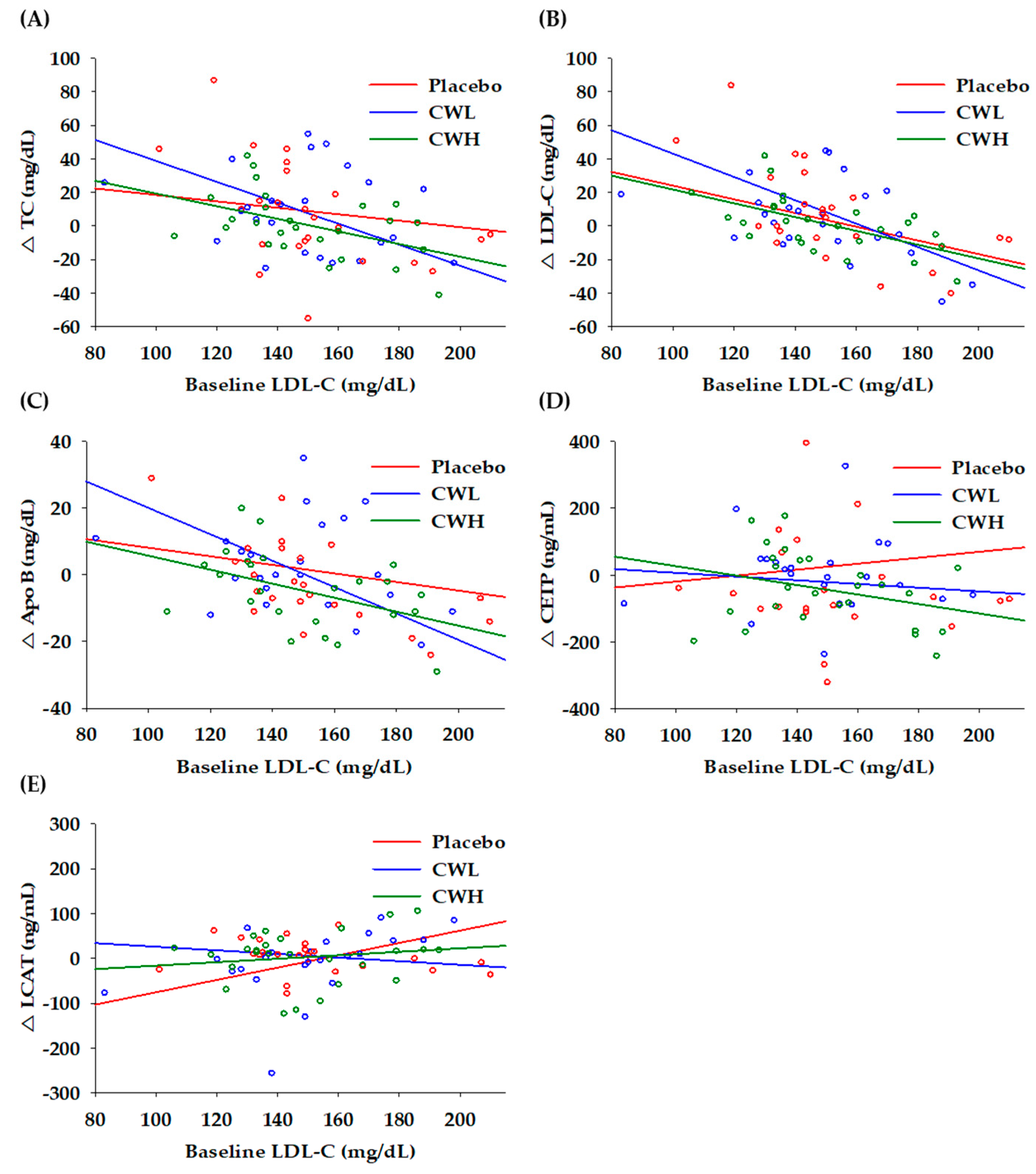
3.2. Correlation to Baseline LDL-C
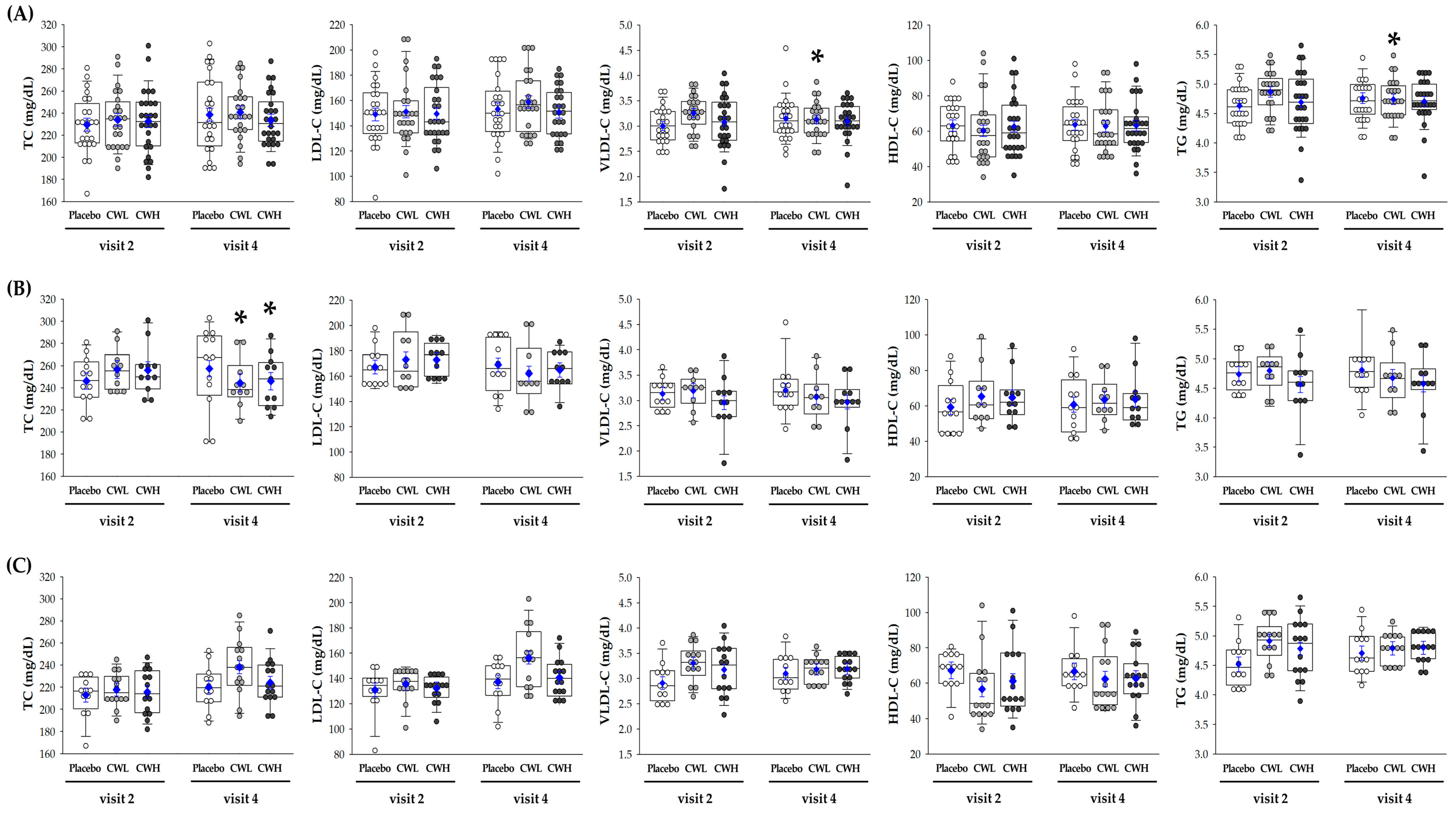
3.3. Lipid Profile
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Kapourchali, F.R.; Surendiran, G.; Goulet, A.; Moghadasian, M.H. The Role of Dietary Cholesterol in Lipoprotein Metabolism and Related Metabolic Abnormalities: A Mini-review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2408–2415. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Davidson, M.H.; Hirsh, B.J.; Kathiresan, S.; Gaudet, D. Genetics and causality of triglyceride-rich lipoproteins in atherosclerotic cardiovascular disease. J. Am. Coll. Cardiol. 2014, 64, 2525–2540. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.R. Saroglitazar for the treatment of dyslipidemia in diabetic patients. Expert Opin. Pharmacother. 2015, 16, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Chew, G.T.; Watts, G.F. Recent advances in pharmacotherapy for hypertriglyceridemia. Prog. Lipid Res. 2014, 56, 47–66. [Google Scholar] [CrossRef] [PubMed]
- Ossoli, A.; Neufeld, E.B.; Thacker, S.G.; Vaisman, B.; Pryor, M.; Freeman, L.A.; Brantner, C.A.; Baranova, I.; Francone, N.O.; Demosky, S.J., Jr.; et al. Lipoprotein X Causes Renal Disease in LCAT Deficiency. PLoS ONE 2016, 11, e0150083. [Google Scholar] [CrossRef] [PubMed]
- Karr, S. Epidemiology and management of hyperlipidemia. Am. J. Manag. Care 2017, 23, S139–S148. [Google Scholar]
- Thongtang, N.; Diffenderfer, M.R.; Ooi, E.M.M.; Barrett, P.H.R.; Turner, S.M.; Le, N.A.; Brown, W.V.; Schaefer, E.J. Metabolism and proteomics of large and small dense LDL in combined hyperlipidemia: Effects of rosuvastatin. J. Lipid Res. 2017, 58, 1315–1324. [Google Scholar] [CrossRef]
- Itoh, H.; Komuro, I.; Takeuchi, M.; Akasaka, T.; Daida, H.; Egashira, Y.; Fujita, H.; Higaki, J.; Hirata, K.I.; Ishibashi, S.; et al. Achieving LDL-C target levels less than 70 mg/dL may provide extra cardiovascular protection in high-risk patients: Exploratory analysis of the Standard Versus Intensive Statin Therapy for Patients With Hypercholesterolemia and Diabetic Retinopathy Study. Diabetes Obes. Metab. 2019, 21, 791–800. [Google Scholar]
- Nordestgaard, B.G.; Chapman, M.J.; Ray, K.; Boren, J.; Andreotti, F.; Watts, G.F.; Ginsberg, H.; Amarenco, P.; Catapano, A.; Descamps, O.S.; et al. Lipoprotein(a) as a cardiovascular risk factor: Current status. Eur. Heart J. 2010, 31, 2844–2853. [Google Scholar] [CrossRef] [PubMed]
- Ham, W.S.; Kim, J.; Park, D.J.; Ryu, H.C.; Jang, Y.P. Discrimination of Cynanchum wilfordii and Cynanchum auriculatum by terahertz spectroscopic analysis. Phytochem. Anal. 2018, 29, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Piao, D.; Zhang, H.; Woo, M.H.; Lee, J.H.; Moon, D.C.; Lee, S.H.; Chang, H.W.; Son, J.K. Quality assessment and discrimination of the roots of Cynanchum auriculatum and Cynanchum wilfordii by HPLC-UV analysis. Arch. Pharm Res. 2013, 36, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.W.; Ahn, S.; Lim, T.G.; Hong, H.D.; Rhee, Y.K.; Yang, D.C.; Jang, M. Cynanchum wilfordii Polysaccharides Suppress Dextran Sulfate Sodium-Induced Acute Colitis in Mice and the Production of Inflammatory Mediators from Macrophages. Mediators Inflamm. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Lee, G.; Shin, J.; Choi, H.; Jo, A.; Pan, S.; Bae, D.; Lee, Y.; Choi, C. Cynanchum wilfordii Ameliorates Testosterone-Induced Benign Prostatic Hyperplasia by Regulating 5alpha-Reductase and Androgen Receptor Activities in a Rat Model. Nutrients 2017, 9, 1070. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, M.H.; Choi, Y.Y.; Hong, J.; Yang, W.M. Effects of Cynanchum wilfordii on osteoporosis with inhibition of bone resorption and induction of bone formation. Mol. Med. Rep. 2018, 17, 3758–3762. [Google Scholar] [CrossRef] [PubMed]
- Uchikura, T.; Tanaka, H.; Sugiwaki, H.; Yoshimura, M.; Sato-Masumoto, N.; Tsujimoto, T.; Uchiyama, N.; Hakamatsuka, T.; Amakura, Y. Preliminary Quality Evaluation and Characterization of Phenolic Constituents in Cynanchi Wilfordii Radix. Molecules 2018, 23, 656. [Google Scholar] [CrossRef]
- Fang, H.; Yue, R.; Ga, Y.; Zhang, Y.; Shan, L.; Zhao, J. Analysis of Cynandione A’s Anti-Ischemic Stroke Effects from Pathways and Protein-Protein Interactome. PLoS ONE 2015, 10, e0124632. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, S.H.; Lee, T.H.; Lee, S.M.; Park, J.H.; Park, K.H.; Jung, M.; Jung, H.; Mohamed, M.A.; Baek, N.I.; Chung, I.S.; Kim, J. Cynandione A attenuates lipopolysaccharide-induced production of inflammatory mediators via MAPK inhibition and NF-kappaB inactivation in RAW264.7 macrophages and protects mice against endotoxin shock. Exp. Biol. Med. (Maywood) 2015, 240, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Kim, J.; Lee, E.H.; Lee, T.H. Cynandione A inhibits lipopolysaccharide-induced cell adhesion via suppression of the protein expression of VCAM1 in human endothelial cells. Int. J. Mol. Med. 2018, 41, 1756–1764. [Google Scholar]
- Huang, L.J.; Wang, B.; Zhang, J.X.; Yuan, C.M.; Gu, W.; Mu, S.Z.; Hao, X.J. Synthesis and evaluation of antifungal activity of C21-steroidal derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 2040–2043. [Google Scholar] [CrossRef]
- Tan, Z.W.; Xie, S.; Hu, S.Y.; Liao, T.; Liu, P.; Peng, K.H.; Yang, X.Z.; He, Z.L.; Tang, H.Y.; Cui, Y.; et al. Caudatin targets TNFAIP1/NF-kappaB and cytochrome c/caspase signaling to suppress tumor progression in human uterine cancer. Int. J. Oncol. 2016, 49, 1638–1650. [Google Scholar] [CrossRef]
- Fei, H.R.; Cui, L.Y.; Zhang, Z.R.; Zhao, Y.; Wang, F.Z. Caudatin inhibits carcinomic human alveolar basal epithelial cell growth and angiogenesis through modulating GSK3beta/beta-catenin pathway. J. Cell. Biochem. 2012, 113, 3403–3410. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.A.; Lee, S.; Sohn, E.H.; Yang, J.; Park, D.W.; Jeong, Y.J.; Kim, I.; Kwon, J.E.; Song, H.S.; Cho, Y.M.; et al. Cynanchum wilfordii Radix attenuates liver fat accumulation and damage by suppressing hepatic cyclooxygenase-2 and mitogen-activated protein kinase in mice fed with a high-fat and high-fructose diet. Nutr. Res. 2016, 36, 914–924. [Google Scholar] [CrossRef]
- Lee, H.S.; Choi, J.H.; Kim, Y.E.; Kim, I.H.; Kim, B.M.; Lee, C.H. Effects of the Cynanchum wilfordii Ethanol Extract on the Serum Lipid Profile in Hypercholesterolemic Rats. Prev. Nutr. Food Sci. 2013, 18, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Lee, Y.J.; Kim, J.S.; Kang, D.G.; Lee, H.S. Cynanchum wilfordii ameliorates hypertension and endothelial dysfunction in rats fed with high fat/cholesterol diets. Immunopharmacol. Immunotoxicol. 2012, 34, 4–11. [Google Scholar] [CrossRef]
- Choi, D.H.; Lee, Y.J.; Oh, H.C.; Cui, Y.L.; Kim, J.S.; Kang, D.G.; Lee, H.S. Improved endothelial dysfunction by Cynanchum wilfordii in apolipoprotein E(-/-) mice fed a high fat/cholesterol diet. J. Med. Food 2012, 15, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S. Effects of Cynanchum wilfordii Extract on Serum Lipid Components and Enzyme Activities in Hyperlipidemic and Streptozotocin-Induced Diabetic Rats. Korean J. Human Ecology 2004, 7, 1–11. [Google Scholar]
- Verhoeven, V.; Lopez, H.M.; Remmen, R.; Wens, J.; Apers, S.; Van Royen, P. Red yeast rice lowers cholesterol in physicians-a double blind, placebo controlled randomized trial. BMC Complement. Altern. Med. 2013, 13, 178. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormesis and medicine. Br. J. Clin. Pharmacol. 2008, 66, 594–617. [Google Scholar] [CrossRef]
- Son, T.G.; Camandola, S.; Mattson, M.P. Hormetic dietary phytochemicals. Neuromolecular Med. 2008, 10, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Merz, C.N.; Brewer, H.B.; Clark, L.T.; Hunninghake, D.B.; Pasternak, R.C.; Smith, S.C.; Stone, N.J.; Coordinating Committee of the National Cholesterol Education Program; et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004, 110, 227–239. [Google Scholar] [CrossRef]
- Argani, H.; Ghorbanihaghjo, A.; Vatankhahan, H.; Rashtchizadeh, N.; Raeisi, S.; Ilghami, H. The effect of red grape seed extract on serum paraoxonase activity in patients with mild to moderate hyperlipidemia. Sao Paulo Med. J. 2016, 134, 234–239. [Google Scholar] [CrossRef]
- Samavat, H.; Newman, A.R.; Wang, R.; Yuan, J.M.; Wu, A.H.; Kurzer, M.S. Effects of green tea catechin extract on serum lipids in postmenopausal women: A randomized, placebo-controlled clinical trial. Am. J. Clin. Nutr. 2016, 104, 1671–1682. [Google Scholar] [CrossRef]
- Tome-Carneiro, J.; Gonzalvez, M.; Larrosa, M.; Garcia-Almagro, F.J.; Aviles-Plaza, F.; Parra, S.; Yanez-Gascon, M.J.; Ruiz-Ros, J.A.; Garcia-Conesa, M.T.; Tomas-Barberan, F.A.; Espin, J.C. Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients undergoing primary prevention of cardiovascular disease: A triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol. Nutr. Food Res. 2012, 56, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.E.; Wagener, G.; Baker, B.F.; Geary, R.S.; Donovan, J.M.; Beuers, U.H.; Nederveen, A.J.; Verheij, J.; Trip, M.D.; Basart, D.C.; et al. Mipomersen, an apolipoprotein B synthesis inhibitor, lowers low-density lipoprotein cholesterol in high-risk statin-intolerant patients: A randomized, double-blind, placebo-controlled trial. Eur. Heart J. 2012, 33, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Youn, Y.; Kim, Y.S. Inhibitory effects of Citrus unshiu pericarpium extracts on palmitate-induced lipotoxicity in HepG2 cells. Food Sci. Biotechnol. 2016, 25, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.J.; Rye, K.A. Cholesteryl Ester Transfer Protein Inhibitors as Agents to Reduce Coronary Heart Disease Risk. Cardiol. Clin. 2018, 36, 299–310. [Google Scholar] [CrossRef]
- Rosales, C.; Gillard, B.K.; Gotto, A.M.; Pownall, H.J. High-Density Lipoprotein Processing and Premature Cardiovascular Disease. Methodist Debakey Cardiovasc. J. 2015, 11, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Miyares, M.A.; Davis, K. Patient considerations and clinical impact of cholesteryl ester transfer protein inhibitors in the management of dyslipidemia: Focus on anacetrapib. Vasc. Health Risk Manag. 2012, 8, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Choi, H.G.; Li, Y.; Park, Y.M.; Lee, J.H.; Kim, D.H.; Lee, J.H.; Son, J.K.; Na, M.; Lee, S.H. Chemical constituents of Cynanchum wilfordii and the chemotaxonomy of two species of the family Asclepiadacease, C. wilfordii and C. auriculatum. Arch. Pharm Res. 2011, 34, 2021–2027. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, E.M.; Thabet, N.M. Beta-sitosterol upregulated paraoxonase-1 via peroxisome proliferator-activated receptor-gamma in irradiated rats. Can. J. Physiol. Pharmacol. 2017, 95, 661–666. [Google Scholar] [CrossRef]
- Litvinov, D.; Mahini, H.; Garelnabi, M. Antioxidant and anti-inflammatory role of paraoxonase 1: Implication in arteriosclerosis diseases. N. Am. J. Med. Sci 2012, 4, 523–532. [Google Scholar] [PubMed]
- Farrell, N.; Norris, G.; Lee, S.G.; Chun, O.K.; Blesso, C.N. Anthocyanin-rich black elderberry extract improves markers of HDL function and reduces aortic cholesterol in hyperlipidemic mice. Food Funct. 2015, 6, 1278–1287. [Google Scholar] [CrossRef]
- Zagayko, A.L.; Kravchenko, G.B.; Krasilnikova, O.A.; Ogai, Y.O. Grape polyphenols increase the activity of HDL enzymes in old and obese rats. Oxid. Med. Cell. Longev. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Tosheska, T.K.; Topuzovska, S. High-density lipoprotein metabolism and reverse cholesterol transport: Strategies for raising HDL cholesterol. Anatol. J. Cardiol. 2017, 18, 149–154. [Google Scholar]
- Bjorck, L.; Rosengren, A.; Bennett, K.; Lappas, G.; Capewell, S. Modelling the decreasing coronary heart disease mortality in Sweden between 1986 and 2002. Eur. Heart J. 2009, 30, 1046–1056. [Google Scholar] [CrossRef]
- Manfrin, A.; Trimarco, V.; Manzi, M.V.; Rozza, F.; Izzo, R. A single blind, multicenter, randomized controlled trial to evaluate the effectiveness and cost of a novel nutraceutical (LopiGLIK((R))) lowering cardiovascular disease risk. Clinicoecon Outcomes Res. 2018, 10, 601–609. [Google Scholar] [CrossRef]
- Bagdade, J.D.; Ritter, M.; Subbaiah, P.V. Marine lipids normalize cholesteryl ester transfer in IDDM. Diabetologia 1996, 39, 487–491. [Google Scholar] [CrossRef]
- Inazu, A.; Brown, M.L.; Hesler, C.B.; Agellon, L.B.; Koizumi, J.; Takata, K.; Maruhama, Y.; Mabuchi, H.; Tall, A.R. Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation. N. Engl. J. Med. 1990, 323, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
| Variables | Placebo 1 (n = 24) | CWL 1 (n = 24) | CWH 1 (n = 26) | p-Value 2 |
|---|---|---|---|---|
| Gender (male/female) | 8/16 | 9/15 | 10/16 | 0.924 |
| Age (y) | 58.1 ± 2.3 | 57.4 ± 1.8 | 58.3 ± 1.8 | 0.940 |
| Body weight (kg) | 60.9 ± 2.5 | 64.0 ± 2.3 | 62.8 ± 1.8 | 0.630 |
| BMI (kg/m2) | 23.6 ± 0.6 | 24.8 ± 0.6 | 23.9 ± 0.5 | 0.257 |
| Dietary intake | ||||
| Energy (kcal/d) | 1343.5 ± 81.7 | 1483.7 ± 72.2 | 1374.9 ± 72.3 | 0.398 |
| Carbohydrate (g/d) | 201.8 ± 12.5 | 217.3 ± 14.0 | 209.9 ± 13.4 | 0.718 |
| Protein (g/d) | 52.5 ± 3.4 | 60.5 ± 3.6 | 61.2 ± 11.2 | 0.632 |
| Fat (g/d) | 35.0 ± 3.2 | 39.7 ± 3.3 | 32.5 ± 2.5 | 0.242 |
| Sodium (mg/d) | 2628.7 ± 233.8 | 2832.4 ± 241.5 | 2692.0 ± 217.4 | 0.818 |
| SBP (mmHg) | 119.8 ± 2.4 | 124.3 ± 2.1 | 121.4 ± 1.8 | 0.338 |
| DBP (mmHg) | 71.5 ± 1.8 | 74.3 ± 1.7 | 74.1 ± 1.7 | 0.467 |
| Blood | ||||
| TC (mg/dL) | 229.5 ± 5.4 | 233.9 ± 5.3 | 232.7 ± 5.7 | 0.844 |
| LDL-C (mg/dL) | 149.0 ± 5.0 | 151.2 ± 5.3 | 149.4 ± 4.6 | 0.948 |
| HDL-C (mg/dL) | 63.2 ± 2.7 | 60.2 ± 3.8 | 62.7 ± 3.3 | 0.796 |
| VLDL-C (mg/dL) | 21.8 ± 1.6 | 27.5 ± 1.9 | 24.7 ± 2.4 | 0.135 |
| TG (mg/dL) | 108.9 ± 8.1 | 137.3 ± 9.4 | 123.3 ± 12.2 | 0.135 |
| Total | Baseline LDL-C≥ 150 mg/dL | Baseline LDL-C< 150 mg/dL | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | CWL 1 (n = 24) | p2 | CWH 1 (n = 26) | p3 | p4 | CWL 1 (n = 10) | p2 | CWH 1 (n = 11) | p3 | p4 | CWL 1 (n = 14) | p2 | CWH 1 (n = 15) | p3 | p4 |
| LP (a) (mg/dL) | |||||||||||||||
| Week 4 | 3.15 ± 3.75 | 0.403 | −0.25 ± 3.65 | 0.945 | 6.07 ± 5.38 | 0.264 | 0.84 ± 5.14 | 0.871 | 0.74 ± 5.33 | 0.890 | −1.33 ± 5.25 | 0.800 | |||
| Week 8 | 4.13 ± 3.78 | 0.276 | 2.50 ± 3.68 | 0.498 | 0.759 | −0.76 ± 5.45 | 0.889 | −0.06 ± 5.22 | 0.991 | 0.690 | 8.10 ± 5.33 | 0.133 | 4.83 ± 5.25 | 0.360 | 0.545 |
| ApoA1 (mg/dL) | |||||||||||||||
| Week 4 | 1.75 ± 4.88 | 0.720 | 0.83 ± 4.85 | 0.864 | −10.73 ± 6.57 | 0.108 | −7.47 ± 6.52 | 0.257 | 12.39 ± 6.82 | 0.073 | 8.73 ± 6.79 | 0.203 | |||
| Week 8 | 2.92 ± 4.88 | 0.551 | 2.21 ± 4.85 | 0.649 | 0.982 | −12.67 ± 6.57 | 0.059 | −6.85 ± 6.40 | 0.289 | 0.351 | 15.31 ± 6.82 | 0.028 | 10.39 ± 6.87 | 0.135 | 0.222 |
| ApoB (mg/dL) | |||||||||||||||
| Week 4 | −5.62 ± 3.90 | 0.151 | −2.58 ± 3.88 | 0.506 | −10.82 ± 5.53 | 0.055 | −6.00 ± 5.49 | 0.278 | −1.51 ± 5.22 | 0.773 | 0.87 ± 5.19 | 0.868 | |||
| Week 8 | −2.04 ± 3.90 | 0.601 | −6.64 ± 3.88 | 0.089 | 0.202 | −13.05 ± 5.53 | 0.022 | −13.39 ± 5.39 | 0.016 | 0.069 | 6.08 ± 5.22 | 0.247 | −0.87 ± 5.25 | 0.869 | 0.382 |
| ApoE (mg/dL) | |||||||||||||||
| Week 4 | −0.25 ± 0.24 | 0.307 | −0.29 ± 0.24 | 0.224 | −0.31 ± 0.34 | 0.368 | −0.18 ± 0.34 | 0.599 | −0.18 ± 0.34 | 0.592 | −0.35 ± 0.34 | 0.304 | |||
| Week 8 | −0.36 ± 0.24 | 0.137 | −0.31 ± 0.24 | 0.194 | 0.547 | −0.52 ± 0.34 | 0.134 | −0.62 ± 0.33 | 0.068 | 0.367 | −0.18 ± 0.34 | 0.606 | −0.02 ± 0.34 | 0.952 | 0.767 |
| CETP (ng/mL) | |||||||||||||||
| Week 8 | −46.15 ± 50.08 | 0.360 | −67.55 ± 48.61 | 0.169 | 0.375 | −134.20 ± 61.51 | 0.037 | −149.68 ± 58.22 | 0.016 | 0.030 | 25.00 ± 75.71 | 0.743 | 4.29 ± 74.59 | 0.954 | 0.935 |
| LCAT (ng/mL) | |||||||||||||||
| Week 8 | 13.25 ± 17.36 | 0.448 | 10.76 ± 16.85 | 0.525 | 0.719 | −28.21 ± 21.37 | 0.197 | −16.15 ± 20.23 | 0.431 | 0.419 | 55.15 ± 24.81 | 0.032 | 42.66 ± 24.44 | 0.089 | 0.085 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youn, J.S.; Ham, Y.M.; Yoon, W.-J.; Choi, H.-C.; Lee, J.E.; Cho, B.; Kim, J.Y. Cynanchum wilfordii Etanolic Extract Controls Blood Cholesterol: A Double-blind, Randomized, Placebo-Controlled, Parallel Trial. Nutrients 2019, 11, 836. https://doi.org/10.3390/nu11040836
Youn JS, Ham YM, Yoon W-J, Choi H-C, Lee JE, Cho B, Kim JY. Cynanchum wilfordii Etanolic Extract Controls Blood Cholesterol: A Double-blind, Randomized, Placebo-Controlled, Parallel Trial. Nutrients. 2019; 11(4):836. https://doi.org/10.3390/nu11040836
Chicago/Turabian StyleYoun, Ji Sun, Young Min Ham, Weon-Jong Yoon, Ho-Chun Choi, Ji Eun Lee, Belong Cho, and Ji Yeon Kim. 2019. "Cynanchum wilfordii Etanolic Extract Controls Blood Cholesterol: A Double-blind, Randomized, Placebo-Controlled, Parallel Trial" Nutrients 11, no. 4: 836. https://doi.org/10.3390/nu11040836
APA StyleYoun, J. S., Ham, Y. M., Yoon, W.-J., Choi, H.-C., Lee, J. E., Cho, B., & Kim, J. Y. (2019). Cynanchum wilfordii Etanolic Extract Controls Blood Cholesterol: A Double-blind, Randomized, Placebo-Controlled, Parallel Trial. Nutrients, 11(4), 836. https://doi.org/10.3390/nu11040836





