Similar Weight Loss Induces Greater Improvements in Insulin Sensitivity and Liver Function among Individuals with NAFLD Compared to Individuals without NAFLD
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Laboratory Analyses
2.3. MR Imaging
2.4. Statistical Analyses
3. Results
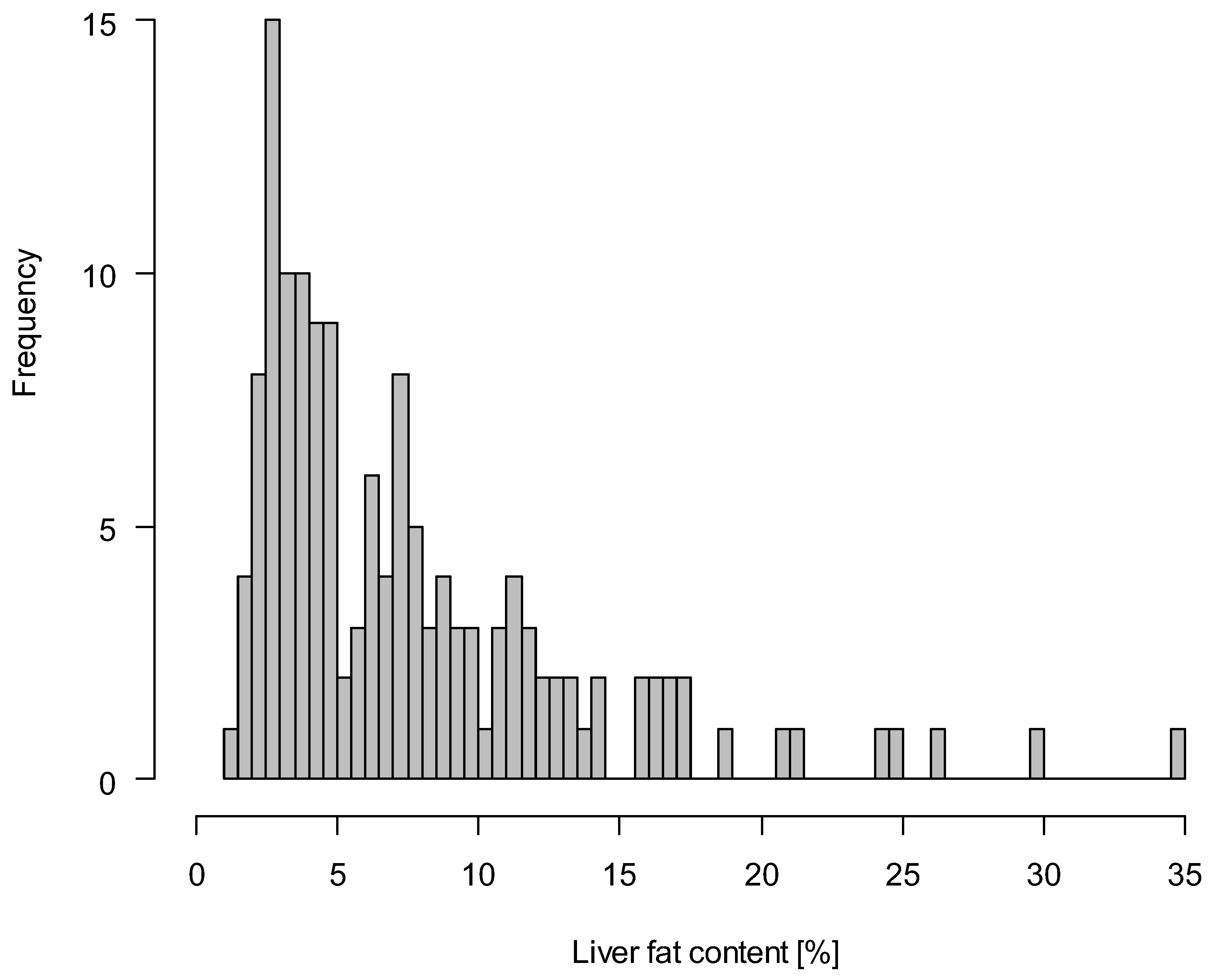
3.1. Characteristics of the Study Population
3.2. Correlations pf Liver Fat with Anthropometric Measures and Body Fat Volumes
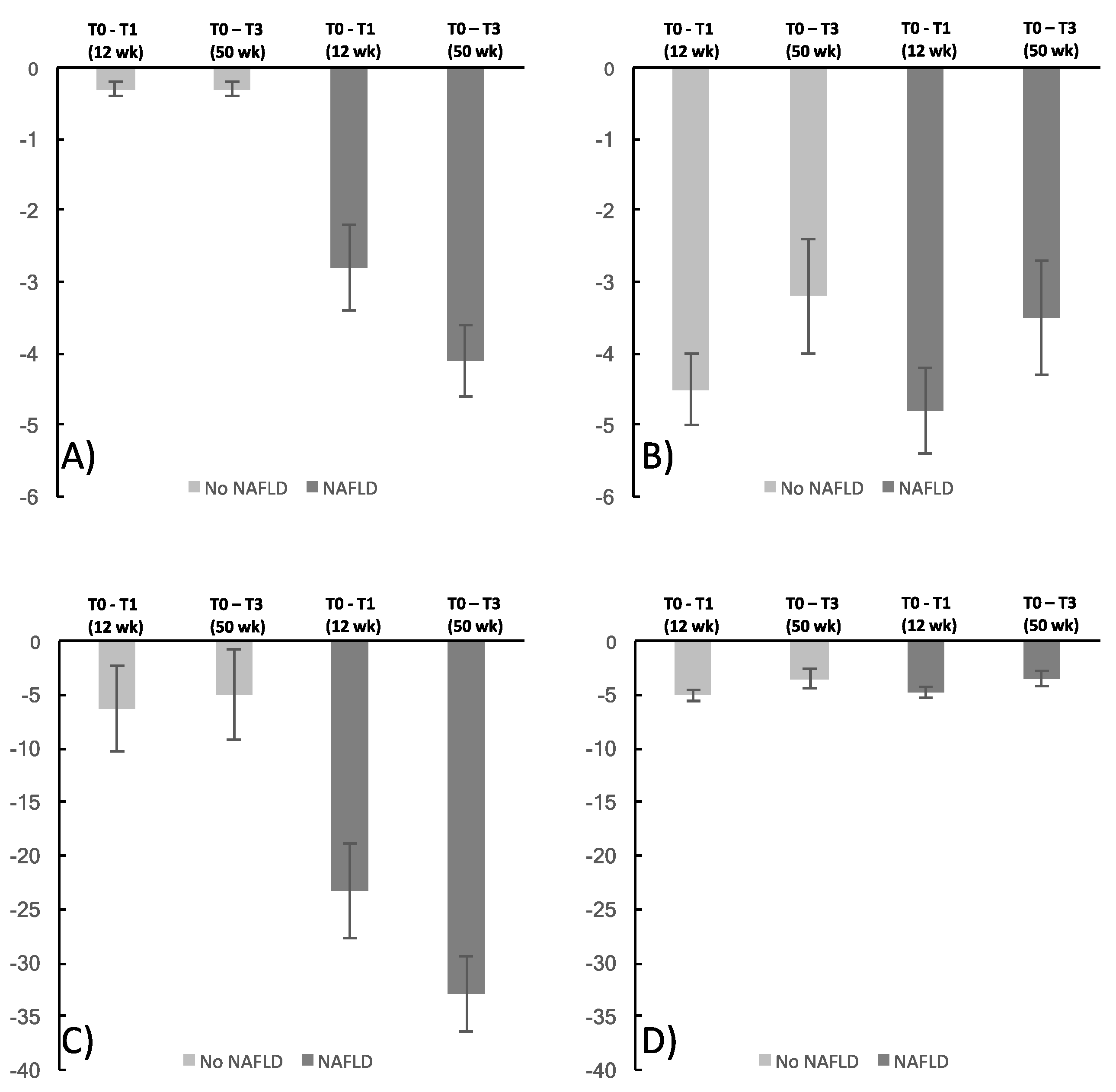
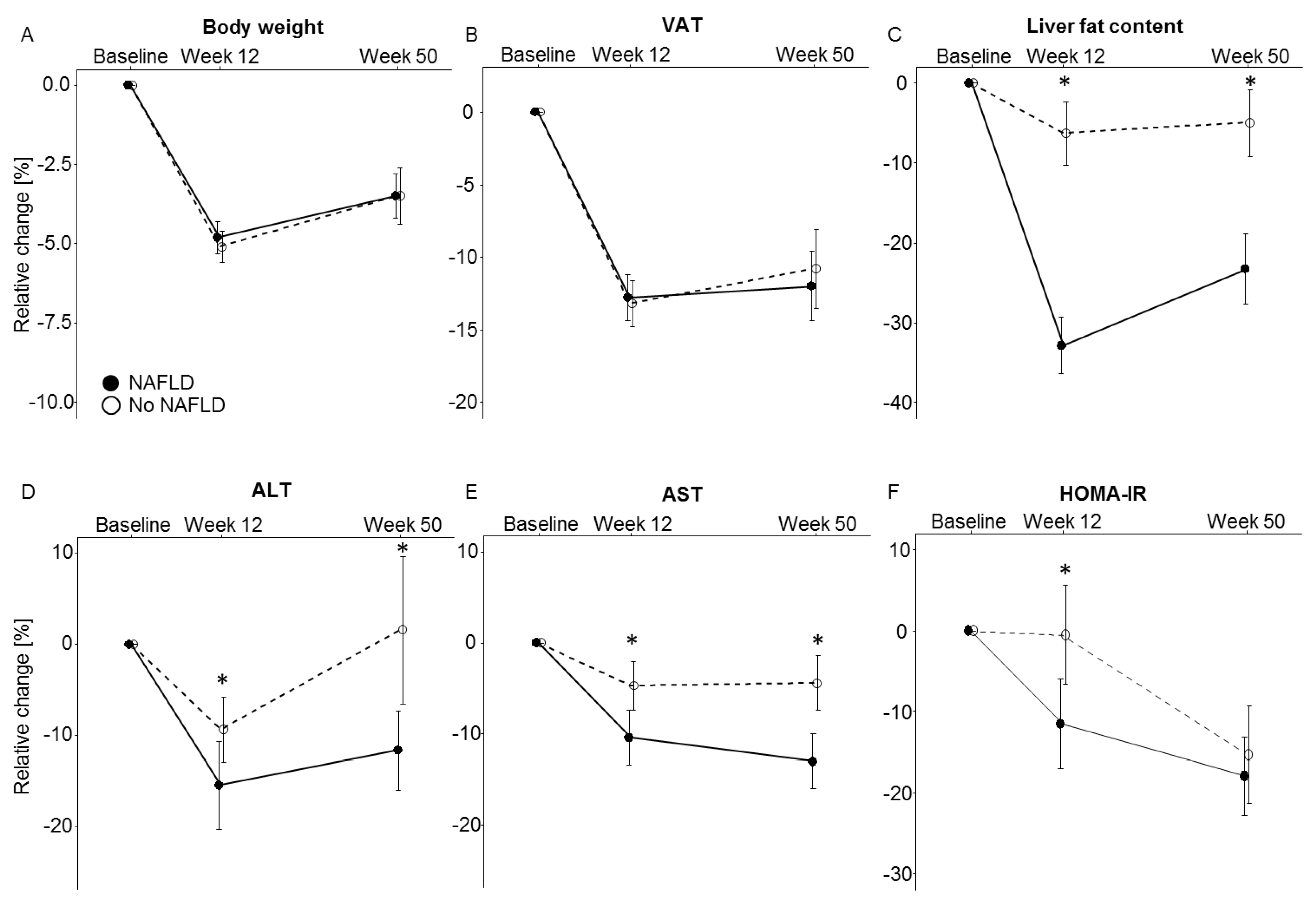
3.3. NAFLD and Metabolic Effects of Weight Loss
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tchernof, A.; Despres, J.P. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Korenblat, K.M.; Fabbrini, E.; Mohammed, B.S.; Klein, S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology 2008, 134, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, L.S.; Nurenberg, P.; Leonard, D.; Browning, J.D.; Reingold, J.S.; Grundy, S.; Hobbs, H.H.; Dobbins, R.L. Magnetic resonance spectroscopy to measure hepatic triglyceride content: Prevalence of hepatic steatosis in the general population. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E462–E468. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Wong, V.W.; Nobili, V.; Day, C.P.; Sookoian, S.; Maher, J.J.; Bugianesi, E.; Sirlin, C.B.; Neuschwander-Tetri, B.A.; Rinella, M.E. Nonalcoholic fatty liver disease. Nat. Rev. Dis. Primers 2015, 1, 15080. [Google Scholar] [CrossRef] [PubMed]
- Lazo, M.; Hernaez, R.; Eberhardt, M.S.; Bonekamp, S.; Kamel, I.; Guallar, E.; Koteish, A.; Brancati, F.L.; Clark, J.M. Prevalence of nonalcoholic fatty liver disease in the United States: The Third National Health and Nutrition Examination Survey, 1988–1994. Am. J. Epidemiol. 2013, 178, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [PubMed]
- Sanna, C.; Rosso, C.; Marietti, M.; Bugianesi, E. Non-Alcoholic Fatty Liver Disease and Extra-Hepatic Cancers. Int. J. Mol. Sci. 2016, 17, 717. [Google Scholar] [CrossRef] [PubMed]
- Thoma, C.; Day, C.P.; Trenell, M.I. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: A systematic review. J. Hepatol. 2012, 56, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Kirk, E.; Reeds, D.N.; Finck, B.N.; Mayurranjan, S.M.; Patterson, B.W.; Klein, S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology 2009, 136, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S. Implications of diet on nonalcoholic fatty liver disease. Curr. Opin. Gastroenterol. 2010, 26, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Magkos, F.; Mohammed, B.S.; Pietka, T.; Abumrad, N.A.; Patterson, B.W.; Okunade, A.; Klein, S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc. Natl. Acad. Sci. USA 2009, 106, 15430–15435. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Fabbrini, E.; Mohammed, B.S.; Patterson, B.W.; Klein, S. Increased whole-body adiposity without a concomitant increase in liver fat is not associated with augmented metabolic dysfunction. Obesity 2010, 18, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, Z.; Pan, L.; Xu, Y.; Shao, J.; Huang, Z.; Chen, Z.; Sun, Q.; Liu, C.; Lin, M.; et al. Hepatic fat content is a determinant of metabolic phenotypes and increased carotid intima-media thickness in obese adults. Sci. Rep. 2016, 6, 21894. [Google Scholar] [CrossRef] [PubMed]
- Schübel, R.; Graf, M.E.; Nattenmuller, J.; Nabers, D.; Sookthai, D.; Gruner, L.F.; Johnson, T.; Schlett, C.L.; von Stackelberg, O.; Kirsten, R.; et al. The effects of intermittent calorie restriction on metabolic health: Rationale and study design of the HELENA Trial. Contemp. Clin. Trials 2016, 51, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Jungvogel, A.; Wendt, I.; Schabethal, K.; Leschik-Bonnet, E.; Oberritter, H. Revised: The 10 DGE Rules. ErnahrungsUmschau 2013, 60, M644–M645. [Google Scholar]
- Oberritter, H.; Schabethal, K.; von Ruesten, A.; Boeing, H. The DGE Nutrition Circle—Presentation and Basis of the Food-Related Recommendations from the German Nutrition Society (DGE). ErnahrungsUmschau 2013, 60, 24–29. [Google Scholar] [CrossRef]
- Schubel, R.; Nattenmuller, J.; Sookthai, D.; Nonnenmacher, T.; Graf, M.E.; Riedl, L.; Schlett, C.L.; von Stackelberg, O.; Johnson, T.; Nabers, D.; et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Nickel, M.D.; Kannengiesser, S.A.; Dale, B.M.; Kiefer, B.; Bashir, M.R. Liver fat quantification using a multi-step adaptive fitting approach with multi-echo GRE imaging. Magn. Reson. Med. 2014, 72, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Desai, A.; Hamilton, G.; Wolfson, T.; Gamst, A.; Lam, J.; Clark, L.; Hooker, J.; Chavez, T.; Ang, B.D.; et al. Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology 2015, 274, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Tan, J.; Sun, M.; Hamilton, G.; Bydder, M.; Wolfson, T.; Gamst, A.C.; Middleton, M.; Brunt, E.M.; Loomba, R.; et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology 2013, 267, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Yokoo, T.; Bydder, M.; Hamilton, G.; Middleton, M.S.; Gamst, A.C.; Wolfson, T.; Hassanein, T.; Patton, H.M.; Lavine, J.E.; Schwimmer, J.B.; et al. Nonalcoholic fatty liver disease: Diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T. Radiology 2009, 251, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Wald, D.; Teucher, B.; Dinkel, J.; Kaaks, R.; Delorme, S.; Boeing, H.; Seidensaal, K.; Meinzer, H.P.; Heimann, T. Automatic quantification of subcutaneous and visceral adipose tissue from whole-body magnetic resonance images suitable for large cohort studies. J. Magn. Reson. Imaging 2012, 36, 1421–1434. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H.; Pickhardt, P.J.; Kliewer, M.A.; Hernando, D.; Chen, G.H.; Zagzebski, J.A.; Reeder, S.B. Accuracy of Liver Fat Quantification With Advanced CT, MRI, and Ultrasound Techniques: Prospective Comparison With MR Spectroscopy. AJR Am. J. Roentgenol. 2017, 208, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Hetterich, H.; Bayerl, C.; Peters, A.; Heier, M.; Linkohr, B.; Meisinger, C.; Auweter, S.; Kannengiesser, S.A.; Kramer, H.; Ertl-Wagner, B.; et al. Feasibility of a three-step magnetic resonance imaging approach for the assessment of hepatic steatosis in an asymptomatic study population. Eur. Radiol. 2016, 26, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Neamat-Allah, J.; Wald, D.; Husing, A.; Teucher, B.; Wendt, A.; Delorme, S.; Dinkel, J.; Vigl, M.; Bergmann, M.M.; Feller, S.; et al. Validation of anthropometric indices of adiposity against whole-body magnetic resonance imaging—A study within the German European Prospective Investigation into Cancer and Nutrition (EPIC) cohorts. PLoS ONE 2014, 9, e91586. [Google Scholar] [CrossRef] [PubMed]
- Troyanskaya, O.; Cantor, M.; Sherlock, G.; Brown, P.; Hastie, T.; Tibshirani, R.; Botstein, D.; Altman, R.B. Missing value estimation methods for DNA microarrays. Bioinformatics 2001, 17, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.E.; Li, C.; Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007, 8, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Kantartzis, K.; Machann, J.; Schick, F.; Fritsche, A.; Haring, H.U.; Stefan, N. The impact of liver fat vs visceral fat in determining categories of prediabetes. Diabetologia 2010, 53, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, W.R. Nonobese Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2017, 15, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Haufe, S.; Haas, V.; Utz, W.; Birkenfeld, A.L.; Jeran, S.; Bohnke, J.; Mahler, A.; Luft, F.C.; Schulz-Menger, J.; Boschmann, M.; et al. Long-lasting improvements in liver fat and metabolism despite body weight regain after dietary weight loss. Diabetes Care 2013, 36, 3786–3792. [Google Scholar] [CrossRef] [PubMed]
- Rachakonda, V.; Wills, R.; DeLany, J.P.; Kershaw, E.E.; Behari, J. Differential Impact of Weight Loss on Nonalcoholic Fatty Liver Resolution in a North American Cohort with Obesity. Obesity 2017, 25, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Tiikkainen, M.; Bergholm, R.; Vehkavaara, S.; Rissanen, A.; Hakkinen, A.M.; Tamminen, M.; Teramo, K.; Yki-Jarvinen, H. Effects of identical weight loss on body composition and features of insulin resistance in obese women with high and low liver fat content. Diabetes 2003, 52, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Idilman, I.S.; Aniktar, H.; Idilman, R.; Kabacam, G.; Savas, B.; Elhan, A.; Celik, A.; Bahar, K.; Karcaaltincaba, M. Hepatic steatosis: Quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology 2013, 267, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Yokoo, T.; Serai, S.D.; Pirasteh, A.; Bashir, M.R.; Hamilton, G.; Hernando, D.; Hu, H.H.; Hetterich, H.; Kuhn, J.P.; Kukuk, G.M.; et al. Linearity, Bias, and Precision of Hepatic Proton Density Fat Fraction Measurements by Using MR Imaging: A Meta-Analysis. Radiology 2018, 286, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Severson, T.J.; Besur, S.; Bonkovsky, H.L. Genetic factors that affect nonalcoholic fatty liver disease: A systematic clinical review. World J. Gastroenterol. 2016, 22, 6742–6756. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.A.; Gallego-Duran, R.; Gallego, P.; Grande, L. Genetic and Epigenetic Regulation in Nonalcoholic Fatty Liver Disease (NAFLD). Int. J. Mol. Sci. 2018, 19, 911. [Google Scholar] [CrossRef] [PubMed]
| No NAFLD at Baseline | NAFLD at Baseline | |
|---|---|---|
| n (women/men) | 68 (40/28) | 75 (31/44) |
| Age, years | 50.7 (44.1,56.8) | 49.7 (44.0,57.3) |
| Weight, kg | 89.4 (79.7,95.7) | 99.4 (87.7,110.0) |
| BMI, kg/m2 | 29.4 (27.5,33.0) | 32.7 (29.5,34.6) |
| Waist circumference, cm | 99.8 (90.8,105.8) | 110.0 (101.5,115.0) |
| Fat distribution | ||
| VAT, L | 3.6 (2.6,4.9) | 5.8 (4.4,7.1) |
| SAT, L | 10.6 (8.9,14.0) | 12.9 (10.0,14.8) |
| Liver fat content, % | 3.4 (2.6,4.2) | 9.8 (7.2,13.5) |
| Liver tests | ||
| ALT, μkat/L | 0.32 (0.27,0.4) | 0.5 (0.38,0.6) |
| AST, μkat/L | 0.35 (0.3,0.41) | 0.38 (0.35,0.47) |
| GGT, μkat/L | 0.32 (0.24,0.47) | 0.43 (0.3,0.67) |
| Metabolism | ||
| Glucose, mmol/L | 5.11 (4.8,5.3) | 5.3 (5.0,5.6) |
| Insulin, pmol/L | 60.4 (47.2,84.0) | 88.2 (64.6,145.8) |
| HOMA-IR | 1.9 (1.5,3.0) | 3.1 (2.2,4.8) |
| HbA1c, % | 5.4 (5.2,5.6) | 5.6 (5.3,5.7) |
| LDL, mmol/L | 3.2. (2.6,3.7) | 3.3 (2.9,3.7) |
| HDL, mmol/L | 1.4 (1.2,1.7) | 1.2 (1.0,1.4) |
| Adipokines | ||
| Leptin, μg/L | 18.7 (8.6,35.6) | 16.0 (8.6,29.4) |
| Resistin, μg/L | 5.4 (4.3,6.5) | 5.2 (3.8,6.5) |
| CRP, nmol/L | 24.8 (12.4,45.7) | 28.6 (15.2,54.3) |
| Alcohol intake, g/day a | 2.2 (0.3,4.3) | 1.9 (0.3,5.8) |
| No NAFLD at Baseline (n = 68) | NAFLD at Baseline (n = 75) | p value a | ||||
|---|---|---|---|---|---|---|
| Baseline to week 12 | Baseline to week 50 | Baseline to week 12 | Baseline to week 50 | Baseline to week 12 | Baseline to week 50 | |
| Weight | −5.1 ± 0.5 | −3.5 ± 0.9 | −4.8 ± 0.5 | −3.5 ± 0.7 | 0.80 | 0.84 |
| Waist circumference, cm | −4.8 ± 0.6 | −1.7 ± 0.8 | −4.3 ± 0.6 | −2.3 ± 0.7 | 0.85 | 0.59 |
| Fat distribution | ||||||
| VAT, L | −13.2 ± 2 | −10.8 ± 2.7 | −12.8 ± 1.6 | −12.0 ± 2.4 | 0.08 | 0.23 |
| SAT, L | −9.8 ± 1.4 | −5.8 ± 2.2 | −10.6 ± 1.3 | −7.5 ± 1.9 | 0.36 | 0.65 |
| Liver fat content, % | −6.3 ± 4.0 | −5.0 ± 4.2 | −32.9 ± 9.5 | −23.3 ± 4.4 | <0.01 | <0.01 |
| Liver function | ||||||
| ALT, μkat/L | −9.3 ± 3.6 | 1.6 ± 8.8 | −15.4 ± 4.8 | −11.6 ± 4.3 | <0.01 | 0.02 |
| AST, μkat/L | −4-7 ± 2.7 | −4.4 ± 3.0 | −10.4 ± 3.0 | −13.0 ± 3.0 | 0.03 | 0.02 |
| GGT, μkat/L | −17.2 ± 2.5 | −0.2 ± 7.9 | −20.8 ± 3.6 | −14.7 ± 3.3 | 0.01 | 0.03 |
| Metabolism | ||||||
| Glucose, mmol/L | −4.3 ± 1.2 | −5.3 ± 1.1 | −5.5 ± 0.9 | −4.4 ± 1.0 | 0.45 | 0.44 |
| Insulin, pmol/L | 2.6 ± 5.9 | −12.2 ± 5.6 | −7.9 ± 5.2 | −15.8 ± 4.7 | 0.06 | 0.08 |
| HOMA-IR | −0.5 ± 6.1 | −15.3 ± 6.0 | −11.5 ± 5.5 | −18.0 ± 4.9 | 0.04 | 0.08 |
| HbA1c, % | 0 ± 0.5 | −1.1 ± 0.5 | −0.7 ± 0.4 | −2.3 ± 0.5 | 0.25 | 0.08 |
| LDL, mmol/L | −9.1 ± 1.6 | 2.6 ± 2.3 | −4.5 ± 2.0 | −0.6 ± 2.3 | 0.20 | 0.25 |
| HDL, mmol/L | −9.4 ± 1.6 | −2.7 ± 1.7 | −8.3 ± 1.7 | −0.1 ± 1.7 | 0.35 | 0.29 |
| Adipokines | ||||||
| Leptin, μg/L | −28.8 ± 4.7 | −15.2 ± 7.4 | −25.9 ± 6.7 | −10.7 ± 6.9 | 0.74 | 0.89 |
| Resistin, μg/L | −27.3 ± 9.5 | 4.5 ± 9.0 | −28.6 ± 7.0 | 6.0 ± 5.7 | 0.59 | 0.20 |
| CRP, nmol/L | −0.6 ± 9.8 | 26.9 ± 21.6 | 12.2 ± 16.4 | 18.5 ± 19.9 | 0.58 | 0.52 |
| Dietary intake b | ||||||
| Energy intake, kcal | −20.4 ± 2.8 | NA | −20.9 ± 2.6 | NA | 0.61 | |
| Fat, % | −13.5 ± 2.7 | −11.9 ± 2.5 | 0.88 | |||
| Carbohydrates, % | 9.9 ± 2.5 | 7.2 ± 2.2 | 0.46 | |||
| Protein, % | 15.5 ± 3.3 | 20.2 ± 3.4 | 0.34 | |||
| Fibers, g/day | 24.8 ± 5.8 | 23.4 ± 5.7 | 0.63 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schübel, R.; Nonnenmacher, T.; Sookthai, D.; Gonzalez Maldonado, S.; Sowah, S.A.; von Stackelberg, O.; Schlett, C.L.; Grafetstätter, M.; Nabers, D.; Johnson, T.; et al. Similar Weight Loss Induces Greater Improvements in Insulin Sensitivity and Liver Function among Individuals with NAFLD Compared to Individuals without NAFLD. Nutrients 2019, 11, 544. https://doi.org/10.3390/nu11030544
Schübel R, Nonnenmacher T, Sookthai D, Gonzalez Maldonado S, Sowah SA, von Stackelberg O, Schlett CL, Grafetstätter M, Nabers D, Johnson T, et al. Similar Weight Loss Induces Greater Improvements in Insulin Sensitivity and Liver Function among Individuals with NAFLD Compared to Individuals without NAFLD. Nutrients. 2019; 11(3):544. https://doi.org/10.3390/nu11030544
Chicago/Turabian StyleSchübel, Ruth, Tobias Nonnenmacher, Disorn Sookthai, Sandra Gonzalez Maldonado, Solomon A. Sowah, Oyunbileg von Stackelberg, Christopher L. Schlett, Mirja Grafetstätter, Diana Nabers, Theron Johnson, and et al. 2019. "Similar Weight Loss Induces Greater Improvements in Insulin Sensitivity and Liver Function among Individuals with NAFLD Compared to Individuals without NAFLD" Nutrients 11, no. 3: 544. https://doi.org/10.3390/nu11030544
APA StyleSchübel, R., Nonnenmacher, T., Sookthai, D., Gonzalez Maldonado, S., Sowah, S. A., von Stackelberg, O., Schlett, C. L., Grafetstätter, M., Nabers, D., Johnson, T., Kirsten, R., Ulrich, C. M., Kaaks, R., Kauczor, H.-U., Kühn, T., & Nattenmüller, J. (2019). Similar Weight Loss Induces Greater Improvements in Insulin Sensitivity and Liver Function among Individuals with NAFLD Compared to Individuals without NAFLD. Nutrients, 11(3), 544. https://doi.org/10.3390/nu11030544






