Inhibition of Tumor Growth by Dietary Indole-3-Carbinol in a Prostate Cancer Xenograft Model May Be Associated with Disrupted Gut Microbial Interactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diet
2.2. Tumor Xenograft Model
2.3. Fecal DNA Extraction and Analysis
2.4. Cecal DNA Extraction, Amplicon Library Construction and Sequencing
2.5. Sequence-Based Microbiome Analysis
2.6. Network Construction and Analysis
3. Results
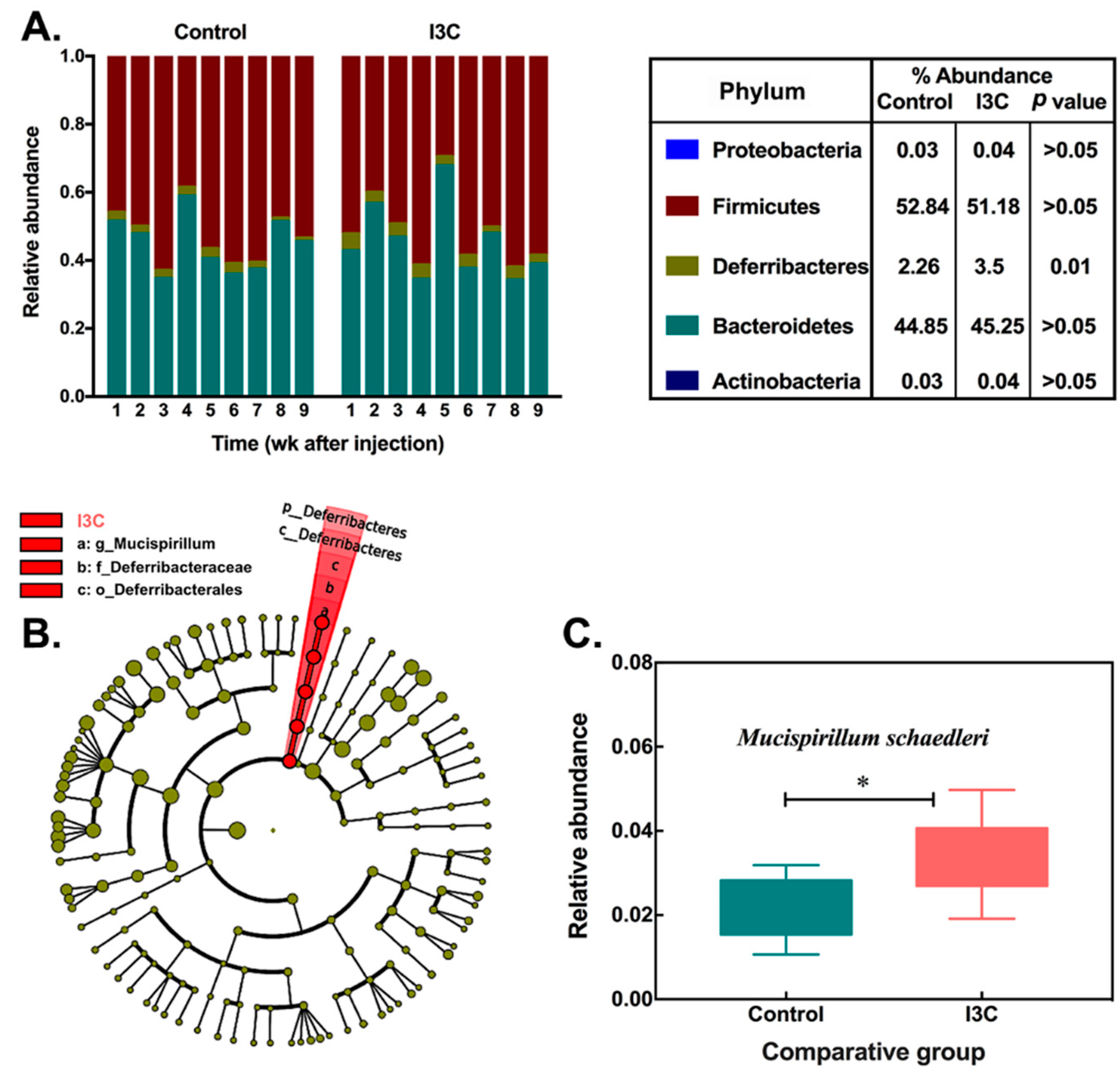
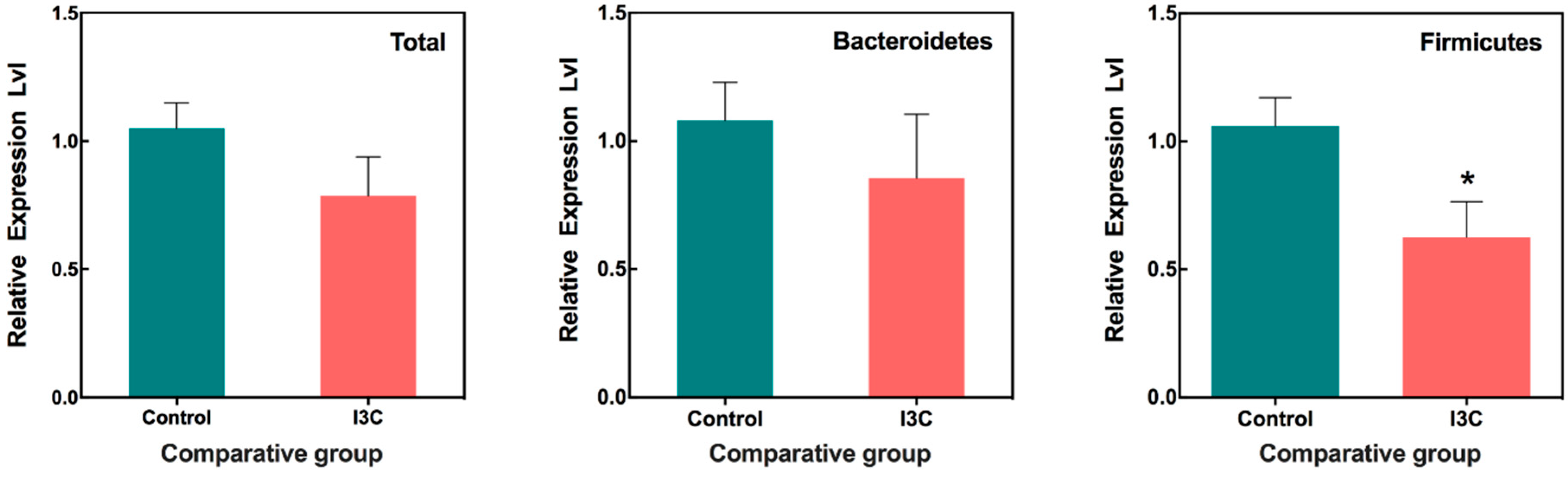
3.1. The Effect of I3C on the Cecal Microbiota in Mice
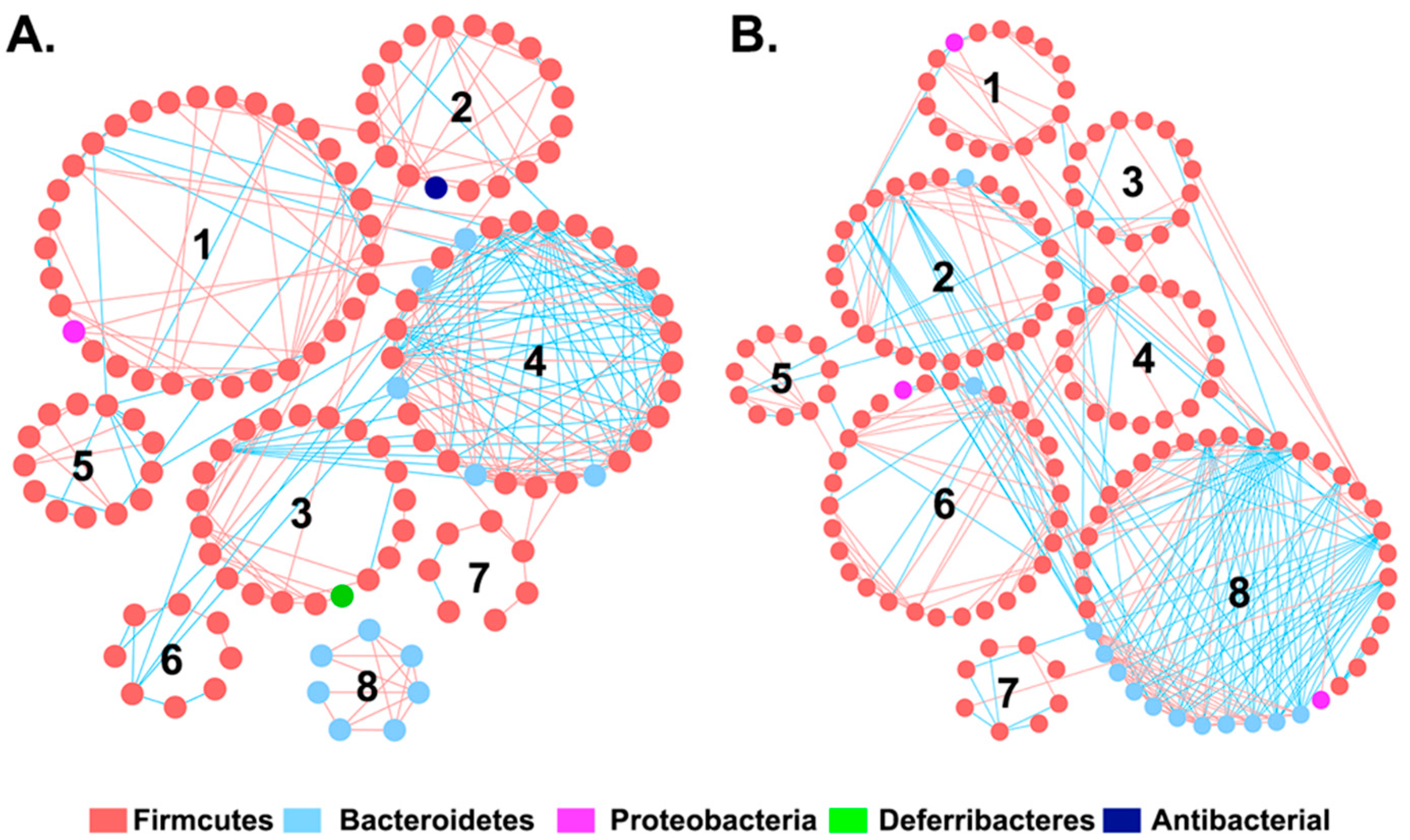
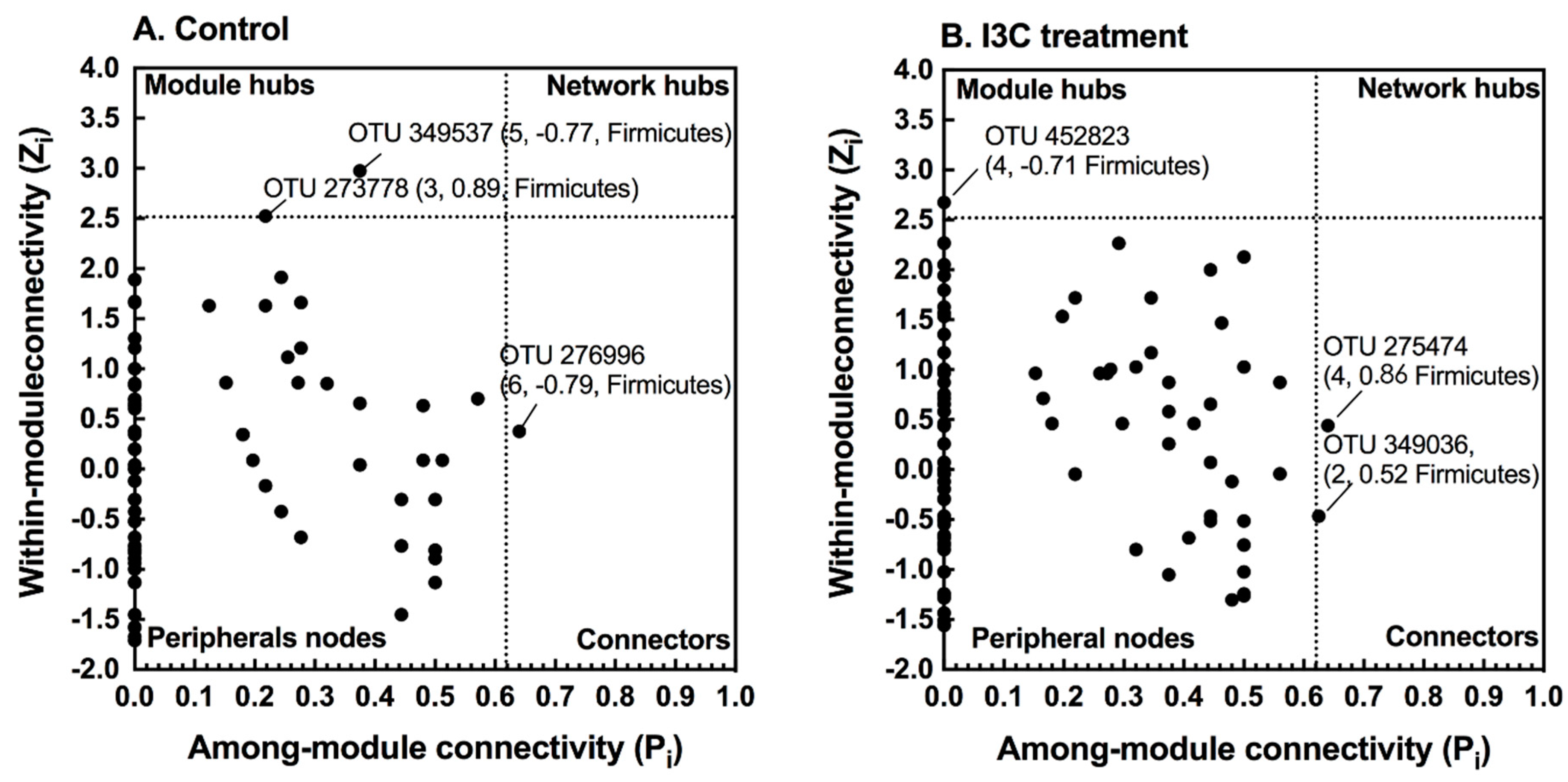
3.2. The Effect of I3C on Microbial Co-Occurrence Network in Mice
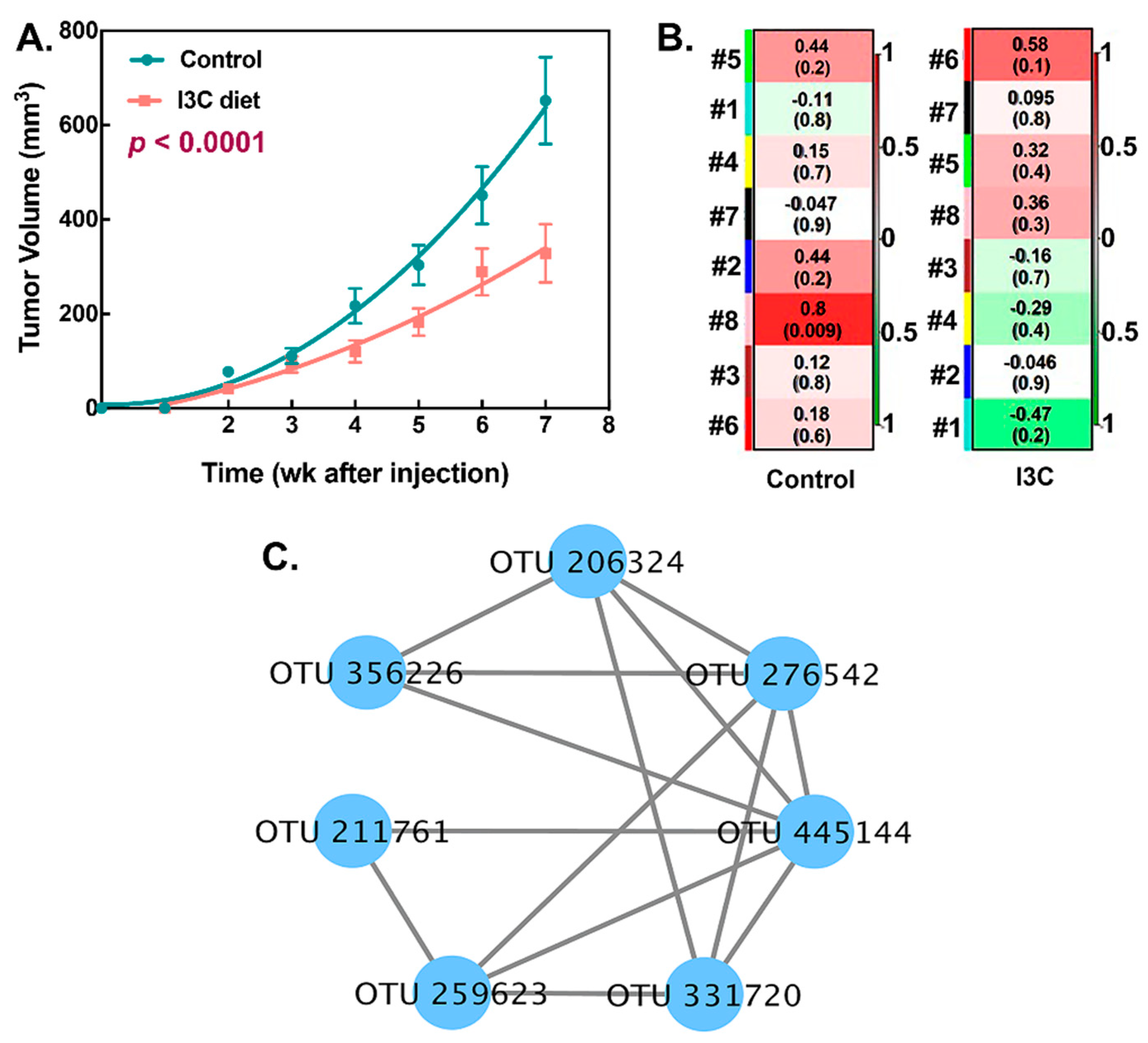
3.3. Association between I3C-Altered Microbial Interactions and Tumor Growth
3.4. The Effect of I3C on the Fecal Microbiota in Mice
4. Discussion
5. Conclusions
Ethical Approval
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018. [Google Scholar] [CrossRef] [PubMed]
- Grönberg, H. Prostate cancer epidemiology. Lancet 2003, 361, 859–864. [Google Scholar] [CrossRef]
- Hayes, R.B.; Liff, J.M.; Pottern, L.M.; Greenberg, R.S.; Schoenberg, J.B.; Schwartz, A.G.; Swanson, G.M.; Silverman, D.T.; Brown, L.M.; Hoover, R.N. Prostate cancer risk in US blacks and whites with a family history of cancer. Int. J. Cancer 1995, 60, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.B.; Ziegler, R.G.; Gridley, G.; Swanson, C.; Greenberg, R.S.; Swanson, G.M.; Schoenberg, J.B.; Silverman, D.T.; Brown, L.M.; Pottern, L.M. Dietary factors and risks for prostate cancer among blacks and whites in the United States. Cancer Epidemiol. Prev. Biomarkers 1999, 8, 25–34. [Google Scholar]
- Syed, D.N.; Khan, N.; Afaq, F.; Mukhtar, H. Chemoprevention of prostate cancer through dietary agents: Progress and promise. Cancer Epidemiol. Prev. Biomarkers 2007, 16, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.H.; Kristal, A.R.; Stanford, J.L. Fruit and vegetable intakes and prostate cancer risk. J. Natl. Cancer Inst. 2000, 92, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, T.A.; Broadbent, H.S. The chemistry and pharmacology of indole-3-carbinol (indole-3-methanol) and 3-(methoxymethyl) indole. [Part I]. Curr. Med. Chem. 1998, 5, 469–491. [Google Scholar] [PubMed]
- Sarkar, F.H.; Li, Y. Indole-3-carbinol and prostate cancer. J. Nutr. 2004, 134, 3493S–3498S. [Google Scholar] [CrossRef]
- Chinni, S.R.; Li, Y.; Upadhyay, S.; Koppolu, P.K.; Sarkar, F.H. Indole-3-carbinol (I3C) induced cell growth inhibition, G1 cell cycle arrest and apoptosis in prostate cancer cells. Oncogene 2001, 20, 2927. [Google Scholar] [CrossRef]
- Kim, E.-K.; Kim, Y.S.; Milner, J.A.; Wang, T.T.Y. Indole-3-carbinol and 3′,3′-diindolylmethane modulate androgen’s effect on CC chemokine ligand 2 and monocyte attraction to prostate cancer cells. Cancer Prev. Res. 2013, 6, 519–529. [Google Scholar] [CrossRef]
- Wang, T.T.Y.; Schoene, N.W.; Milner, J.A.; Kim, Y.S. Broccoli-derived phytochemicals indole-3-carbinol and 3, 3′-diindolylmethane exerts concentration-dependent pleiotropic effects on prostate cancer cells: Comparison with other cancer preventive phytochemicals. Mol. Carcinog. 2012, 51, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.-R.; Tsai, C.-H.; Kulp, S.K.; Chen, C.-S. Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Lett. 2008, 262, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2017, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.L.; Clarke, T.B. The regulation of host defences to infection by the microbiota. Immunology 2017, 150, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M. The gut microbiota and host health: A new clinical frontier. Gut 2015. [Google Scholar] [CrossRef]
- Golombos, D.M.; Ayangbesan, A.; O’Malley, P.; Lewicki, P.; Barlow, L.; Barbieri, C.E.; Chan, C.; DuLong, C.; Abu-Ali, G.; Huttenhower, C. The role of gut microbiome in the pathogenesis of prostate cancer: A prospective, pilot study. Urology 2018, 111, 122–128. [Google Scholar] [CrossRef]
- Frugé, A.D.; Ptacek, T.; Tsuruta, Y.; Morrow, C.D.; Azrad, M.; Desmond, R.A.; Hunter, G.R.; Rais-Bahrami, S.; Demark-Wahnefried, W. Dietary changes impact the gut microbe composition in overweight and obese men with prostate cancer undergoing radical prostatectomy. J. Acad. Nutr. Diet. 2016, 118, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Paturi, G.; Mandimika, T.; Butts, C.A.; Zhu, S.; Roy, N.C.; McNabb, W.C.; Ansell, J. Influence of dietary blueberry and broccoli on cecal microbiota activity and colon morphology in mdr1a−/− mice, a model of inflammatory bowel diseases. Nutrition 2012, 28, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Hudson, T.S.; Perkins, S.N.; Hursting, S.D.; Young, H.A.; Kim, Y.S.; Wang, T.-C.; Wang, T.T.Y. Inhibition of androgen-responsive LNCaP prostate cancer cell tumor xenograft growth by dietary phenethyl isothiocyanate correlates with decreased angiogenesis and inhibition of cell attachment. Int. J. Oncol. 2012, 40, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, H.K.; Martin, G.R. Matrigel: Basement membrane matrix with biological activity. In Proceedings of the Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2005; Volume 15, pp. 378–386. [Google Scholar]
- Mirsepasi, H.; Persson, S.; Struve, C.; Andersen, L.O.B.; Petersen, A.M.; Krogfelt, K.A. Microbial diversity in fecal samples depends on DNA extraction method: EasyMag DNA extraction compared to QIAamp DNA stool mini kit extraction. BMC Res. Notes 2014, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J.A.; Reimer, R.A. Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR: LA-cp rats. Br. J. Nutr. 2012, 107, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Bose, S.; Kim, G.-C.; Hong, S.-U.; Kim, J.-H.; Kim, J.; Kim, H. Flos Lonicera ameliorates obesity and associated endotoxemia in rats through modulation of gut permeability and intestinal microbiota. PLoS ONE 2014, 9, e86117. [Google Scholar] [CrossRef]
- Li, R.W.; Wu, S.; Li, W.; Navarro, K.; Couch, R.D.; Hill, D.; Urban, J.F. Alterations in the porcine colon microbiota induced by the gastrointestinal nematode Trichuris suis. Infect. Immun. 2012. [Google Scholar] [CrossRef]
- Li, R.W.; Wu, S.; Li, W.; Huang, Y.; Gasbarre, L.C. Metagenome plasticity of the bovine abomasal microbiota in immune animals in response to Ostertagia ostertagi infection. PLoS ONE 2011, 6, e24417. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, Y.; Luo, F.; He, Z.; Yang, Y. Phylogenetic molecular ecological network of soil microbial communities in response to elevated CO2. MBio 2011, 2, e00122-11. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, Y.-H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analyses. BMC Bioinformatics 2012, 13, 113. [Google Scholar] [CrossRef]
- Olesen, J.M.; Stefanescu, C.; Traveset, A. Strong, long-term temporal dynamics of an ecological network. PLoS ONE 2011, 6, e26455. [Google Scholar] [CrossRef] [PubMed]
- Wooley, J.C.; Godzik, A.; Friedberg, I. A primer on metagenomics. PLoS Comput. Biol. 2010, 6, e1000667. [Google Scholar] [CrossRef] [PubMed]
- Loy, A.; Pfann, C.; Steinberger, M.; Hanson, B.; Herp, S.; Brugiroux, S.; Neto, J.C.G.; Boekschoten, M.V.; Schwab, C.; Urich, T. Lifestyle and horizontal gene transfer-mediated evolution of Mucispirillum schaedleri, a core member of the murine gut microbiota. Msystems 2017, 2, e00171-16. [Google Scholar] [CrossRef] [PubMed]
- Serino, M.; Luche, E.; Gres, S.; Baylac, A.; Bergé, M.; Cenac, C.; Waget, A.; Klopp, P.; Iacovoni, J.; Klopp, C. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut 2011. [Google Scholar] [CrossRef] [PubMed]
- Palm, N.W.; de Zoete, M.R.; Cullen, T.W.; Barry, N.A.; Stefanowski, J.; Hao, L.; Degnan, P.H.; Hu, J.; Peter, I.; Zhang, W. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 2014, 158, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Bunker, J.J.; Flynn, T.M.; Koval, J.C.; Shaw, D.G.; Meisel, M.; McDonald, B.D.; Ishizuka, I.E.; Dent, A.L.; Wilson, P.C.; Jabri, B. Innate and adaptive humoral responses coat distinct commensal bacteria with immunoglobulin A. Immunity 2015, 43, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Galluzzi, L.; Viaud, S.; Vétizou, M.; Daillère, R.; Merad, M.; Kroemer, G. Cancer and the gut microbiota: An unexpected link. Sci. Transl. Med. 2015, 7, ps1–ps271. [Google Scholar] [CrossRef] [PubMed]
- Rooks, M.G.; Veiga, P.; Wardwell-Scott, L.H.; Tickle, T.; Segata, N.; Michaud, M.; Gallini, C.A.; Beal, C.; van Hylckama-Vlieg, J.E.T.; Ballal, S.A. Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission. ISME J. 2014, 8, 1403. [Google Scholar] [CrossRef]
| Bacteria | Direction | Sequence (5′–3′) |
|---|---|---|
| Total bacteria | Forward | ACTCCTACGGGAGGCAG |
| Reverse | GTATTACCGCGGCTGCTG | |
| Bifidobacteria | Forward | TCGCGTCYGGTGTGAAAG |
| Reverse | CCACATCCAGCRTCCAC | |
| Lactobacillus | Forward | GAGGCAGCAGTAGGGAATCTTC |
| Reverse | GGCCAGTTACTACCTCTATCCTTCTTC | |
| Prevotella | Forward | TCCTACGGGAGGCAGCAGT |
| Reverse | CAATCGGAGTTCTTCGTG | |
| Enterobacteriaceae | Forward | CATTGACGTTACCCGCAGAAGAAGC |
| Reverse | CTCTACGAGACTCAAGCTTGC | |
| Ruminococcus | Forward | GGCGGCCTACTGGGCTTT |
| Reverse | CCAGGTGGATAACTTATTGTGTTAA | |
| Bacteroidetes | Forward | GGARCATGTGGTTTAATTCGATGAT |
| Reverse | AGCTGACGACAACCATGCAG | |
| Firmicutes | Forward | GGAGYATGTGGTTTAATTCGAAGCA |
| Reverse | AGCTGACGACAACCATGCAC |
| Greengenes ID | Control | I3C | LDA log10 Score | Annotation |
|---|---|---|---|---|
| 198238 | 0.29 ± 0.28 | 0.92 ± 0.59 | 3.5 | Bacteria|Firmicutes|Clostridia|Clostridiales |
| 311286 | 0.13 ± 0.01 | 0.05 ± 0.04 | 2.6 | Bacteria|Firmicutes|Clostridia|Clostridiales|Lachnospiraceae |
| 343923 | 0.02 ± 0.02 | 1.11 ± 2.15 | 3.8 | Bacteria|Firmicutes|Clostridia|Clostridiales|Lachnospiraceae |
| 195621 | 0.30 ± 0.31 | 1.05 ± 0.68 | 3.6 | Bacteria|Firmicutes|Clostridia|Clostridiales |
| 1510067 | 0.86 ± 0.37 | 0.44 ± 0.36 | 3.4 | Bacteria|Firmicutes|Clostridia|Clostridiales |
| 274697 | 0.15 ± 0.15 | 0.51 ± 0.34 | 3.3 | Bacteria|Firmicutes|Clostridia|Clostridiales |
| 747987 | 0.02 ± 0.06 | 0.20 ± 0.34 | 2.8 | Bacteria|Firmicutes|Clostridia|Clostridiales |
| 272757 | 0.17 ± 0.09 | 0.29 ± 0.13 | 2.8 | Bacteria|Firmicutes|Clostridia|Clostridiales |
| 332854 | 0.08 ± 0.05 | 0.03 ± 0.02 | 2.4 | Bacteria|Firmicutes|Clostridia|Clostridiales |
| 330219 | 0.03 ± 0.01 | 0.05 ± 0.02 | 2.1 | Bacteria|Firmicutes|Clostridia|Clostridiales |
| 314188 | 0.03 ± 0.01 | 0.05 ± 0.02 | 2.1 | Bacteria|Firmicutes|Clostridia|Clostridiales |
| 337494 | 0.01 ± 0.01 | 0.03 ± 0.02 | 2.1 | Bacteria|Firmicutes|Clostridia|Clostridiales |
| 1136443 | 2.26 ± 0.76 | 3.50 ± 0.93 | 3.8 | Bacteria|Deferribacteres|Deferribacteres|Deferribacterales|Deferribacteraceae|Mucispirillum|Schaedleri |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Li, R.W.; Huang, H.; Fletcher, A.; Yu, L.; Pham, Q.; Yu, L.; He, Q.; Wang, T.T.Y. Inhibition of Tumor Growth by Dietary Indole-3-Carbinol in a Prostate Cancer Xenograft Model May Be Associated with Disrupted Gut Microbial Interactions. Nutrients 2019, 11, 467. https://doi.org/10.3390/nu11020467
Wu Y, Li RW, Huang H, Fletcher A, Yu L, Pham Q, Yu L, He Q, Wang TTY. Inhibition of Tumor Growth by Dietary Indole-3-Carbinol in a Prostate Cancer Xenograft Model May Be Associated with Disrupted Gut Microbial Interactions. Nutrients. 2019; 11(2):467. https://doi.org/10.3390/nu11020467
Chicago/Turabian StyleWu, Yanbei, Robert W. Li, Haiqiu Huang, Arnetta Fletcher, Lu Yu, Quynhchi Pham, Liangli Yu, Qiang He, and Thomas T. Y. Wang. 2019. "Inhibition of Tumor Growth by Dietary Indole-3-Carbinol in a Prostate Cancer Xenograft Model May Be Associated with Disrupted Gut Microbial Interactions" Nutrients 11, no. 2: 467. https://doi.org/10.3390/nu11020467
APA StyleWu, Y., Li, R. W., Huang, H., Fletcher, A., Yu, L., Pham, Q., Yu, L., He, Q., & Wang, T. T. Y. (2019). Inhibition of Tumor Growth by Dietary Indole-3-Carbinol in a Prostate Cancer Xenograft Model May Be Associated with Disrupted Gut Microbial Interactions. Nutrients, 11(2), 467. https://doi.org/10.3390/nu11020467





