How Does Feeding Development and Progression onto Solid Foods in PKU Compare with Non-PKU Children During Weaning?
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Feeding Development Diary
2.4. Weaning Protein Substitute and Weaning Diet
2.5. Ethical Approval
2.6. Data Analysis
3. Results
3.1. Subjects
3.2. First Weaning Foods and Textures
3.3. Fluid Intake
3.4. Independent Self-Feeding Skills
3.5. Feeding Environment
3.6. Frequency of Meals and Snacks
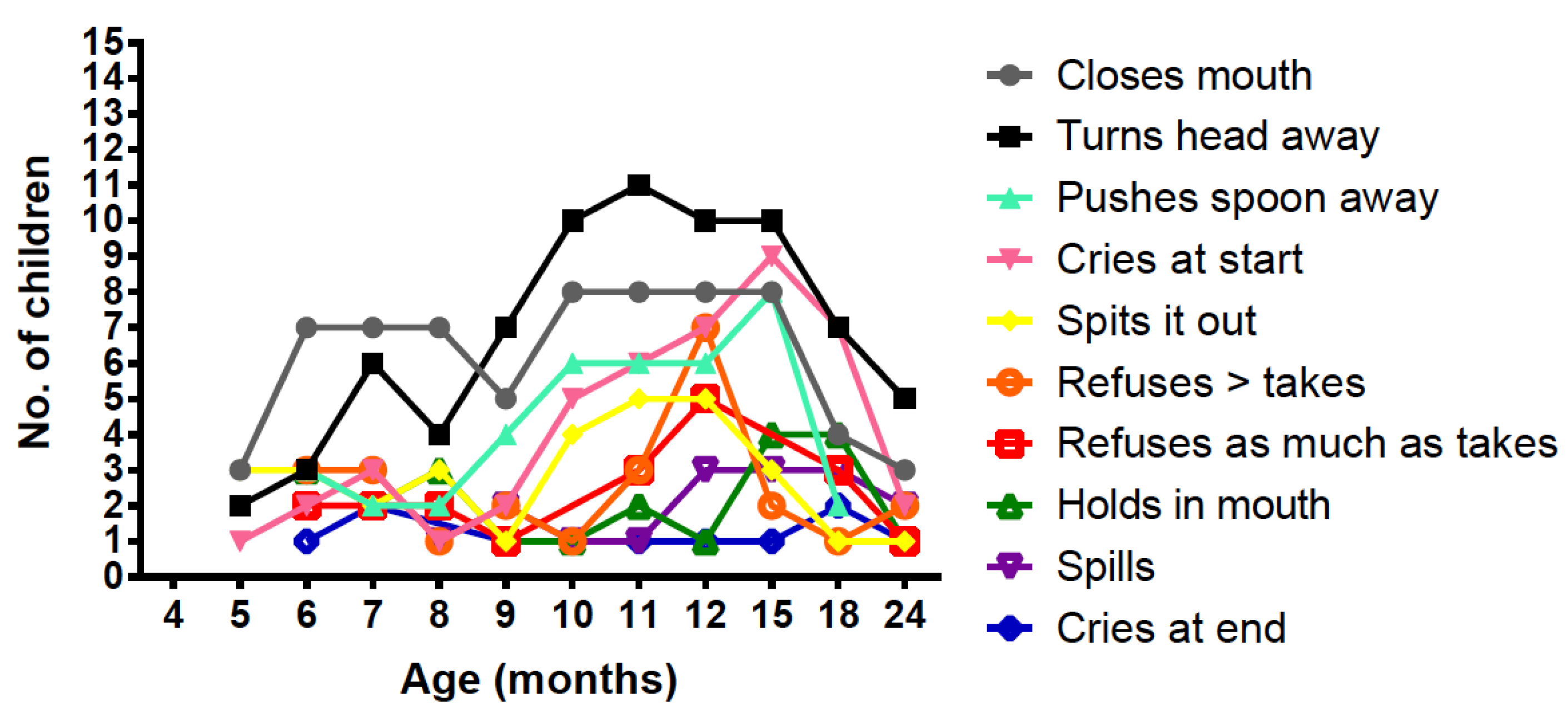
3.7. Negative Behaviour with Protein Substitute (PKU Group)
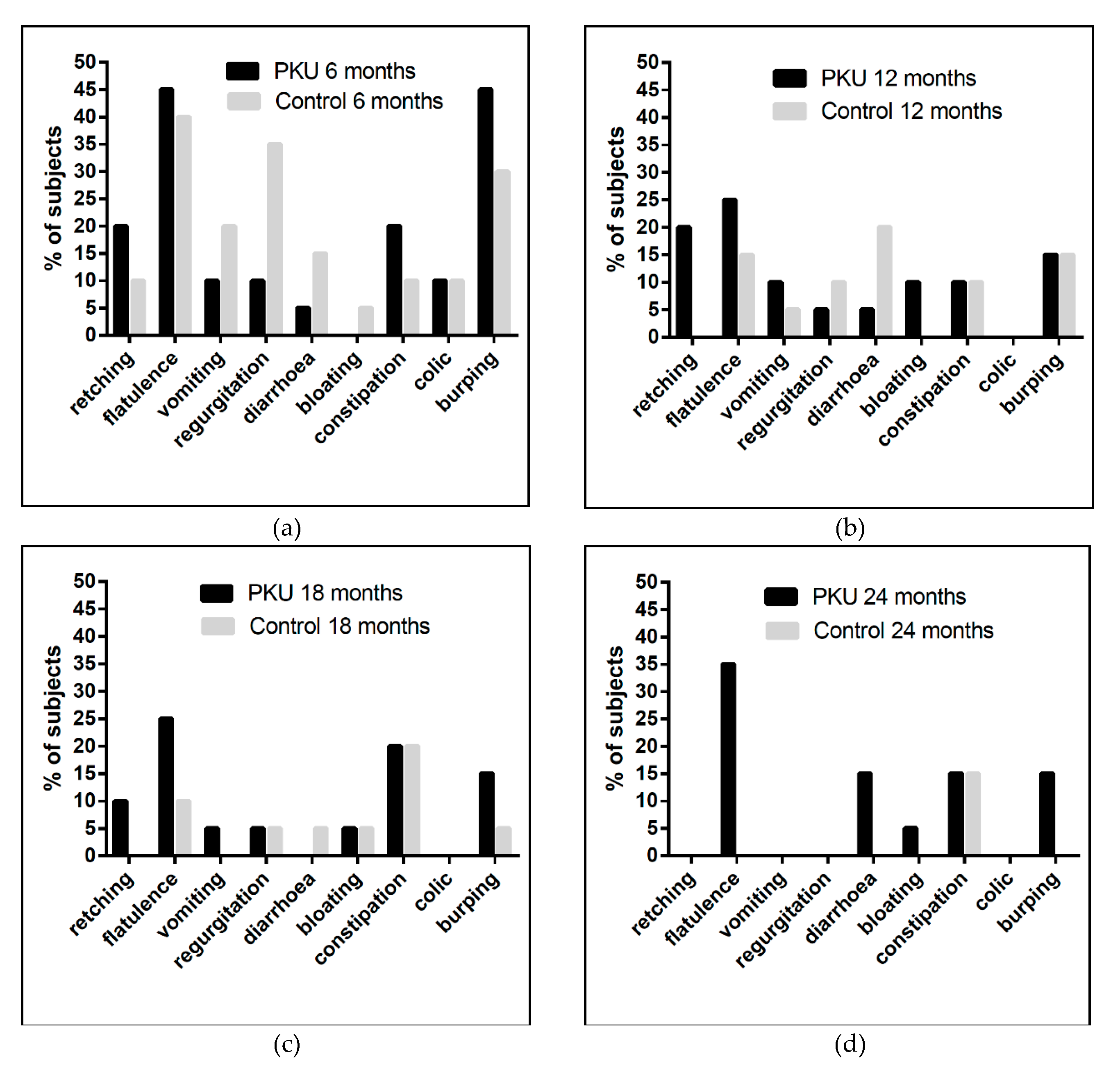
3.8. Gastrointestinal Symptoms
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Spronsen, F.J.; Van Wegberg, A.M.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. Key European guidelines for the diagnosis and management of patients with phenylketonuria. Lancet 2017, 5, 743–756. [Google Scholar] [CrossRef]
- Smith, I.; Cockburn, F.; Barwell, B.; Brenton, D.; Chapple, J.; Clark, B.; Curzon, G.; Davidson, D.; Heeley, A.; Laing, S.; et al. Recommendations on the dietary management of phenylketonuria. Report of Medical Research Council Working Party on Phenylketonuria. Arch. Dis. Child. 1993, 68, 426–427. [Google Scholar]
- British Nutrition Founation Introducing Solid Foods to Your Baby. Available online: https://www.nutrition.org.uk/healthyliving/nutrition4baby/complementaryfeeding.html (accessed on 20 February 2019).
- Macdonald, A.; Daly, A.; Davies, P.; Asplin, D.; Hall, S.K.; Rylance, G.; Chakrapani, A. Protein substitutes for PKU: What’s new? J. Inherit. Metab. Dis. 2004, 27, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.; Daly, A.; Macdonald, J.; Pinto, A.; Macdonald, A. Fifteen years of using a second stage protein substitute for weaning in phenylketonuria: A retrospective study. J. Hum. Nutr. Diet. 2017, 31, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Decsi, T.; Fewtrell, M.; Goulet, O.; Kolaček, S.; Koletzko, B.; Michaelsen, K.F.; Moreno, L.; Puntis, J.; Rigo, J.; et al. Complementary Feeding: A Commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Vail, B.; Prentice, P.; Dunger, D.B.; Hughes, I.A.; Acerini, C.L.; Ong, K.K. Age at Weaning and Infant Growth: Primary Analysis and Systematic Review. J. Pediatr. 2015, 167, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, R.D.; Allaire, J.H. The Development of Normal Feeding and Swallowing. Pediatr. Clin. N. Am. 1991, 38, 1439–1453. [Google Scholar] [CrossRef]
- Alles, M.S.; Eussen, S.R.; Van Der Beek, E.M. Nutritional Challenges and Opportunities during the Weaning Period and in Young Childhood. Ann. Nutr. Metab. 2014, 64, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.; Daly, A.; Wildgoose, J.; Cochrane, B.; Chahal, S.; Ashmore, C.; Loveridge, N.; MacDonald, A. Growth, protein, and energy intake in children with PKU taking a weaning protein substitute in the first 2 years of life: A case-control study. Nutrients 2019, in press. [Google Scholar]
- Northstone, K.; Nethersole, F.; Emmett, P. The effect of age of introduction to lumpy solids on foods eaten and reported feeding difficulties at 6 and 15 months. J. Hum. Nutr. Diet. 2001, 14, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, A.; Harris, G.; Rylance, G.; Asplin, D.; Booth, I.W. Abnormal feeding behaviours in phenylketonuria. J. Hum. Nutr. Diet. 1997, 10, 163–170. [Google Scholar] [CrossRef]
- Pinto, A.; Adams, S.; Ahring, K.; Allen, H.; Almeida, M.; Garcia-Arenas, D.; Arslan, N.; Assoun, M.; Altınok, Y.A.; Barrio-Carreras, D.; et al. Early feeding practices in infants with phenylketonuria across Europe. Mol. Genet. Metab. Rep. 2018, 16, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, A.; Rylance, G.; Asplin, D.; Hall, K.; Harris, G.; Booth, I. Feeding problems in young PKU children. Acta. Paediatr. 1994, 83, 73–74. [Google Scholar] [CrossRef]
| PKU | Control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject No. | Sex | Age at Weaning (months) | PKU Siblings | Mother’s marital Status | Age Stopped Breast Feeding (mths) * | Sex | Age at Weaning (months) | PKU Siblings | Mother’s marital Status | Age Stopped Breast Feeding (mths) * |
| 1 | M | 4.1 | Married | M | 4.6 | 3 | Married | |||
| 2 | M | 5.0 | Married | F | 5.0 | Married | 10 | |||
| 3 | F | 3.3 | Single | M | 5.5 | Married | 9 | |||
| 4 | M | 3.4 | 1 | Married | F | 4.4 | Married | 7 | ||
| 5 | F | 5.0 | Partner | M | 5.7 | Married | 8 | |||
| 6 | F | 3.7 | Partner | M | 5.8 | Married | ||||
| 7 | M | 3.9 | Married | M | 6.0 | Single | ||||
| 8 | F | 5.0 | Married | M | 3.8 | Partner | ||||
| 9 | F | 4.5 | Single | M | 5.7 | Partner | ||||
| 10 | M | 2.8 | Single | F | 3.7 | Single | ||||
| 11 | M | 3.7 | Married | 5 | F | 4.5 | Single | |||
| 12 | F | 3.7 | Married | M | 4.0 | Partner | 12 | |||
| 13 | M | 3.3 | Married | F | 5.0 | Partner | ||||
| 14 | M | 4.7 | Married | 8 | F | 4.5 | Partner | 18 | ||
| 15 | M | 4.4 | 2 | Married | F | 5.0 | Married | |||
| 16 | M | 4.4 | 2 | Married | M | 4.5 | Married | |||
| 17 | M | 4.5 | Married | M | 5.0 | Partner | ||||
| 18 | M | 5.0 | Partner | M | 4.4 | Married | 7 | |||
| 19 | M | 4.0 | Married | F | 5.0 | Married | 10 | |||
| 20 | M | 4.0 | Married | 8 | M | 5.0 | Married | 18 | ||
| PKU % | Control % | |
|---|---|---|
| 1st Weaning Foods | ||
| Baby rice/porridge | 0 | 75 |
| Puree fruit | 85 | 55 |
| Puree vegetables | 65 | 30 |
| Rusks- regular | 0 | 25 |
| Low protein rusks | 45 | 0 |
| Mashed potato | 5 | 20 |
| Stage 1 baby food jar | 0 | 20 |
| Weaning Textures | ||
| Thin puree | 70 | 70 |
| Thick puree | 60 | 45 |
| Mashed | 15 | 25 |
| Finger food | 5 | 15 |
| Mean Age in Months (Range) | |||
|---|---|---|---|
| PKU | Control | p value * | |
| Thin puree | 4.5 (3–6) | 5.0 (3–8) | 0.03 |
| Thick puree | 5.8 (3–7) | 5.4 (3–8) | 0.29 |
| Weaning foods | 5.2 (4–8) | 5.3 (4–7) | 0.82 |
| Normal family foods | 7.6 (5–11) | 7.1 (4–10) | 0.46 |
| Mean Volume ml (Range) | |||||||
|---|---|---|---|---|---|---|---|
| Age (m) | PKU Phe-Free Infant Formula | n | PKU Standard Infant Formula | n | PKU Total Volume | Control Total Volume | n |
| 4 | 493 (160–870) | 11 * | 304 (170–540) | 11 * | 796 (400–1170) | 981 (750–1260) | 9 * |
| 5 | 510 (190–900) | 20 | 260 (90–480) | 20 | 744 (435–1200) | 776 (550–1110) | 18 * |
| 6 | 509 (210–720) | 20 | 181 (60–340) | 20 | 623 (450–750) | 663 (450–840) | 20 |
| 7 | 567 (300–840) | 19 | 126 (600–380) | 11 | 641 (360–930) | 653 (240–1000) | 20 |
| 8 | 511 (200–810) | 19 | 90 (90–90) | 2 | 521 (200–810) | 614 (270–900) | 20 |
| 9 | 498 (250–840) | 19 | 90 | 1 | 503 (250–930) | 559 (285–1010) | 20 |
| 10 | 397 (240–600) | 19 | 90 | 1 | 403 (240–600) | 485 (120–890) | 20 |
| 11 | 387 (110–640) | 18 | 90 | 1 | 393 (110–640) | 501 (180–900) | 17 |
| 12 | 347 (80–840) | 18 | 0 | 0 | 347 (80–840) | 502 (240–1160) | 11 |
| 15 | 448 (210–840) | 13 | 0 | 0 | 448 (210–840) | 488 (270–600) | 6 |
| 18 | 311 (120–840) | 10 | 0 | 0 | 311 (120–840) | 383 (300–450) | 4 |
| 24 | 420 (240–750) | 4 | 0 | 0 | 420 (240–750) | 540 | 1 |
| PKU Control | 4 m n = 13 n = 11 | 5 m n = 19 n = 20 | 6 m n = 20 n = 20 | 7 m n = 20 n = 20 | 8 m n = 20 n = 20 | 9 m n = 20 n = 20 | 10 m n = 20 n = 20 | 11 m n = 20 n = 20 | 12 m n = 20 n = 20 | 15 m n = 20 n = 20 | 18 m n = 20 n = 20 | 24 m n = 20 n = 20 | p value # | |
| Spoon fed by parent | PKU Control | 100 89 | 100 100 | 100 100 | 100 100 | 100 100 | 100 100 | 100 95 | 100 95 | 100 85 | 95 80 | 90 55 | 85 25 | 0.008 |
| Spoon feeds self | PKU Control | 8 0 | 11 6 | 10 5 | 15 15 | 5 10 | 20 5 | 10 30 | 30 25 | 50 40 | 65 55 | 80 85 | 100 90 | 0.29 |
| Fork feeds self | PKU Control | 0 0 | 0 0 | 0 0 | 0 0 | 0 0 | 0 5 | 0 5 | 0 5 | 15 5 | 10 40 | 40 60 | 80 85 | 0.25 |
| Finger fed by parent | PKU Control | 0 44 | 21 39 | 40 50 | 65 40 | 55 50 | 60 70 | 70 75 | 70 85 | 65 70 | 50 65 | 60 25 | 35 5 | 0.78 |
| Finger feeds self | PKU Control | 5 15 | 15 40 | 45 80 | 90 85 | 100 90 | 100 95 | 100 95 | 95 100 | 100 100 | 100 100 | 100 100 | 100 90 | 0.76 |
| Drinks from bottle | PKU Control | 100 67 * | 95 61 | 100 75 | 95 70 | 100 100 | 100 100 | 100 85 | 90 90 | 85 95 | 90 80 | 100 65 | 85 30 | 0.006 |
| Drinks from cup | PKU Control | 8 0 | 16 17 | 30 35 | 40 55 | 45 70 | 55 75 | 60 85 | 65 85 | 65 90 | 80 95 | 85 95 | 85 90 | 0.004 |
| Drinks from straw | PKU Control | 0 11 | 0 6 | 0 5 | 0 5 | 0 10 | 10 20 | 10 25 | 15 25 | 30 30 | 30 40 | 45 50 | 70 70 | 0.002 |
| Mean Age in Months (Range) | |||
|---|---|---|---|
| PKU | Control | p value * | |
| Self finger-feeding | 6.5 (4–8) | 5.7 (4–9) | 0.01 |
| Self spoon-feeding | 12.8 (5-24) | 12.6 (5–18) 1 still not | 0.99 |
| Self fork-feeding | 19.2 (12–24) 3 still not | 18.1 (12–24) 2 still not | 0.41 |
| Drinking from a cup | 8.8 (4–15) 2 still not | 7.8 (5–15) | 0.43 |
| Drinking from a straw | 14.7 (9–24) 6 still not | 14.9 (4–24) 4 still not | 0.94 |
| 4 m | 5 m | 6 m | 7 m | 8 m | 9 m | 10 m | 11 m | 12 m | 15 m | 18 m | 24 m | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Highchair | PKU Control | 38 11 | 53 33 | 70 60 | 80 90 | 85 85 | 85 90 | 80 95 | 90 100 | 100 100 | 80 80 | 70 75 | 50 45 |
| Lap | PKU Control | 31 56 | 26 28 | 30 25 | 15 20 | 10 10 | 10 20 | 15 20 | 15 20 | 5 0 | 10 5 | 15 0 | 5 0 |
| Bouncer * | PKU Control | 38 67 | 26 67 | 15 40 | 0 20 | 5 5 | 5 5 | 5 5 | 0 5 | 5 0 | 5 0 | 5 0 | 0 0 |
| Table/Booster ** | PKU Control | 0 0 | 0 0 | 0 0 | 0 0 | 5 10 | 0 5 | 0 0 | 0 0 | 0 0 | 5 25 | 15 40 | 30 85 |
| Other (sofa, cot, pushchair, floor) | PKU Control | 8 11 | 21 6 | 0 10 | 10 15 | 10 5 | 15 10 | 15 10 | 5 0 | 5 0 | 5 10 | 10 10 | 15 5 |
| Total Number of Meals/Snacks | ||||||||||||
| 4 m | 5 m | 6 m | 7 m | 8 m | 9 m | 10 m | 11 m | 12 m | 15 m | 18 m | 24 m | |
| PKU | 6 | 6.5 | 6 | 6 | 6 | 6 | 6 | 6 | 5 | 6 | 5 | 6 |
| Control | 9 | 8 | 7 | 8 | 7.5 | 7 | 7 | 7 | 7 | 7 | 6 | 6 |
| p value * | 0.01 | 0.07 | 0.37 | 0.01 | 0.02 | 0.07 | 0.02 | 0.06 | 0.04 | 0.03 | 0.12 | 0.80 |
| Number of Solid Meals/Snacks | ||||||||||||
| PKU | 2 | 3 | 3 | 3.5 | 3 | 3.5 | 4 | 4 | 4 | 4 | 4 | 4 |
| Control | 1 | 2 | 3 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| p value * | 0.05 | 0.27 | 0.57 | 0.38 | 0.30 | 0.01 | 0.02 | 0.04 | 0.09 | 0.02 | 0.03 | 0.16 |
| Number Fluid Breastmilk/Formula Feeds | ||||||||||||
| PKU | 6 | 6 | 5 | 5 | 4 | 4 | 3 | 3 | 2 | 2 | 1 | 0 |
| Control | 7.5 | 5 | 4 | 4 | 4 | 3 | 2.5 | 3 | 2 | 0 | 0 | 0 |
| p value * | 0.005 | 0.22 | 0.04 | 0.33 | 0.80 | 0.12 | 0.05 | 0.76 | 0.12 | 0.06 | 0.04 | 0.25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, S.; Daly, A.; Wildgoose, J.; Cochrane, B.; Chahal, S.; Ashmore, C.; Loveridge, N.; MacDonald, A. How Does Feeding Development and Progression onto Solid Foods in PKU Compare with Non-PKU Children During Weaning? Nutrients 2019, 11, 529. https://doi.org/10.3390/nu11030529
Evans S, Daly A, Wildgoose J, Cochrane B, Chahal S, Ashmore C, Loveridge N, MacDonald A. How Does Feeding Development and Progression onto Solid Foods in PKU Compare with Non-PKU Children During Weaning? Nutrients. 2019; 11(3):529. https://doi.org/10.3390/nu11030529
Chicago/Turabian StyleEvans, Sharon, Anne Daly, Jo Wildgoose, Barbara Cochrane, Satnam Chahal, Catherine Ashmore, Nik Loveridge, and Anita MacDonald. 2019. "How Does Feeding Development and Progression onto Solid Foods in PKU Compare with Non-PKU Children During Weaning?" Nutrients 11, no. 3: 529. https://doi.org/10.3390/nu11030529
APA StyleEvans, S., Daly, A., Wildgoose, J., Cochrane, B., Chahal, S., Ashmore, C., Loveridge, N., & MacDonald, A. (2019). How Does Feeding Development and Progression onto Solid Foods in PKU Compare with Non-PKU Children During Weaning? Nutrients, 11(3), 529. https://doi.org/10.3390/nu11030529






