Vitamin B12 Status in Pregnant Adolescents and Their Infants
Abstract
:1. Introduction
2. Materials and Methods
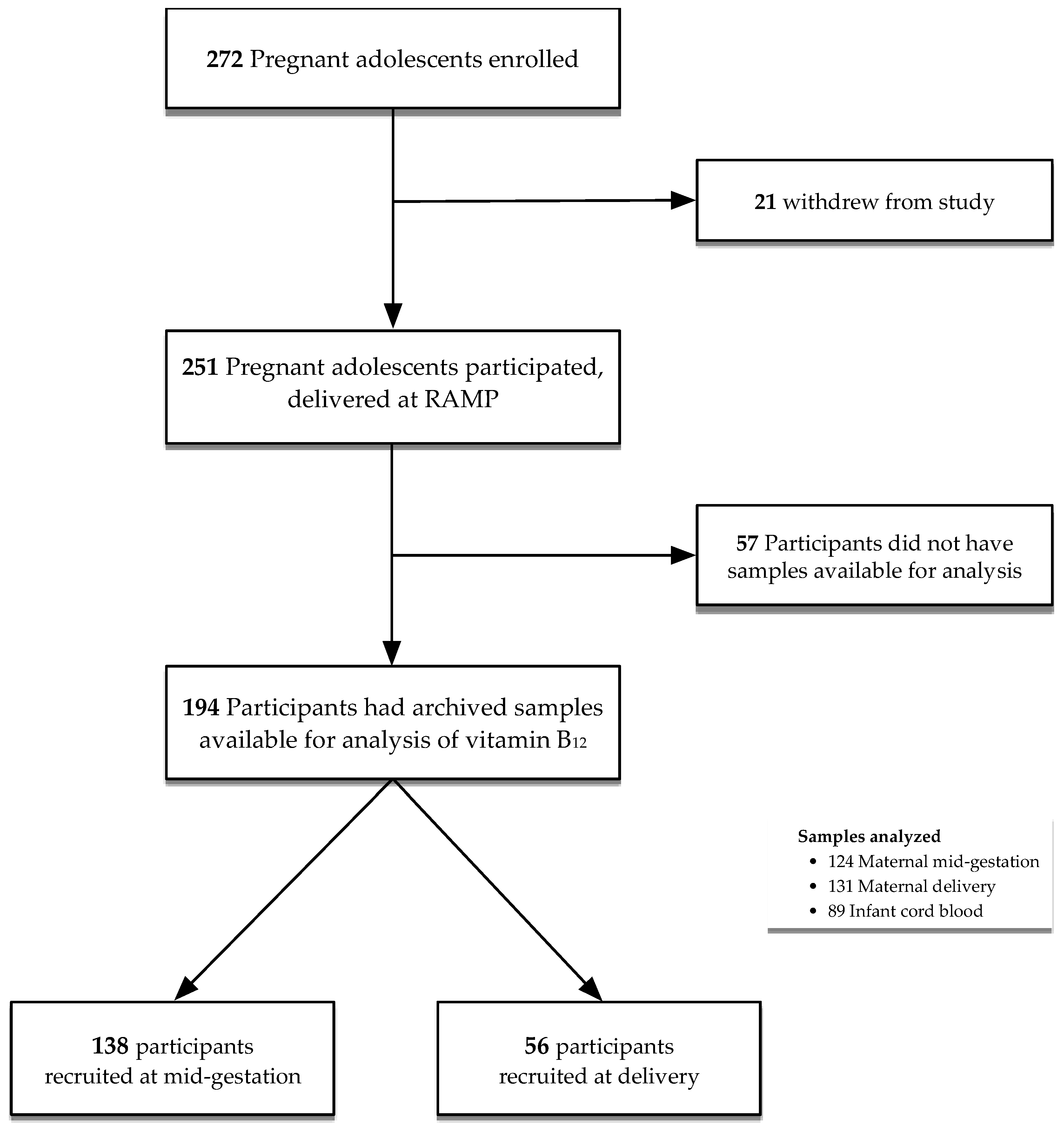
2.1. Study Population
2.2. Ethics
2.3. Follow-Up Procedures
2.4. Laboratory Analyses
2.5. Definitions of Outcomes
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Maternal and Neonatal Vitamin B12 Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Allen, L.H. How common is vitamin B-12 deficiency? Am. J. Clin. Nutr. 2009, 89, 693S–696S. [Google Scholar] [CrossRef] [PubMed]
- McLean, E.; de Benoist, B.; Allen, L.H. Review of the magnitude of folate and vitamin B12 deficiencies worldwide. Food Nutr. Bull. 2008, 29, S38–S51. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.H.; Rosenberg, I.H.; Oakley, G.P.; Omenn, G.S. Considering the case for vitamin B12 fortification of flour. Food Nutr. Bull. 2010, 31, S36–S46. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.L.; Layden, A.J.; Stover, P.J. Vitamin B-12 and Perinatal Health. Adv. Nutr. 2015, 6, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Ratan, S.K.; Rattan, K.N.; Pandey, R.M.; Singhal, S.; Kharab, S.; Bala, M.; Singh, V.; Jhanwar, A. Evaluation of the levels of folate, vitamin B12, homocysteine and fluoride in the parents and the affected neonates with neural tube defect and their matched controls. Pediatr. Surg. Int. 2008, 24, 803–808. [Google Scholar] [CrossRef]
- Adams, M.J., Jr.; Khoury, M.J.; Scanlon, K.S.; Stevenson, R.E.; Knight, G.J.; Haddow, J.E.; Sylvester, G.C.; Cheek, J.E.; Henry, J.P.; Stabler, S.P.; et al. Elevated midtrimester serum methylmalonic acid levels as a risk factor for neural tube defects. Teratology 1995, 51, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Platt, R.; Wu, Q.; Leclerc, D.; Christensen, B.; Yang, H.; Gravel, R.A.; Rozen, R. A common variant in methionine synthase reductase combined with low cobalamin (vitamin B12) increases risk for spina bifida. Mol. Genet. Metab. 1999, 67, 317–323. [Google Scholar] [CrossRef]
- Gu, Q.; Li, Y.; Cui, Z.L.; Luo, X.P. Homocysteine, folate, vitamin B12 and B6 in mothers of children with neural tube defects in Xinjiang, China. Acta Paediatr. 2012, 101, e486–e490. [Google Scholar] [CrossRef]
- Ray, J.G.; Wyatt, P.R.; Thompson, M.D.; Vermeulen, M.J.; Meier, C.; Wong, P.Y.; Farrell, S.A.; Cole, D.E. Vitamin B12 and the risk of neural tube defects in a folic-acid-fortified population. Epidemiology 2007, 18, 362–366. [Google Scholar] [CrossRef]
- Molloy, A.M.; Kirke, P.N.; Troendle, J.F.; Burke, H.; Sutton, M.; Brody, L.C.; Scott, J.M.; Mills, J.L. Maternal vitamin B12 status and risk of neural tube defects in a population with high neural tube defect prevalence and no folic Acid fortification. Pediatrics 2009, 123, 917–923. [Google Scholar] [CrossRef]
- Rowland, A.S.; Baird, D.D.; Shore, D.L.; Weinberg, C.R.; Savitz, D.A.; Wilcox, A.J. Nitrous oxide and spontaneous abortion in female dental assistants. Am. J. Epidemiol. 1995, 141, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Hubner, U.; Alwan, A.; Jouma, M.; Tabbaa, M.; Schorr, H.; Herrmann, W. Low serum vitamin B12 is associated with recurrent pregnancy loss in Syrian women. Clin. Chem. Lab. Med. 2008, 46, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Reznikoff-Etievant, M.F.; Zittoun, J.; Vaylet, C.; Pernet, P.; Milliez, J. Low Vitamin B(12) level as a risk factor for very early recurrent abortion. Eur. J. Obs. Gynecol. Reprod. Biol. 2002, 104, 156–159. [Google Scholar] [CrossRef]
- Hogeveen, M.; Blom, H.J.; den Heijer, M. Maternal homocysteine and small-for-gestational-age offspring: Systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Muthayya, S.; Kurpad, A.V.; Duggan, C.P.; Bosch, R.J.; Dwarkanath, P.; Mhaskar, A.; Mhaskar, R.; Thomas, A.; Vaz, M.; Bhat, S.; et al. Low maternal vitamin B12 status is associated with intrauterine growth retardation in urban South Indians. Eur. J. Clin. Nutr. 2006, 60, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Black, M.M. Effects of vitamin B12 and folate deficiency on brain development in children. Food Nutr. Bull. 2008, 29, S126–S131. [Google Scholar] [CrossRef]
- Pepper, M.R.; Black, M.M. B12 in fetal development. Semin. Cell Dev. Biol. 2011, 22, 619–623. [Google Scholar] [CrossRef]
- Obeid, R.; Morkbak, A.L.; Munz, W.; Nexo, E.; Herrmann, W. The cobalamin-binding proteins transcobalamin and haptocorrin in maternal and cord blood sera at birth. Clin. Chem. 2006, 52, 263–269. [Google Scholar] [CrossRef]
- Muthayya, S.; Dwarkanath, P.; Mhaskar, M.; Mhaskar, R.; Thomas, A.; Duggan, C.; Fawzi, W.W.; Bhat, S.; Vaz, M.; Kurpad, A. The relationship of neonatal serum vitamin B12 status with birth weight. Asia Pac. J. Clin. Nutr. 2006, 15, 538–543. [Google Scholar]
- Balci, Y.I.; Ergin, A.; Karabulut, A.; Polat, A.; Dogan, M.; Kucuktasci, K. Serum vitamin B12 and folate concentrations and the effect of the Mediterranean diet on vulnerable populations. Pediatr. Hematol. Oncol. 2014, 31, 62–67. [Google Scholar] [CrossRef]
- Adaikalakoteswari, A.; Vatish, M.; Lawson, A.; Wood, C.; Sivakumar, K.; McTernan, P.G.; Webster, C.; Anderson, N.; Yajnik, C.S.; Tripathi, G.; et al. Low maternal vitamin B12 status is associated with lower cord blood HDL cholesterol in white Caucasians living in the UK. Nutrients 2015, 7, 2401–2414. [Google Scholar] [CrossRef] [PubMed]
- Radunovic, N.; Lockwood, C.J.; Stanojlovic, O.; Steric, M.; Kontic-Vucinic, O.; Sulovic, N.; Hrncic, D.; Ackerman Iv, W.E. Fetal and maternal plasma homocysteine levels during the second half of uncomplicated pregnancy. J. Matern.-Fetal Neonatal Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Bergen, N.E.; Schalekamp-Timmermans, S.; Jaddoe, V.W.; Hofman, A.; Lindemans, J.; Russcher, H.; Tiemeier, H.; Steegers-Theunissen, R.P.; Steegers, E.A. Maternal and neonatal markers of the homocysteine pathway and fetal growth: The Generation R Study. Paediatr. Perinat. Epidemiol. 2016, 30, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Kalay, Z.; Islek, A.; Parlak, M.; Kirecci, A.; Guney, O.; Koklu, E.; Kalay, S. Reliable and powerful laboratory markers of cobalamin deficiency in the newborn: Plasma and urinary methylmalonic acid. J. Matern.-Fetal Neonatal Med. 2016, 29, 60–63. [Google Scholar] [CrossRef]
- Hay, G.; Clausen, T.; Whitelaw, A.; Trygg, K.; Johnston, C.; Henriksen, T.; Refsum, H. Maternal folate and cobalamin status predicts vitamin status in newborns and 6-month-old infants. J. Nutr. 2010, 140, 557–564. [Google Scholar] [CrossRef]
- Murphy, M.M.; Molloy, A.M.; Ueland, P.M.; Fernandez-Ballart, J.D.; Schneede, J.; Arija, V.; Scott, J.M. Longitudinal study of the effect of pregnancy on maternal and fetal cobalamin status in healthy women and their offspring. J. Nutr. 2007, 137, 1863–1867. [Google Scholar] [CrossRef] [PubMed]
- Deegan, K.L.; Jones, K.M.; Zuleta, C.; Ramirez-Zea, M.; Lildballe, D.L.; Nexo, E.; Allen, L.H. Breast milk vitamin B-12 concentrations in Guatemalan women are correlated with maternal but not infant vitamin B-12 status at 12 months postpartum. J. Nutr. 2012, 142, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.L.; Kurpad, A.V.; Thomas, T.; Srinivasan, K.; Duggan, C. Vitamin B12 status in pregnant women and their infants in South India. Eur. J. Clin. Nutr. 2017, 71, 1046–1053. [Google Scholar] [CrossRef]
- Varsi, K.; Ueland, P.M.; Torsvik, I.K.; Bjorke-Monsen, A.L. Maternal serum cobalamin at 18 weeks of pregnancy predicts infant cobalamin status at 6 months—A prospective, observational study. J. Nutr. 2018, 148, 738–745. [Google Scholar] [CrossRef]
- Lindblad, B.; Zaman, S.; Malik, A.; Martin, H.; Ekstrom, A.; Amu, S.; Holmgren, A.; Norman, M. Folate, vitamin B12, and homocysteine levels in South Asian women with growth-retarded fetuses. Acta Obstet. Gynecol. Scand. 2005, 84, 1055–1061. [Google Scholar] [CrossRef]
- Bjorke Monsen, A.L.; Ueland, P.M.; Vollset, S.E.; Guttormsen, A.B.; Markestad, T.; Solheim, E.; Refsum, H. Determinants of cobalamin status in newborns. Pediatrics 2001, 108, 624–630. [Google Scholar] [CrossRef]
- Guerra-Shinohara, E.M.; Paiva, A.A.; Rondo, P.H.; Yamasaki, K.; Terzi, C.A.; D’Almeida, V. Relationship between total homocysteine and folate levels in pregnant women and their newborn babies according to maternal serum levels of vitamin B12. BJOG 2002, 109, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Koc, A.; Kocyigit, A.; Soran, M.; Demir, N.; Sevinc, E.; Erel, O.; Mil, Z. High frequency of maternal vitamin B12 deficiency as an important cause of infantile vitamin B12 deficiency in Sanliurfa province of Turkey. Eur. J. Nutr. 2006, 45, 291–297. [Google Scholar] [CrossRef]
- Jacquemyn, Y.; Ajaji, M.; Karepouan, N.; Jacquemyn, N.; Van Sande, H. Vitamin B12 and folic acid status of term pregnant women and newborns in the Antwerp region, Belgium. Clin. Exp. Obstet. Gynecol. 2014, 41, 141–143. [Google Scholar] [PubMed]
- Mittal, M.; Bansal, V.; Jain, R.; Dabla, P.K. Perturbing Status of Vitamin B12 in Indian Infants and Their Mothers. Food Nutr. Bull. 2017, 38, 209–215. [Google Scholar] [CrossRef]
- Dilli, D.; Dogan, N.N.; Orun, U.A.; Koc, M.; Zenciroglu, A.; Karademir, S.; Akduman, H. Maternal and neonatal micronutrient levels in newborns with CHD. Cardiol. Young 2018, 28, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Chebaya, P.; Karakochuk, C.D.; March, K.M.; Chen, N.N.; Stamm, R.A.; Kroeun, H.; Sophonneary, P.; Borath, M.; Shahab-Ferdows, S.; Hampel, D.; et al. Correlations between maternal, breast milk, and infant vitamin b12 concentrations among mother-infant dyads in Vancouver, Canada and Prey Veng, Cambodia: An exploratory analysis. Nutrients 2017, 9, 270. [Google Scholar] [CrossRef] [PubMed]
- Coban, S.; Yilmaz Keskin, E.; Igde, M. Association between maternal and infantile markers of cobalamin status during the first month post-delivery. Indian J. Pediatr. 2018, 85, 517–522. [Google Scholar] [CrossRef]
- Williams, A.M.; Stewart, C.P.; Shahab-Ferdows, S.; Hampel, D.; Kiprotich, M.; Achando, B.; Lin, A.; Null, C.A.; Allen, L.H.; Chantry, C.J. Infant serum and maternal milk vitamin b-12 are positively correlated in Kenyan infant-mother dyads at 1–6 months postpartum, irrespective of infant feeding practice. J. Nutr. 2018, 148, 86–93. [Google Scholar] [CrossRef]
- Bellows, A.L.; Smith, E.R.; Muhihi, A.; Briegleb, C.; Noor, R.A.; Mshamu, S.; Sudfeld, C.; Masanja, H.; Fawzi, W.W. Micronutrient deficiencies among breastfeeding infants in Tanzania. Nutrients 2017, 9, 1258. [Google Scholar] [CrossRef]
- Baker, P.N.; Wheeler, S.J.; Sanders, T.A.; Thomas, J.E.; Hutchinson, C.J.; Clarke, K.; Berry, J.L.; Jones, R.L.; Seed, P.T.; Poston, L. A prospective study of micronutrient status in adolescent pregnancy. Am. J. Clin. Nutr. 2009, 89, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Baron, M.A.; Solano, L.; Pena, E.; Moron, A. (Nutritional status of folate, vitamin B12 and iron in pregnant adolescents). Arch. Latinoam. Nutr. 2003, 53, 150–156. [Google Scholar] [PubMed]
- Gadowsky, S.; Gale, K.; Wolfe, S.A.; Jory, J.; Gibson, R.; O’Connor, D. Biochemical folate, B12, and iron status of a group of pregnant adolescents accessed through the public health system in southern Ontario. J. Adolsc. Health 1995, 16, 465–474. [Google Scholar] [CrossRef]
- Davis, L.M.; Chang, S.C.; Mancini, J.; Nathanson, M.S.; Witter, F.R.; O’Brien, K.O. Vitamin D insufficiency is prevalent among pregnant African American adolescents. J. Pediatr. Adolesc. Gynecol. 2010, 23, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Moran, V. A systematic review of dietary assessments of pregnant adolescents in industrialised countries. Br. J. Nutr. 2007, 97, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.D. American Academy of Pediatrics Committee on, A. Adolescent pregnancy: Current trends and issues. Pediatrics 2005, 116, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Sedgh, G.; Finer, L.B.; Bankole, A.; Eilers, M.A.; Singh, S. Adolescent Pregnancy, Birth, and abortion rates across countries: Levels and recent trends. J. Adolesc. Health 2015, 56, 223–230. [Google Scholar] [CrossRef]
- Lee, S.; Guillet, R.; Cooper, E.M.; Westerman, M.; Orlando, M.; Kent, T.; Pressman, E.; O’Brien, K.O. Prevalence of anemia and associations between neonatal iron status, hepcidin, and maternal iron status among neonates born to pregnant adolescents. Pediatr. Res. 2016, 79, 42–48. [Google Scholar] [CrossRef]
- Lee, S.; Guillet, R.; Cooper, E.M.; Westerman, M.; Orlando, M.; Pressman, E.; O’Brien, K.O. Maternal inflammation at delivery affects assessment of maternal iron status. J. Nutr. 2014, 144, 1524–1532. [Google Scholar] [CrossRef]
- Layden, A.J.; O’Brien, K.O.; Pressman, E.K.; Cooper, E.M.; Kent, T.R.; Finkelstein, J.L. Vitamin B12 and placental expression of transcobalamin in pregnant adolescents. Placenta 2016, 45, 1–7. [Google Scholar] [CrossRef]
- Yetley, E.A.; Pfeiffer, C.M.; Phinney, K.W.; Bailey, R.L.; Blackmore, S.; Bock, J.L.; Brody, L.C.; Carmel, R.; Curtin, L.R.; Durazo-Arvizu, R.A.; et al. Biomarkers of vitamin B-12 status in NHANES: A roundtable summary. Am. J. Clin. Nutr. 2011, 94, 313S–321S. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.M.; Mei, Z.; Grummer-Strawn, L.; Parvanta, I. Haemoglobin adjustments to define anaemia. Trop. Med. Int. Health 2008, 13, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- WHO. Physical Status: The Use and Interpretation of Anthropometry; Report of a WHO Expert Committee; World Health Organization: Geneva, Switzerland, 1995; p. 329. [Google Scholar]
- Spiegelman, D.; Hertzmark, E. Easy SAS calculations for risk or prevalence ratios and differences. Am. J. Epidemiol. 2005, 162, 199–200. [Google Scholar] [CrossRef] [PubMed]
- Wacholder, S. Binomial regression in GLIM: Estimating risk ratios and risk differences. Am. J. Epidemiol. 1986, 123, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Zou, G. A modified poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 2004, 159, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Durrleman, S.; Simon, R. Flexible regression models with cubic splines. Stat. Med. 1989, 8, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Govindarajulu, U.S.; Spiegelman, D.; Thurston, S.W.; Ganguli, B.; Eisen, E.A. Comparing smoothing techniques in Cox models for exposure-response relationships. Stat. Med. 2007, 26, 3735–3752. [Google Scholar] [CrossRef]
- Greenland, S. Modeling and variable selection in epidemiologic analysis. Am. J. Public Health 1989, 79, 340–349. [Google Scholar] [CrossRef]
- Miettinen, O. Theoretical Epidemiology; John Wiley & Sons: New York, NY, USA, 1985; Volume 107. [Google Scholar]
- Garcia-Casal, M.N.; Osorio, C.; Landaeta, M.; Leets, I.; Matus, P.; Fazzino, F.; Marcos, E. High prevalence of folic acid and vitamin B12 deficiencies in infants, children, adolescents and pregnant women in Venezuela. Eur. J. Clin. Nutr. 2005, 59, 1064–1070. [Google Scholar] [CrossRef]
- Green, R.; Allen, L.H.; Bjorke-Monsen, A.L.; Brito, A.; Gueant, J.L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.H.; et al. Vitamin B12 deficiency. Nat. Rev. Dis. Prim. 2017, 3, 17040. [Google Scholar] [CrossRef]
- Tucker, K.L.; Rich, S.; Rosenberg, I.; Jacques, P.; Dallal, G.; Wilson, P.W.; Selhub, J. Plasma vitamin B-12 concentrations relate to intake source in the Framingham Offspring study. Am. J. Clin. Nutr. 2000, 71, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Visentin, C.E.; Masih, S.P.; Plumptre, L.; Schroder, T.H.; Sohn, K.J.; Ly, A.; Lausman, A.Y.; Berger, H.; Croxford, R.; Lamers, Y.; et al. Low serum vitamin b-12 concentrations are prevalent in a cohort of pregnant Canadian women. J. Nutr. 2016, 146, 1035–1042. [Google Scholar] [CrossRef]
- Wadhwani, N.S.; Pisal, H.R.; Mehendale, S.S.; Joshi, S.R. A prospective study of maternal fatty acids, micronutrients and homocysteine and their association with birth outcome. Matern. Child Nutr. 2015, 11, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, N.; Rafnsson, S.B.; Kandala, N.B.; Bhopal, R.; Yajnik, C.S.; Saravanan, P. Prevalence of vitamin B-12 insufficiency during pregnancy and its effect on offspring birth weight: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 103, 1232–1251. [Google Scholar] [CrossRef] [PubMed]
- Rush, E.C.; Katre, P.; Yajnik, C.S. Vitamin B12: One carbon metabolism, fetal growth and programming for chronic disease. Eur. J. Clin. Nutr. 2013, 68, 2–7. [Google Scholar] [CrossRef] [PubMed]
- De-Regil, L.M.; Harding, K.B.; Roche, M.L. Preconceptional nutrition interventions for adolescent girls and adult women: Global guidelines and gaps in evidence and policy with emphasis on micronutrients. J. Nutr. 2016, 146, 1461S–1470S. [Google Scholar] [CrossRef] [PubMed]
| Variables a | Original Cohort (n = 251) | Current Study (n = 194) | Recruited at Mid-Gestation (n = 138) | Recruited at Delivery (n = 56) |
|---|---|---|---|---|
| Maternal | ||||
| Age at enrollment, years | 17.3 (16.5, 18.1) | 17.3 (16.5, 18.1) | 17.3 (16.4, 18.1) | 17.3 (16.6, 18.1) |
| Age at delivery, years | 17.5 (16.7, 18.3) | 17.6 (16.8, 18.4) | 17.6 (16.7, 18.4) | 17.4 (16.9, 18.2) |
| <16 years, % (n) | 12.0 (30) | 9.8 (19) | 9.4 (13) | 10.7 (6) |
| Gestational age at delivery, weeks | 39.9 (38.7, 40.7) | 40 (39.0, 40.9) | 40.0 (38.9, 40.9) | 40.0 (39.2, 41.0) |
| Pre-term (<37 weeks), % (n) | 8.0 (20) | 7.8 (15) | 8.8 (12) | 5.4 (3) |
| Parity ≥1, % (n) | 17.3 (43) | 15.1 (29) | 8.7 (12) | 30.9 (17) |
| Smoking at enrollment, % (n) | ||||
| Never a smoker | 77.8 (189) | 78.5 (150) | 77.5 (107) | 81.1 (43) |
| Past smoker | 15.2 (37) | 14.4 (27) | 12.3 (17) | 18.9 (10) |
| Current smoker | 7.0 (17) | 7.3 (14) | 10.1 (14) | 0.0 (0) |
| Relationship status b, % (n) | 13.5 (33) | 10.5 (20) | 1.5 (2) | 34.0 (18) |
| WIC c program participant | 60.9 (148) | 63.2 (120) | 80.0 (100) | 37.7 (20) |
| Self-reported prenatal supplement use, % (n) | ||||
| ≥2 pills per week | 54.1 (131) | 55.5 (106) | 56.6 (77) | 52.7 (29) |
| Dietary folate, µg/day | 617.2 (397.0, 948.9) | 617.2 (400.8, 950.45) | 692.7 (464.2, 1020.6) | 415.3 (283.9, 624.7) |
| Dietary vitamin B12, µg/day | 4.6 (2.7, 6.5) | 4.6 (2.7, 6.6) | 5.0 (3.7, 6.9) | 2.8 (1.4, 5.2) |
| Pre-pregnancy BMI, kg/m2 | 23.5 (20.8, 28.0) | 23.7 (20.8, 28.0) | 23.3 (20.8, 28.1) | 24.7 (20.8, 27.9) |
| <18.5 kg/m2, % (n) | 6.9 (17) | 7.3 (14) | 6.62 (9) | 9.1 (5) |
| ≥18.5 to <25 kg/m2, % (n) | 54.3 (133) | 52.4 (100) | 55.2 (75) | 45.5 (25) |
| ≥25.0 to <30 kg/m2, % (n) | 20.8 (51) | 21.5 (41) | 19.9 (27) | 25.5 (14) |
| ≥30 kg/m2, % (n) | 18.0 (44) | 18.9 (36) | 18.4 (25) | 20.0 (11) |
| Gestational weight gain (GWG), kg | 15.9 (11.8, 20.5) | 16.4 (11.8, 20.5) | 15.5 (11.8, 20.5) | 17.3 (12.3, 21.4) |
| Inadequate d GWG, % (n) | 15.0 (36) | 13.9 (26) | 14.3 (19) | 13.0 (7) |
| Within IOM range, % (n) | 22.9 (55) | 24.0 (45) | 26.3 (35) | 18.5 (10) |
| Excessive GWG, % (n) | 62.1 (149) | 62.0 (116) | 59.0 (79) | 68.5 (37) |
| Race, % (n) | ||||
| Caucasian | 27.9 (70) | 29.4 (57) | 33.3 (36) | 19.6 (11) |
| African American | 71.3 (179) | 69.6 (135) | 65.2 (90) | 80.4 (45) |
| Native American | 0.8 (2) | 1.0 (2) | 1.5 (2) | 0.0 (0) |
| Ethnicity, % (n) | ||||
| Hispanic | 24.3 (61) | 26.3 (51) | 24.6 (34) | 30.4 (17) |
| Infant | ||||
| Birthweight, g | 3206.0 (2904.0, 3550.0) | 3266.0 (2928.0, 3581.0) | 3258.0 (2892.0, 3581.0) | 3318.5 (3055.5, 3584.0) |
| Birth length, cm | 51.0 (49.0, 52.7) | 51.3 (49.5, 52.9) | 51.0 (49.5, 52.5) | 52.0 (50.0, 53.5) |
| Weight-for-length z-score < −2, % (n) | 27.0 (60) | 27.0 (47) | 27.3 (35) | 26.1 (12) |
| Ponderal index, g/cm3 × 100 | 2.4 (2.2, 2.7) | 2.4 (2.3, 2.7) | 2.4 (2.2, 2.7) | 2.4 (2.3, 2.6) |
| Male sex, % (n) | 52.8 (132) | 51.0 (99) | 50.7 (70) | 51.8 (29) |
| Maternal | Infant | ||||||
|---|---|---|---|---|---|---|---|
| Mid-Gestation | Delivery | Cord Blood | |||||
| Variables a | Total | Total | Recruited at Mid-Gestation | Recruited at Delivery | Total | Mothers Recruited at Mid-Gestation | Mothers Recruited at Delivery |
| n | 124 | 131 | 75 | 56 | 89 | 58 | 31 |
| Serum vitamin B12, pmol/L | 343.7 (237.8, 400.7) | 216.2 (161.6, 297.8) | 216.2 (173.4, 311.8) | 211.2 (158.7, 267.0) | 597.0 (471.6, 796.3) | 569.4 (478.6, 844.3) | 602.9 (406.6, 722.1) |
| <148.0 pmol/L | 1.6 (2) | 15.3 (20) | 14.7 (11) | 16.1 (9) | 0.0 (0) | 0.0 (0) | 0.0 (0) |
| ≥148 to <221.0 pmol/L | 21.0 (26) | 38.2 (50) | 37.3 (28) | 39.3 (22) | 2.3 (2) | 0.0 (0) | 6.5 (2) |
| ≥221 pmol/L | 77.4 (96) | 46.6 (61) | 48.0 (36) | 44.6 (25) | 99.8 (87) | 100.0 (58) | 93.5 (29) |
| n | 122 | 130 | 74 | 56 | 86 | 55 | 31 |
| Serum folate, nmol/L | 39.3 (31.7, 50.5) | 39.7 (31.8, 50.4) | 42.8 (32.2, 51.4) | 37.7 (28.8, 48.4) | 66.7 (53.1, 85.5) | 66.3 (52.1, 84.4) | 67.7 (55.5, 98.4) |
| ≤29.45b nmol/L | 19.7 (24) | 20.0 (26) | 13.5 (10) | 28.6 (16) | 2.3 (2) | 3.6 (2) | 0.0 (0) |
| >29.45, ≤35.79 nmol/L | 20.5 (25) | 16.9 (22) | 18.9 (14) | 14.3 (8) | 2.3 (2) | 3.6 (2) | 0.0 (0) |
| >35.79, ≤43.94 nmol/L | 19.7 (24) | 20.0 (26) | 17.6 (13) | 23.2 (13) | 4.7 (4) | 5.5 (3) | 3.2 (1) |
| >43.94, ≤52.66 nmol/L | 19.7 (24) | 22.3 (29) | 29.7 (22) | 12.5 (7) | 14.0 (12) | 12.7 (7) | 16.1 (5) |
| >52.66 nmol/L | 20.5 (25) | 20.8 (27) | 20.3 (15) | 21.4 (12) | 76.7 (66) | 74.6 (41) | 80.7 (25) |
| Univariate b | Multivariate c | Multivariate d | ||||||
|---|---|---|---|---|---|---|---|---|
| Maternal Variables | Time-Point | n | β (SE) | p-Value | β (SE) | p-Value | β (SE) | p-Value |
| Serum vitamin B12, a pmol/L | Mid-gestation | 54 | 0.29 (0.17) | 0.09 | 0.28 (0.16) | 0.08 | 0.31 (0.16) | 0.06 |
| Delivery (All) | 64 | 0.85 (0.12) | <0.0001 | 0.74 (0.12) | <0.0001 | 0.77 (0.12) | <0.001 | |
| Delivery (Recruited at mid-gestation) | 33 | 0.57 (0.20) | 0.004 | 0.53 (0.18) | 0.003 | 0.53 (0.16) | 0.001 | |
| Delivery (Recruited at delivery) | 31 | 1.09 (0.13) | <0.0001 | 0.97 (0.14) | <0.0001 | 1.02 (0.12) | <0.001 | |
| <148.0 pmol/L | Mid-gestation | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
| Delivery (All) | 64 | −0.65 (0.16) | <0.0001 | −0.54 (0.14) | 0.0002 | −0.62 (0.15) | <0.0001 | |
| Delivery (Recruited at mid-gestation) | 33 | −0.63 (0.22) | 0.004 | −0.60 (0.19) | 0.002 | −0.56 (0.18) | 0.002 | |
| Delivery (Recruited at delivery) | 31 | −0.72 (0.22) | 0.001 | −0.59 (0.22) | 0.008 | −0.67 (0.21) | 0.002 | |
| <221.0 pmol/L | Mid-gestation | 54 | −0.16 (0.15) | 0.28 | −0.18 (0.14) | 0.20 | −0.18 (0.14) | 0.21 |
| Delivery (All) | 64 | −0.42 (0.12) | 0.0007 | −0.30 (0.12) | 0.01 | −0.33 (0.12) | 0.008 | |
| Delivery (Recruited at mid-gestation) | 33 | −0.23 (0.17) | 0.17 | −0.19 (0.16) | 0.22 | −0.26 (0.15) | 0.07 | |
| Delivery (Recruited at delivery) | 31 | −0.61 (0.17) | 0.0004 | −0.44 (0.17) | 0.01 | −0.41 (0.19) | 0.03 | |
| Serum folate a, nmol/L | Mid-gestation | 53 | −0.24 (0.14) | 0.09 | −0.24 (0.14) | 0.09 | −0.28 (0.15) | 0.06 |
| Delivery (All) | 64 | 0.12 (0.16) | 0.47 | 0.07 (0.16) | 0.68 | 0.09 (0.17) | 0.61 | |
| Delivery (Recruited at mid-gestation) | 33 | 0.09 (0.22) | 0.69 | 0.12 (0.23) | 0.60 | 0.16 (0.26) | 0.54 | |
| Delivery (Recruited at delivery) | 31 | 0.12 (0.25) | 0.63 | 0.06 (0.23) | 0.81 | 0.11 (0.22) | 0.62 | |
| <40.0 nmol/L | Mid-gestation | 53 | 0.10 (0.12) | 0.40 | 0.11 (0.12) | 0.37 | 0.14 (0.13) | 0.28 |
| Delivery (All) | 64 | −0.06 (0.13) | 0.65 | 0.02 (0.13) | 0.87 | 0.02 (0.13) | 0.88 | |
| Delivery (Recruited at mid-gestation) | 33 | −0.05 (0.17) | 0.76 | −0.08 (0.17) | 0.64 | 0.04 (0.20) | 0.86 | |
| Delivery (Recruited at delivery) | 31 | −0.08 (0.21) | 0.69 | 0.11 (0.18) | 0.56 | 0.15 (0.17) | 0.39 | |
| Univariate b | Multivariate c | Multivariate d | ||||||
|---|---|---|---|---|---|---|---|---|
| Maternal Variables | Time-Point | n | β (SE) | p-Value | β (SE) | p-Value | β (SE) | p-Value |
| Serum vitamin B12,a pmol/L | Mid-gestation | 51 | −0.04 (0.16) | 0.79 | −0.19 (0.14) | 0.17 | −0.16 (0.13) | 0.22 |
| Delivery (All) | 61 | −0.02 (0.11) | 0.88 | −0.08 (0.11) | 0.48 | −0.08 (0.11) | 0.45 | |
| Delivery (Recruited at mid-gestation) | 30 | −0.20 (0.15) | 0.18 | −0.20 (0.13) | 0.13 | −0.22 (0.12) | 0.07 | |
| Delivery (Recruited at delivery) | 31 | 0.14 (0.16) | 0.37 | 0.04 (0.15) | 0.78 | 0.06 (0.15) | 0.67 | |
| <148.0 pmol/L | Mid-gestation | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
| Delivery (All) | 61 | 0.07 (0.13) | 0.60 | 0.16 (0.12) | 0.16 | 0.18 (0.12) | 0.14 | |
| Delivery (Recruited at mid-gestation) | 30 | 0.26 (0.17) | 0.13 | 0.26 (0.14) | 0.07 | 0.38 (0.13) | 0.005 | |
| Delivery (Recruited at delivery) | 31 | −0.10 (0.18) | 0.57 | 0.07 (0.17) | 0.65 | 0.03 (0.17) | 0.88 | |
| <221.0 pmol/L | Mid-gestation | 51 | −0.05 (0.14) | 0.71 | 0.01 (0.13) | 0.91 | 0.07 (0.12) | 0.54 |
| Delivery (All) | 61 | −0.01 (0.09) | 0.90 | 0.05 (0.09) | 0.58 | 0.07 (0.09) | 0.47 | |
| Delivery (Recruited at mid-gestation) | 30 | 0.18 (0.12) | 0.12 | 0.22 (0.11) | 0.04 | 0.27 (0.10) | 0.006 | |
| Delivery (Recruited at delivery) | 31 | −0.19 (0.13) | 0.15 | −0.09 (0.12) | 0.45 | −0.12 (0.13) | 0.37 | |
| Serum folate a, nmol/L | Mid-gestation | 50 | 0.27 (0.14) | 0.06 | 0.05 (0.13) | 0.69 | 0.003 (0.13) | 0.98 |
| Delivery (All) | 61 | 0.54 (0.09) | <0.0001 | 0.47 (0.10) | <0.0001 | 0.50 (0.10) | <0.0001 | |
| Delivery (Recruited at mid-gestation) | 30 | 0.54 (0.11) | <0.0001 | 0.55 (0.13) | <0.001 | 0.53 (0.15) | 0.0003 | |
| Delivery (Recruited at delivery) | 31 | 0.57 (0.13) | <0.0001 | 0.45 (0.13) | 0.0005 | 0.44 (0.12) | 0.0003 | |
| <40.0 nmol/L | Mid-gestation | 50 | −0.25 (0.12) | 0.03 | −0.13 (0.10) | 0.21 | −0.09 (0.10) | 0.39 |
| Delivery (All) | 61 | −0.42 (0.08) | <0.0001 | −0.40 (0.08) | <0.0001 | −0.42 (0.08) | <0.0001 | |
| Delivery (Recruited at mid-gestation) | 30 | −0.44 (0.09) | <0.0001 | −0.43 (0.10) | <0.0001 | −0.51 (0.12) | <0.0001 | |
| Delivery (Recruited at delivery) | 31 | −0.41 (0.12) | 0.0006 | −0.32 (0.12) | 0.01 | −0.33 (0.12) | 0.006 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finkelstein, J.L.; Guillet, R.; Pressman, E.K.; Fothergill, A.; Guetterman, H.M.; Kent, T.R.; O’Brien, K.O. Vitamin B12 Status in Pregnant Adolescents and Their Infants. Nutrients 2019, 11, 397. https://doi.org/10.3390/nu11020397
Finkelstein JL, Guillet R, Pressman EK, Fothergill A, Guetterman HM, Kent TR, O’Brien KO. Vitamin B12 Status in Pregnant Adolescents and Their Infants. Nutrients. 2019; 11(2):397. https://doi.org/10.3390/nu11020397
Chicago/Turabian StyleFinkelstein, Julia L., Ronnie Guillet, Eva K. Pressman, Amy Fothergill, Heather M. Guetterman, Tera R. Kent, and Kimberly O. O’Brien. 2019. "Vitamin B12 Status in Pregnant Adolescents and Their Infants" Nutrients 11, no. 2: 397. https://doi.org/10.3390/nu11020397
APA StyleFinkelstein, J. L., Guillet, R., Pressman, E. K., Fothergill, A., Guetterman, H. M., Kent, T. R., & O’Brien, K. O. (2019). Vitamin B12 Status in Pregnant Adolescents and Their Infants. Nutrients, 11(2), 397. https://doi.org/10.3390/nu11020397





