Acute Effects of Hibiscus sabdariffa Calyces on Postprandial Blood Pressure, Vascular Function, Blood Lipids, Biomarkers of Insulin Resistance and Inflammation in Humans
Abstract
:1. Introduction
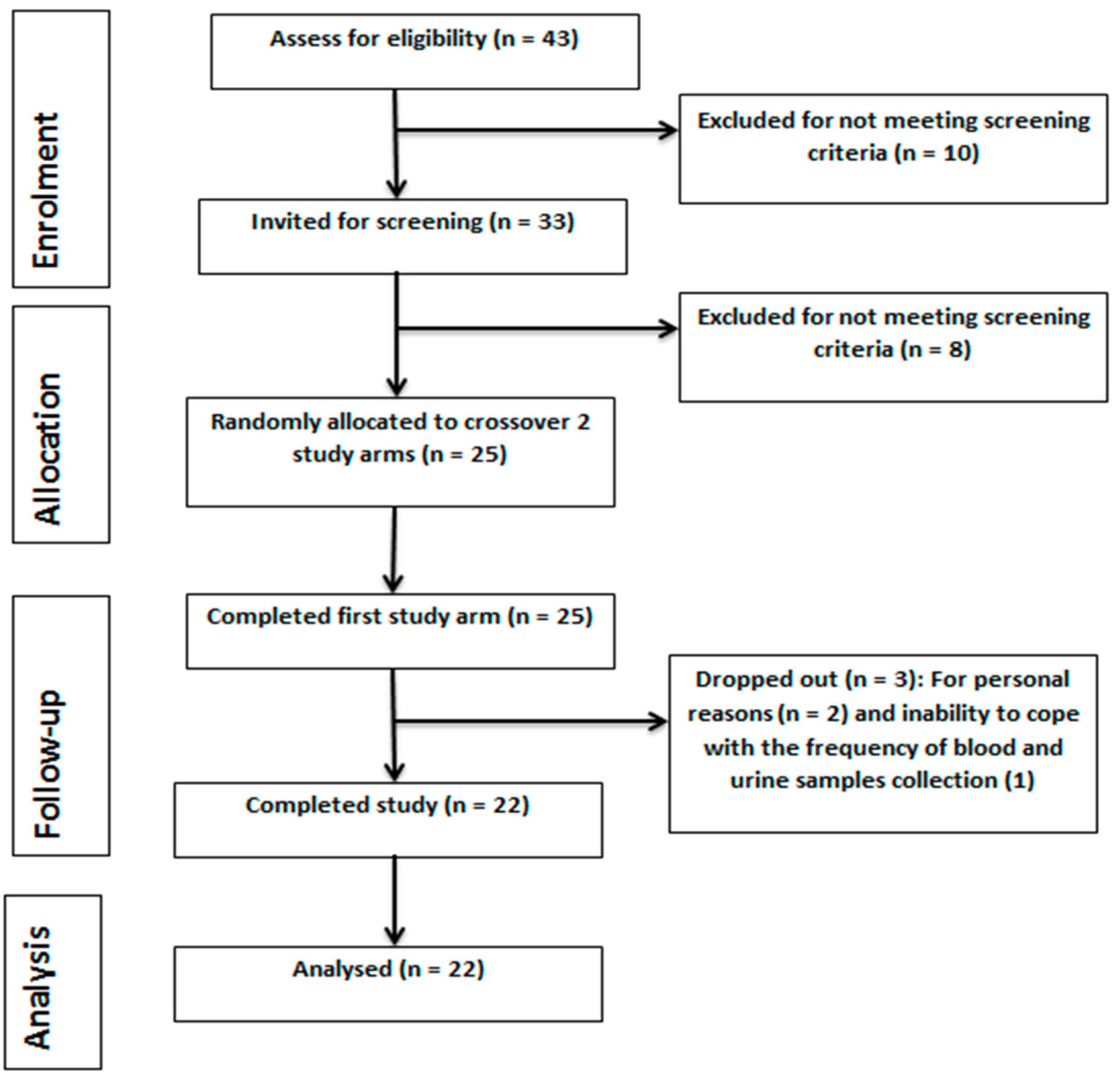
2. Subjects and Methods
2.1. Ethical Approval
2.2. Study Design
2.3. Sample Size and Power Calculation
2.4. Subjects Enrolment
2.5. CVD Risk Calculation
2.6. Hibiscus Drink Preparation
2.7. Blood and Urine Samples Collection and Processing
2.8. Measurement of FMD of the Brachial Artery
2.9. Assessment of Arterial Stiffness
2.10. BP Measurement
2.11. Solid Phase Extraction (SPE) and HPLC Analysis of Plasma Polyphenols
2.12. Plasma and Urine Nitric Oxide Determination
2.13. Biochemical Analyses
2.14. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Volunteers
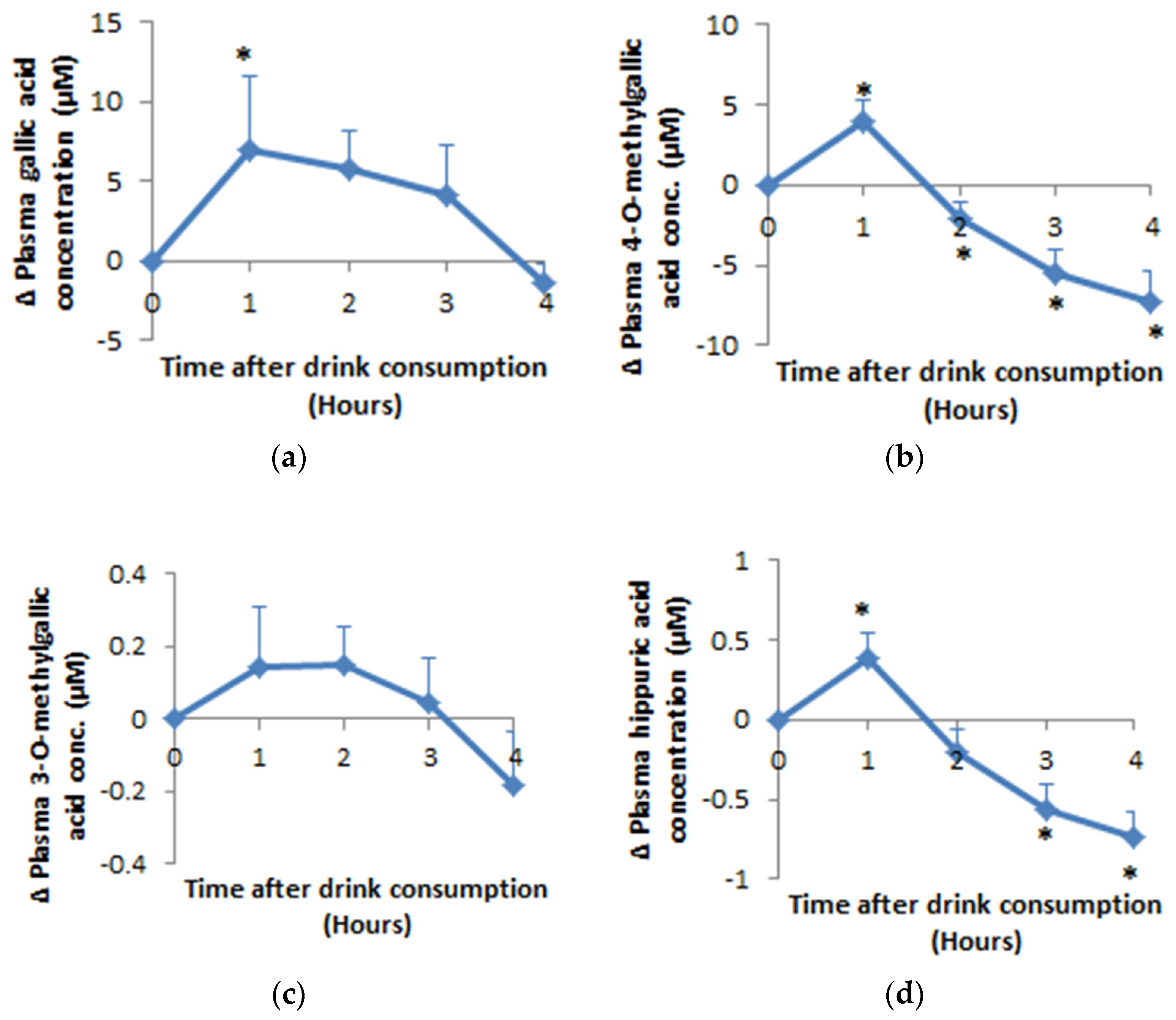
3.2. Bioavailability of Hippuric Acid, Gallic Acid and Its Metabolites
3.3. Impact of Hibiscus Drink Consumption on Systolic (SBP), Diastolic (DBP) and Pulse Pressure (PP) and Heart Rate
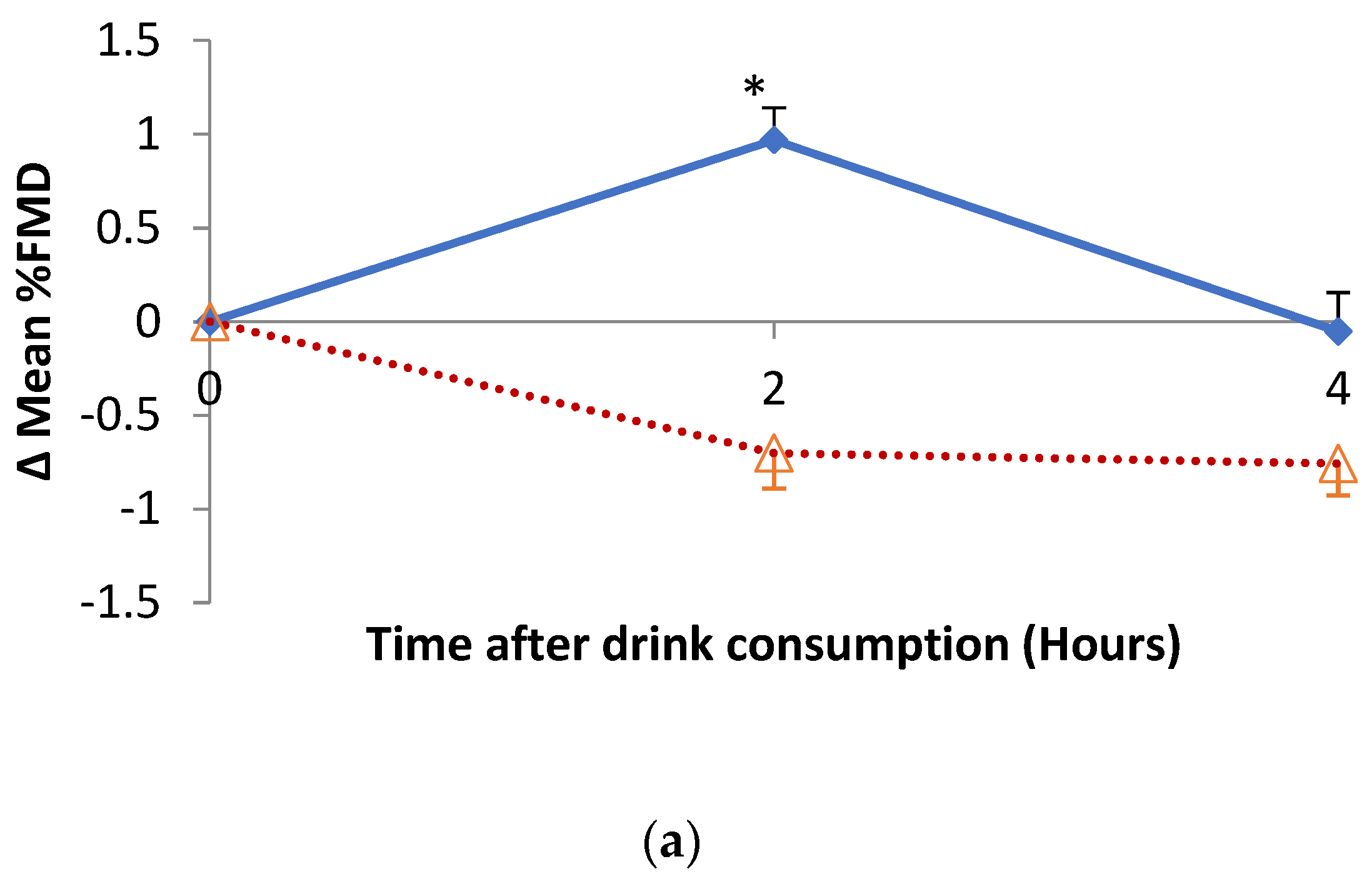
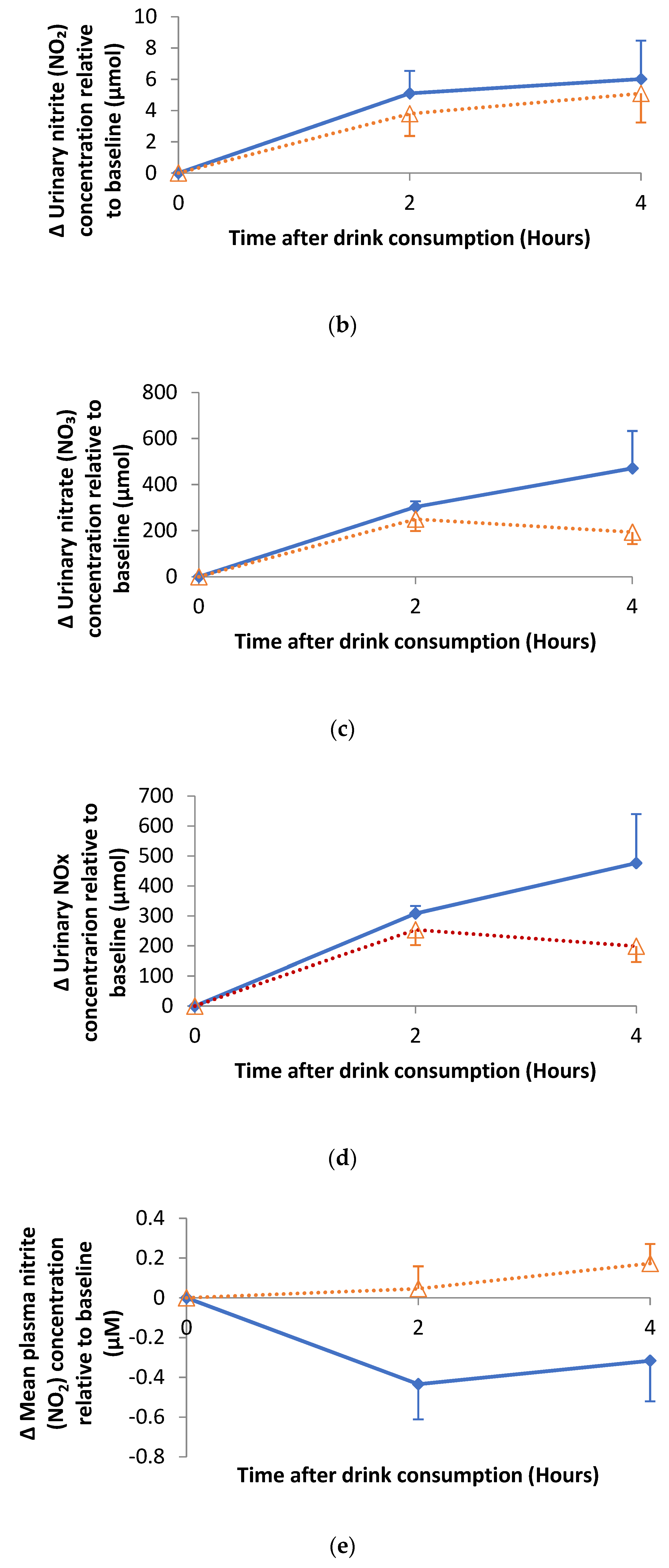
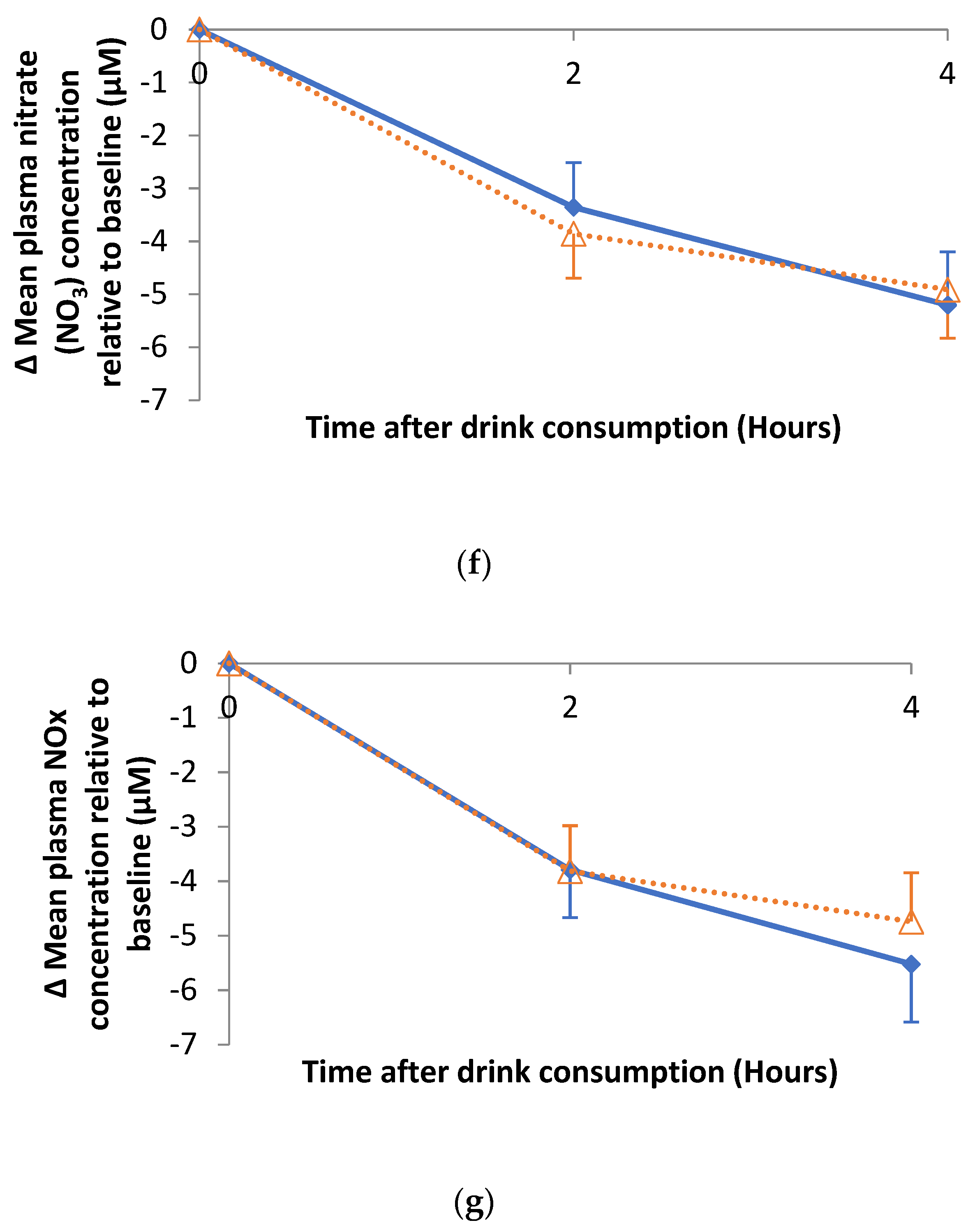
3.4. Impact of Hibiscus Drink Consumption on FMD of the Branchial Artery, Augmentation Index, Plasma and Urine Nitrate and Nitrite
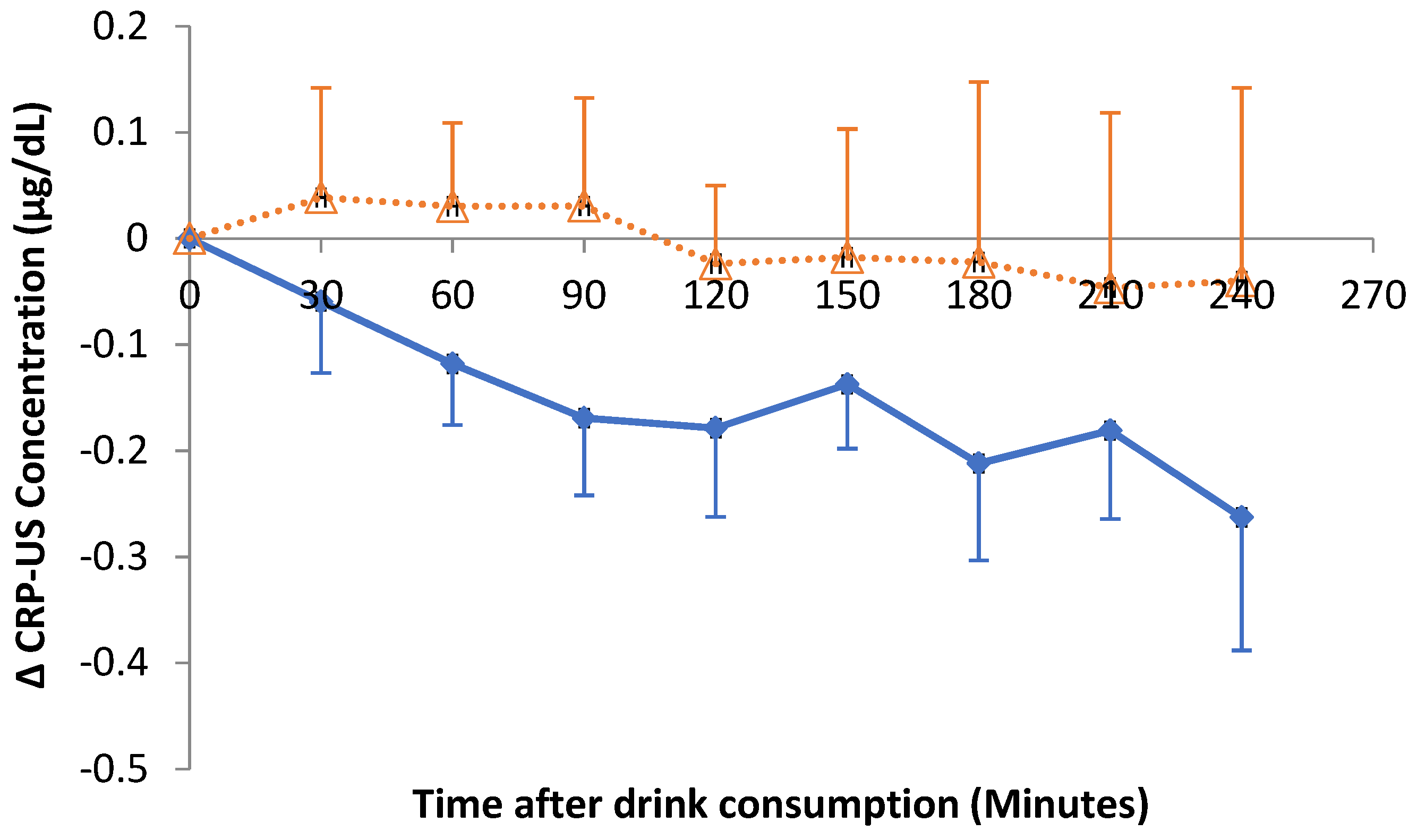
3.5. Impact of Hibiscus Drink Consumption on Postprandial Lipids, Biomarkers of Insulin Resistance and Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vontilainen, S.; Nurmi, T.; Mursu, J.; Rissanen, T.H. Carotenoids and Cardiovascular Disease. Am. J. Clin. Nutr. 2006, 83, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Ajani, U.A.; Croft, J.B. Explaining the Decrease in U.S. Death from Coronary Disease 1980–2000. N. Engl. J. Med. 2007, 365, 2388–2398. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, T.A. The Growing Burden of Cardiovascular Disease in the Developing World. Health Aff. 2007, 26, 13–24. [Google Scholar] [CrossRef] [PubMed]
- British Heart Foundation. BHF Statistics Factsheet. Available online: https://www.bhf.org.uk/ (accessed on 20 January 2019).
- WHO. World Health Statistics 2012; WHO Reports; World Health Organisation: Geneva, Switzerland, 2012. [Google Scholar]
- Hopkins, A.L.; Lamm, M.G.; Funk, J.L.; Ritenbaugh, C. Hibiscus sabdariffa L. in the treatment of hypertension and hyperlipidemia: A comprehensive review of animal and human studies. Fitoterapia 2013, 85, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Z.; Wong, S.Y.; Chong, N.J. Effects of Hibiscus sabdariffa L. on serum lipids: A systematic review and meta-analysis. J. Ethnopharmacol. 2013, 150, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Hibiscus sabdariffa: An ideal yet under-exploited candidate for nutraceutical applications. Biomed. Prev. Nutr. 2014, 4, 23–27. [Google Scholar] [CrossRef]
- Adegunloye, B.J.; Omoniyi, J.O.; Owolabi, O.A.; Ajagbonna, O.P.; Sofola, O.A.; Coker, H.A. Mechanisms of the blood pressure lowering effect of the calyx extract of Hibiscus sabdariffa in rats. Afr. J. Med. Med. Sci. 1996, 25, 235–238. [Google Scholar]
- Ajay, M.; Chai, H.J.; Mustafa, A.M.; Gilani, A.H.; Mustafa, M.R. Mechanisms of the anti-hypertensive effect of Hibiscus sadariffa L. calyces. J. Ethnopharmacol. 2007, 10, 388–393. [Google Scholar] [CrossRef]
- Jonadet, M.; Bastide, J.; Bastide, P.; Boyer, B.; Carnat, A.P.; Lamaison, J.L. Enzyme-inhibiting activities invitro and angioprotective activity invivo of roselle (Hibiscus-sabdariffa L.) extracts. J. Pharm. Belg. 1990, 45, 120–124. [Google Scholar]
- Ojeda, D.; Jimenez-Ferrer, E.; Zamilpa, A.; Herrera-Arellano, A.; Tortoriello, J.; Alvarez, L. Inhibition of angiotensin convertin enzyme (ACE) activity by the anthocyanins delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J. Ethnopharmacol. 2010, 127, 7–10. [Google Scholar] [CrossRef]
- Herrera-Arellano, A.; Miranda-Sanchez, J.; Avila-Castro, P.; Herrera-Alvarez, S.; Jimenez-Ferrer, J.E.; Zamilpa, A.; Roman-Ramos, R.; Ponce-Monter, H.; Tortoriello, J. Clinical effects produced by a standardized herbal medicinal product of Hibiscus sabdariffa on patients with hypertension. A randomized, double-blind, lisinopril-controlled clinical trial. Planta Medica 2007, 73, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Debón, R.; Rodríguez-Gallego, E.; Fernández-Arroyo, S.; Senan-Campos, O.; Massucci, F.A.; Hernández-Aguilera, A.; Sales-Pardo, M.; Guimerà, R.; Camps, J.; Menendez, J.A.; et al. The acute impact of polyphenols from Hibiscus sabdariffa in metabolic homeostasis: An approach combining metabolomics and gene-expression analyses. Food Funct. 2015, 6, 2957–2966. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.F.F.; Macdonald, R.; Lovegrove, J.A. Fruit polyphenols and CVD risk: A review of human intervention studies. Br. J. Nutr. 2010, 104, S28–S39. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Kunz, C.; Herrmann, J.; Borsch, C.H.; Abel, G.; Fröhling, B.; Dietrich, H.; Rudloff, S. Anthocyanins from fruit juices improve the antioxidant status of healthy young female volunteers without affecting anti-inflammatory parameters: Results from the randomised, double-blind, placebo-controlled, cross-over ANTHONIA (ANTHOcyanins in Nutrition Investigation Alliance) study. Br. J. Nutr. 2014, 112, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Woodside, J.V.; Young, I.S.; McKinley, M.C. Fruit and vegetable intake and risk of cardiovascular disease. Proc. Nutr. Soc. 2013, 72, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Okuda, N.; Miura, K.; Okayama, A.; Okamura, T.; Abbott, R.D.; Nishi, N.; Fujiyoshi, A.; Kita, Y.; Nakamura, Y.; Miyagawa, N.; et al. Fruit and vegetable intake and mortality from cardiovascular disease in Japan: A 24-year follow-up of the NIPPON DATA80 Study. Eur. J. Clin. Nutr. 2015, 69, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Vlachojannis, C.; Erne, P.; Schoenenberger, A.W.; Chrubasik-Hausmann, S. A Critical Evaluation of the Clinical Evidence for Pomegranate Preparations in the Prevention and Treatment of Cardiovascular Diseases. Phytother. Res. 2015, 29, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Del Bo, C.; Martini, D.; Porrini, M.; Klimis-Zacas, D.; Riso, P. Berries and oxidative stress markers: An overview of human intervention studies. Food Funct. 2015, 6, 2890–2917. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Chen, C.Y.O.; Zampariello, C.A.; Blumberg, J.B. Flavonoids and phenolic acids from cranberry juice are bioavailable and bioactive in healthy older adults. Food Chem. 2015, 168, 233–240. [Google Scholar] [CrossRef]
- Wightman, J.D.; Heuberger, R.A. Effect of grape and other berries on cardiovascular health. J. Sci. Food Agric. 2015, 95, 1584–1597. [Google Scholar] [CrossRef]
- Bozzetto, L.; Annuzzi, G.; Pacini, G.; Costabile, G.; Vetrani, C.; Vitale, M.; Griffo, E.; Giacco, A.; De Natale, C.; Cocozza, S.; et al. Polyphenol-rich diets improve glucose metabolism in people at high cardiometabolic risk: A controlled randomised intervention trial. Diabetologia 2015, 58, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Bondia-Pons, I.; Pöhö, P.; Bozzetto, L.; Vetrani, C.; Patti, L.; Aura, A.M.; Annuzzi, G.; Hyötyläinen, T.; Rivellese, A.A.; Orešič, M. Isoenergetic diets differing in their n-3 fatty acid and polyphenol content reflect different plasma and HDL-fraction lipidomic profiles in subjects at high cardiovascular risk. Mol. Nutr. Food Res. 2014, 58, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Rimm, E.; Medina-Remón, A.; Martínez-González, M.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 639–647. [Google Scholar] [CrossRef]
- Arts, I.C.; Hollman, P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef] [PubMed]
- Velderrain-Rodríguez, G.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.; Chen, C.O.; Robles-Sánchez, M.; Astiazaran-García, H.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Phenolic compounds: Their journey after intake. Food Funct. 2014, 5, 189–197. [Google Scholar] [CrossRef]
- Frank, T.; Janßen, M.; Netzel, M.; Straß, G.; Kler, A.; Kriesl, E.; Bitsch, I. Pharmacokinetics of Anthocyanidin-3-Glycosides Following Consumption of Hibiscus sabdariffa L. Extract. J. Clin. Pharmacol. 2005, 45, 203–210. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191. [Google Scholar] [CrossRef] [PubMed]
- Millum, J.; Wendler, D.; Emanuel, E.J. The 50th anniversary of the Declaration of Helsinki: Progress but many remaining challenges. JAMA 2013, 310, 2143–2144. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Noordzij, M.; Tripepi, G.; Dekker, F.W.; Zoccali, C.; Tanck, M.W.; Jager, K.J. Sample size calculations: Basic principles and common pitfalls. Nephrol. Dial. Transplant. 2010, 25, 1388–1393. [Google Scholar] [CrossRef]
- Florey, C. Sample size for beginners. BMJ 1993, 306, 1181. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Coupland, C.; Vinogradova, Y.; Robson, J.; Minhas, R.; Sheikh, A.; Brindle, P. Predicting cardiovascular risk in England and Wales: Prospective derivation and validation of QRISK2. BMJ 2008, 336, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef]
- Barton, M.; Turner, A.T.; Newens, K.J.; Williams, C.M.; Thompson, A.K. Minimum recovery time between reactive hyperemia stimulus in the repeated measurement of brachial flow-mediated dilatation. Ultrasound Med. Biol. 2011, 37, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Kizhakekuttu, T.J.; Gutterman, D.D.; Phillips, S.A.; Jurva, J.W.; Arthur, E.I.; Das, E.; Widlansky, M.E. Measuring FMD in the brachial artery: How important is QRS gating? J. Appl. Physiol. 2010, 109, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Rendeiro, C.; Bergillos-Meca, T.; Tabatabaee, S.; George, T.W.; Heiss, C.; Spencer, J.P. Intake and time dependence of blueberry flavonoid–induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013, 98, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Vafeiadou, K.; Weech, M.; Altowaijri, H.; Todd, S.; Yaqoob, P.; Jackson, K.G.; Lovegrove, J.A. Replacement of saturated with unsaturated fats had no impact on vascular function but beneficial effects on lipid biomarkers, E-selectin, and blood pressure: Results from the randomized, controlled Dietary Intervention and VAScular function (DIVAS) study. Am. J. Clin. Nutr. 2015, 102, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Newens, K.; Thompson, A.; Jackson, K.; Williams, C. Endothelial function and insulin sensitivity during acute non-esterified fatty acid elevation: Effects of fat composition and gender. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 575–581. [Google Scholar] [CrossRef]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef]
- Pedersen, T.; Pedersen, E.; Munk, K.; Hjortdal, V.; Emmertsen, K.; Andersen, N.H. High pulse pressure is not associated with abnormal activation of the renin–angiotensin–aldosterone system in repaired aortic coarctation. J. Hum. Hypertens. 2015, 29, 268–273. [Google Scholar] [CrossRef]
- de Simone, G.; Roman, M.J.; Koren, M.J.; Mensah, G.A.; Ganau, A.; Devereux, R.B. Stroke volume/pulse pressure ratio and cardiovascular risk in arterial hypertension. Hypertension 1999, 33, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Milbury, P.E.; Vita, J.A.; Blumberg, J.B. Anthocyanins are Bioavailable in Humans following an Acute Dose of Cranberry Juice. J. Nutr. 2010, 140, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, J.I.; Momma, T.Y.; Kuhnle, G.K.; Keen, C.L.; Schroeter, H. Structurally related (−)-epicatechin metabolites in humans: Assessment using de novo chemically synthesized authentic standards. Free Radic. Biol. Med. 2012, 52, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.S.; Grisham, M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med. 2007, 43, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Polhemus, D.J.; Li, Z.; Pattillo, C.B.; Goodchild, T.T.; Gojon, G.; Giordano, T.; Krum, H. A Novel Hydrogen Sulfide Prodrug, SG1002, Promotes Hydrogen Sulfide and Nitric Oxide Bioavailability in Heart Failure Patients. Cardiovasc. Ther. 2015, 33, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.G.; Walden, C.M.; Murray, P.; Smith, A.M.; Lovegrove, J.A.; Minihane, A.M.; Williams, C.M. A sequential two meal challenge reveals abnormalities in postprandial TAG but not glucose in men with increasing numbers of metabolic syndrome components. Atherosclerosis 2012, 220, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar]
- Okabe, H.; Uji, Y.; Nagashima, K.; Noma, A. Enzymic determination of free fatty acids in serum. Clin. Chem. 1980, 26, 1540–1543. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- McKay, D.L.; Chen, C.-Y.O.; Saltzman, E.; Blumberg, J.B. Hibiscus sabdariffa, L. Tea (Tisane) Lowers Blood Pressure in Prehypertensive and Mildly Hypertensive Adults. J. Nutr. 2010, 140, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari-Khosravi, H.; Jalali-Khanabadi, B.-A.; Afkhami-Ardekani, M.; Fatehi, F. Effects of sour tea (Hibiscus sabdariffa) on lipid profile and lipoproteins in patients with type II diabetes. J. Altern. Complement. Med. 2009, 15, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Arellano, A.; Flores-Romero, S.; Chávez-Soto, M.A.; Tortoriello, J. Effectiveness and tolerability of a standardized extract from Hibiscus sabdariffa in patients with mild to moderate hypertension: A controlled and randomized clinical trial. Phytomedicine 2004, 11, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Haji Faraji, M.; Haji Tarkhani, A.H. The effect of sour tea (Hibiscus sabdariffa) on essential hypertension. J. Ethnopharmacol. 1999, 65, 231–236. [Google Scholar] [CrossRef]
- Collaboration, P.S. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar]
- George, T.W.; Waroonphan, S.; Niwat, C.; Gordon, M.H.; Lovegrove, J.A. The Glu298Asp single nucleotide polymorphism in the endothelial nitric oxide synthase gene differentially affects the vascular response to acute consumption of fruit and vegetable puree based drinks. Mol. Nutr. Food Res. 2012, 56, 1014–1024. [Google Scholar] [CrossRef]
- George, T.W.; Waroonphan, S.; Niwat, C.; Gordon, M.H.; Lovegrove, J.A. Effects of acute consumption of a fruit and vegetable puree-based drink on vasodilation and oxidative status. Br. J. Nutr. 2013, 109, 1442–1452. [Google Scholar] [CrossRef]
- Fernández-Arroyo, S.; Herranz-López, M.; Beltrán-Debón, R.; Borrás-Linares, I.; Barrajón-Catalán, E.; Joven, J.; Fernández-Gutiérrez, A.; Segura-Carretero, A.; Micol, V. Bioavailability study of a polyphenol-enriched extract from Hibiscus sabdariffa in rats and associated antioxidant status. Mol. Nutr. Food Res. 2012, 56, 1590–1595. [Google Scholar] [CrossRef]
- Hollman, P.C. Absorption, bioavailability, and metabolism of flavonoids. Pharm. Biol. 2004, 42, 74–83. [Google Scholar] [CrossRef]
- Joven, J.; March, I.; Espinel, E.; Fernandez-Arroyo, S.; Rodriguez-Gallego, E.; Aragones, G.; Beltrán-Debón, R.; Alonso-Villaverde, C.; Rios, L.; Martin-Paredero, V.; et al. Hibiscus sabdariffa extract lowers blood pressure and improves endothelial function. Mol. Nutr. Food Res. 2014, 58, 1374–1378. [Google Scholar] [CrossRef]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W.S. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef] [PubMed]

| Breakfast | Lunch | |
|---|---|---|
| Energy (MJ) | 3.21 | 2.36 |
| Fat (Saturated) (g) | 50.1 (32.3) | 24.9 (11.4) |
| Carbohydrate (g) | 70.2 | 70.1 |
| Protein (g) | 8.6 | 12.8 |
| Sodium salt (g) | 1.7 | 1.5 |
| Characteristics | Water Consumption Visit | Hibiscus Consumption Visit | p-Values for Water vs. Hibiscus |
|---|---|---|---|
| CVD risk, age and anthropometric measures | |||
| Age (Years) | 49 ± 2.0 | 49 ± 2.0 | 1 |
| BMI (kg/m2) | 26.9 ± 0.7 | 26.7 ± 0.8 | 0.227 |
| Blood pressure and vascular function measures | |||
| SBP (mmHg) | 126± 3 | 129 ± 3 | 0.063 |
| DBP (mmHg) | 74 ± 2 | 75 ± 2 | 0.225 |
| Pulse Pressure (mmHg) | 52 ± 2 | 54 ± 2 | 0.381 |
| Heart Rate (Pulse/minute) | 63 ± 3 | 65 ± 3 | 0.788 |
| FMD (%) | 3.67 ± 0.3 | 3.36 ± 0.2 | 0.838 |
| Augmentation Index | 3.45 ± 1.00 | 3.51 ± 0.9 | 0.371 |
| Biochemical data | |||
| TC (mmol/L) | 5.35 ± 0.2 | 5.37 ± 0.2 | 0.679 |
| HDL-C (mmol/L) | 1.29 ± 0.1 | 1.32 ± 0.1 | 0.645 |
| TC:HDL-C | 4.30 ± 0.2 | 4.23 ± 0.2 | 0.881 |
| LDL-C (mmol/L) | 3.28 ± 0.2 | 3.32 ± 0.2 | 0.67 |
| TAG (mmol/L) | 1.71 ± 0.3 | 1.62 ± 0.2 | 0.901 |
| NEFA (mmol/L) | 454.9 ± 36.4 | 454.9 ± 38.7 | 0.819 |
| Glucose (mmol/L) | 5.51 ± 0.1 | 5.44 ± 0.1 | 0.843 |
| Insulin (pmol/L) | 51.90 ± 7.9 | 55.34 ± 9.8 | 0.449 |
| HOMA2 IR | 0.99 ± 0.2 | 1.05 ± 0.2 | 0.465 |
| SBP | DBP | Heart Rate | Pulse Pressure | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hibiscus | Water | p Value | Hibiscus | Water | p Value | Hibiscus | Water | p Value | Hibiscus | Water | p Value | |
| AUC0–240min | 31,134 ± 496 | 30,992 ± 571 | 0.426 | 17,924 ± 325 | 17,692 ± 338 | 0.312 | 14,580 ± 341 | 14,347 ± 374 | 0.324 | 13,210 ± 383 | 13,300 ± 413 | 0.437 |
| IAUC0–240min | 219 ± 361 | 658 ± 346 | 0.193 | −86 ± 217 | −61 ± 183 | 0.466 | −343 ± 632 | −690 ± 416 | 0.345 | 305 ± 283 | 719 ± 351 | 0.182 |
| Tmax0–240min | 142 ± 16 | 108 ± 18 | 0.083 | 101 ± 18 | 119 ± 17 | 0.237 | 95 ± 20 | 65 ± 17 | 0.133 | 138 ± 18 | 109 ± 18 | 0.130 |
| Pmax0–240min | 138 ± 3 | 135 ± 3 | 0.191 | 79 ± 1 | 78 ± 2 | 0.325 | 68 ± 2 | 66 ± 2 | 0.246 | 62 ± 2 | 61 ± 2 | 0.343 |
| Tmin0–240min | 95 ± 18 | 90 ± 19 | 0.428 | 130 ± 16 | 119 ± 18 | 0.328 | 91 ± 14 | 112 ± 17 | 0.179 | 92 ± 17 | 82 ± 20 | 0.344 |
| Pmin0–240min | 123 ± 2 | 122 ± 2 | 0.425 | 69 ± 1 | 69 ± 1 | 0.387 | 57 ± 1 | 56 ± 2 | 0.300 | 50 ± 1 | 49 ± 2 | 0.424 |
| Glucose | Insulin | TAG | TAC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hibiscus | Water | p Value | Hibiscus | Water | p Value | Hibiscus | Water | p Value | Hibiscus | Water | p Value | |
| AUC 0–240 min | 1384 ± 47 | 1455 ± 56 | 0.058 | 49,242 ± 8666 | 50,017 ± 7661 | 0.424 | 547± 55 | 605 ± 71 | 0.188 | 343 ± 50 | 318 ± 40 | 0.105 |
| IAUC 0–240 min | 80 ± 40 | 134 ± 51 | 0.090 | 35,962 ± 6588 | 37,561 ± 6554 | 0.465 | 157 ± 28 | 196 ± 26 | 0.128 | 79.0 ± 38 | 75.8 ± 25 | 0.478 |
| Tmax 0–240 min | 139 ± 14.5 | 135 ± 15 | 0.408 | 126 ± 13 | 123 ± 14 | 0.500 | 196 ± 6 | 196 ± 6 | 0.380 | 153 ± 14 | 175 ± 10 | 0.100 |
| Cmax 0–240 min | 7.5 ± 0.3 | 7.6 ± 0.3 | 0.052 | 387 ± 61 | 403 ± 49 | 0.482 | 3.3 ± 0.3 | 3.6 ± 0.4 | 0.246 | 2.2 ± 0.3 | 2.3 ± 0.4 | 0.460 |
| AUC 0–120 min | 706 ± 23 | 722 ± 30 | 0.143 | 23,629 ± 3932 | 23,388 ± 3551 | 0.303 | 222 ± 23 | 324 ± 34 | 0.143 | 150 ± 17 * | 130 ± 11 | 0.026 |
| IAUC 0–120 min | 53.8 ± 19.3 | 61.8 ± 24.6 | 0.209 | 16,988 ± 2935 | 17,160 ± 3100 | 0.383 | 27.5 ± 6.8 | 46.1 ± 19.5 | 0.209 | 18.4 ± 10.9 | 9.4 ± 5.3 | 0.209 |
| Tmax0–120 min | 68.4 ± 5.3 | 53.2 ± 5.2 | 0.059 | 64.4 ± 4.3 | 65.5 ± 5.1 | 0.191 | 110 ± 5 | 196 ± 6 | 0.060 | 96.3 ± 7.4 * | 75.0 ± 8.1 | 0.007 |
| Cmax0–120 min | 6.9 ± 0.3 | 7.1 ± 0.3 | 0.201 | 339 ± 60 | 338 ± 49 | 0.344 | 2.4 ± 0.2 | 3.3 ± 0.3 | 0.201 | 1.7 ± 0.3 | 1.4 ± 0.1 | 0.056 |
| AUC 121–240 min | 678 ± 37 | 733± 31 | 0.065 | 25,614 ± 4838 | 26,629 ± 4288 | 0.472 | 239 ± 29 | 365 ± 43 | 0.065 | 192 ± 33 | 187 ± 30 | 0.340 |
| IAUC 121–240 min | 95 ± 36 | 109 ± 33 | 0.272 | 10,457 ± 2477 | 10,782 ± 2141 | 0.449 | 34.7 ± 7.9 | 76.3 ± 16.2 | 0.272 | 21.5 ± 16.7 | 50.9 ± 15.6 | 0.067 |
| Tmax121–240 min | 189 ± 7 | 188± 6 | 0.398 | 185 ± 5 | 177 ± 4 | 0.072 | 108 ± 6 | 196 ± 6 | 0.398 | 176 ± 8 | 187 ± 8 | 0.140 |
| Cmax121–240 min | 7.2 ± 0.3 * | 7.5 ± 0.3 | 0.040 | 365 ± 61 | 347 ± 46 | 0.218 | 2.5 ± 0.3 | 3.6 ± 0.4 | 0.040 | 2.1 ± 0.4 | 2.2 ± 0.4 | 0.440 |
| Hibiscus | Water | p Value | |
|---|---|---|---|
| Tmin0–90 min | 76.8 ± 4.3 | 76.4 ± 4.7 | 0.413 |
| Cmin0–90 min | 230 ± 16 | 221 ± 14 | 0.367 |
| AUC 91–240 min | 46,593 ± 3313 | 46,523 ± 3284 | 0.462 |
| IAUC 91–240 min | 9665 ± 2711 | 10,364 ± 2507 | 0.388 |
| Tmax91–240 min | 186 ± 9 | 195 ± 9 | 0.216 |
| Cmax91–240 min | 423 ± 26 | 410 ± 25 | 0.317 |
| Tmin91–240 min | 127 ± 10 | 130 ± 12 | 0.198 |
| Cmin91–240 min | 216 ± 15 | 215 ± 14 | 0.459 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abubakar, S.M.; Ukeyima, M.T.; Spencer, J.P.E.; Lovegrove, J.A. Acute Effects of Hibiscus sabdariffa Calyces on Postprandial Blood Pressure, Vascular Function, Blood Lipids, Biomarkers of Insulin Resistance and Inflammation in Humans. Nutrients 2019, 11, 341. https://doi.org/10.3390/nu11020341
Abubakar SM, Ukeyima MT, Spencer JPE, Lovegrove JA. Acute Effects of Hibiscus sabdariffa Calyces on Postprandial Blood Pressure, Vascular Function, Blood Lipids, Biomarkers of Insulin Resistance and Inflammation in Humans. Nutrients. 2019; 11(2):341. https://doi.org/10.3390/nu11020341
Chicago/Turabian StyleAbubakar, Salisu M., Moses T. Ukeyima, Jeremy P. E. Spencer, and Julie A. Lovegrove. 2019. "Acute Effects of Hibiscus sabdariffa Calyces on Postprandial Blood Pressure, Vascular Function, Blood Lipids, Biomarkers of Insulin Resistance and Inflammation in Humans" Nutrients 11, no. 2: 341. https://doi.org/10.3390/nu11020341
APA StyleAbubakar, S. M., Ukeyima, M. T., Spencer, J. P. E., & Lovegrove, J. A. (2019). Acute Effects of Hibiscus sabdariffa Calyces on Postprandial Blood Pressure, Vascular Function, Blood Lipids, Biomarkers of Insulin Resistance and Inflammation in Humans. Nutrients, 11(2), 341. https://doi.org/10.3390/nu11020341





