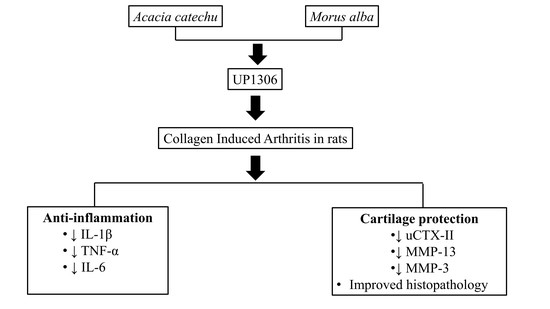
UP1306: A Composition Containing Standardized Extracts of Acacia catechu and Morus alba for Arthritis Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Composition
2.2. Model Induction and Treatment
2.3. Clinical Observation
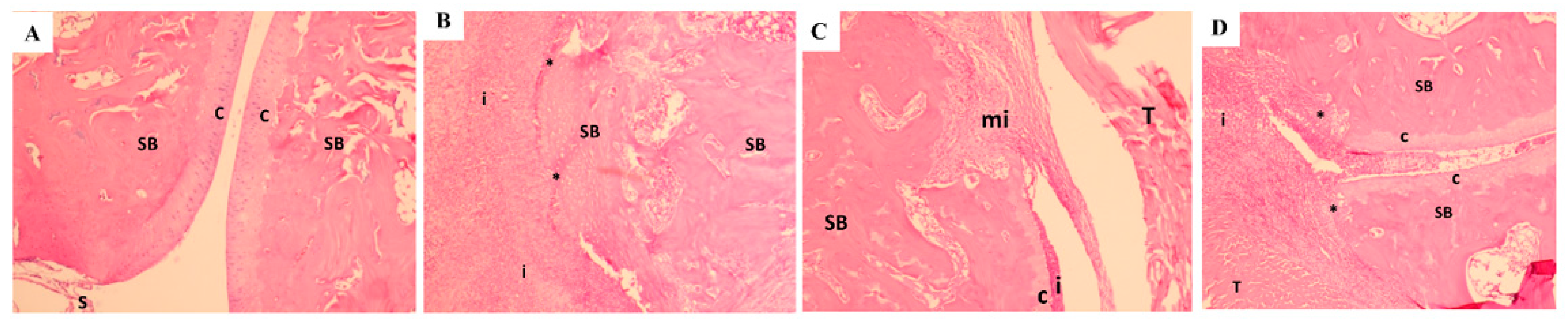
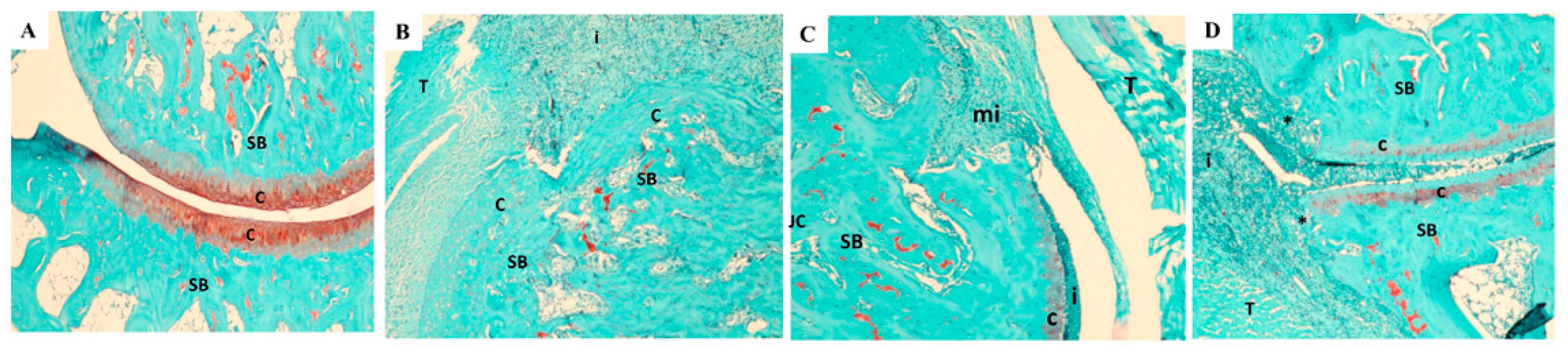
2.4. Histopathology
2.5. Assays
2.5.1. Urine CTX-II-
2.5.2. Serum IL-1β, TNF-α and IL-6 ELISA
2.5.3. Synovial MMP-13 and Serum MMP-3 ELISA
2.6. Statistical Analysis
3. Results
3.1. Arthritis Severity Index
3.2. Ankle Diameter
3.3. Paw Thickness
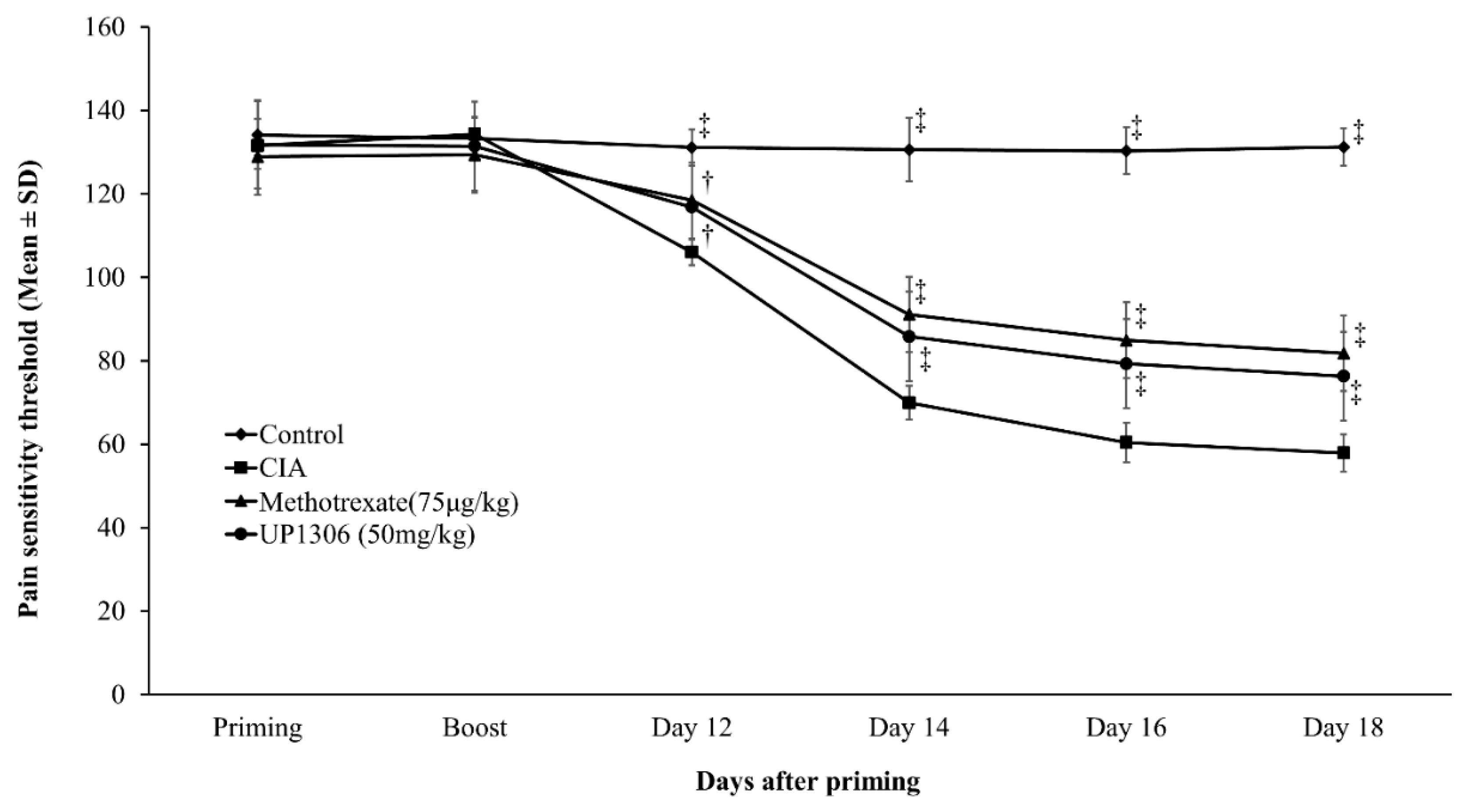
3.4. Pain Sensitivity
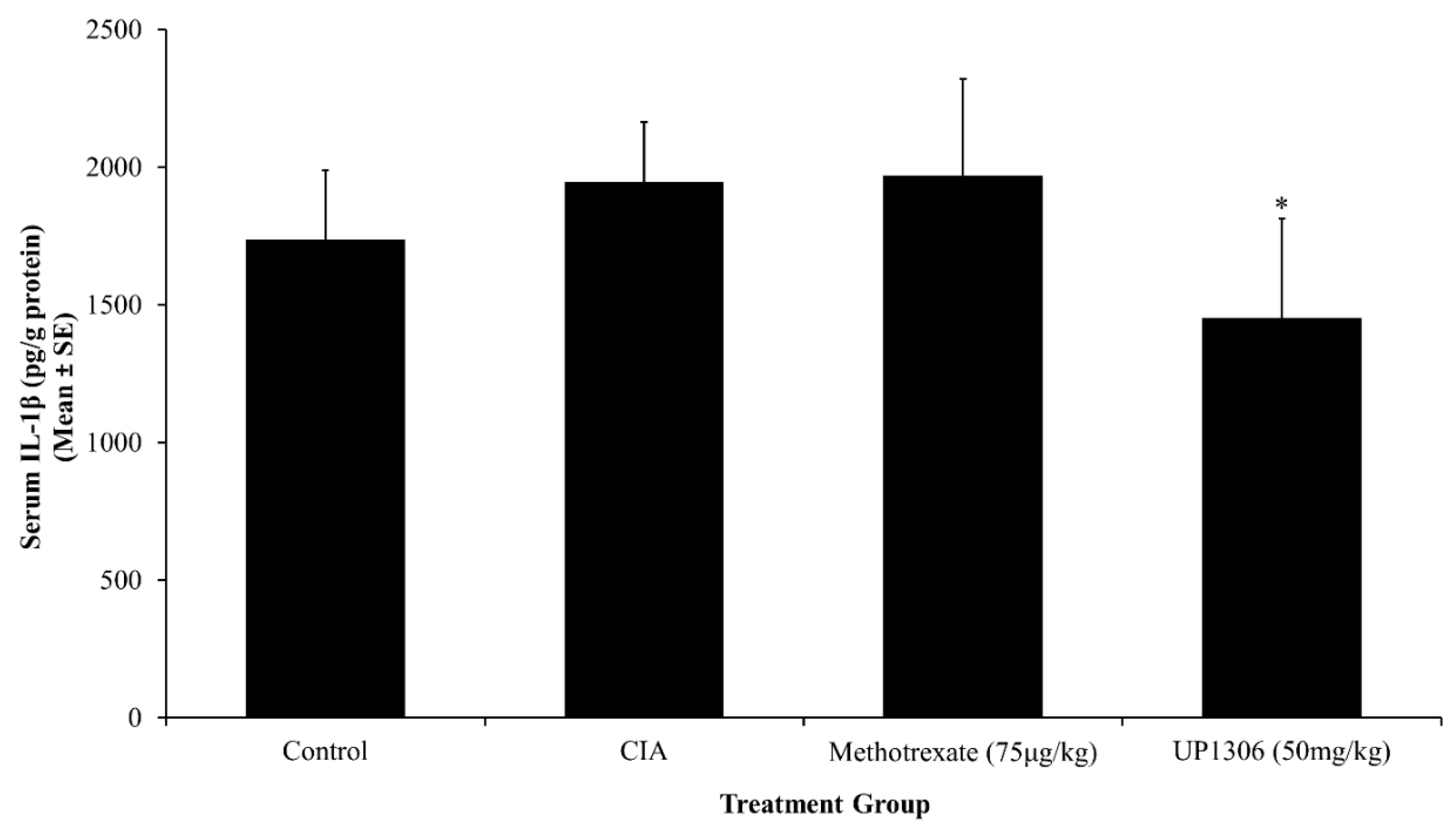
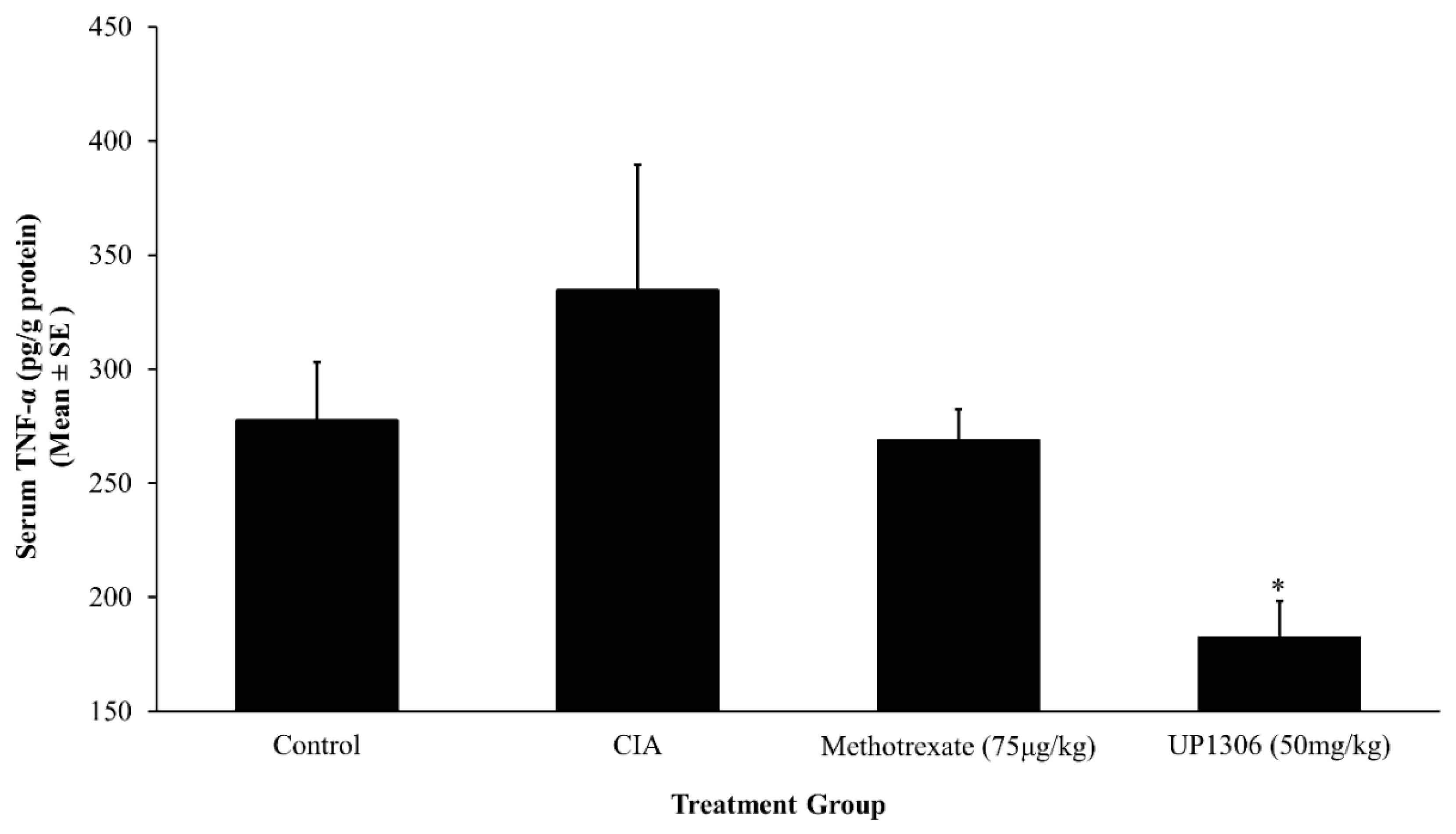
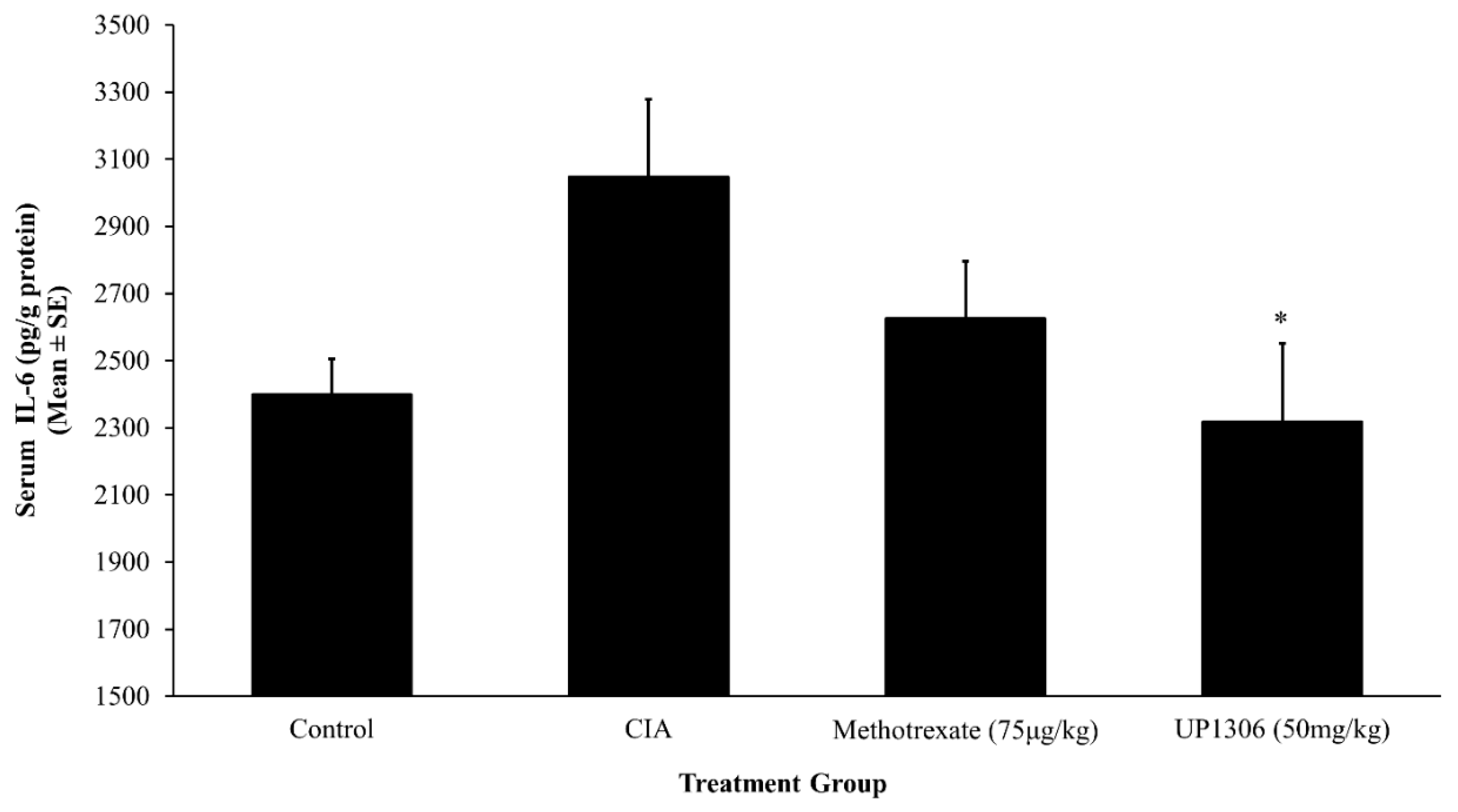
3.5. Serum Pro-Inflammatory Cytokines
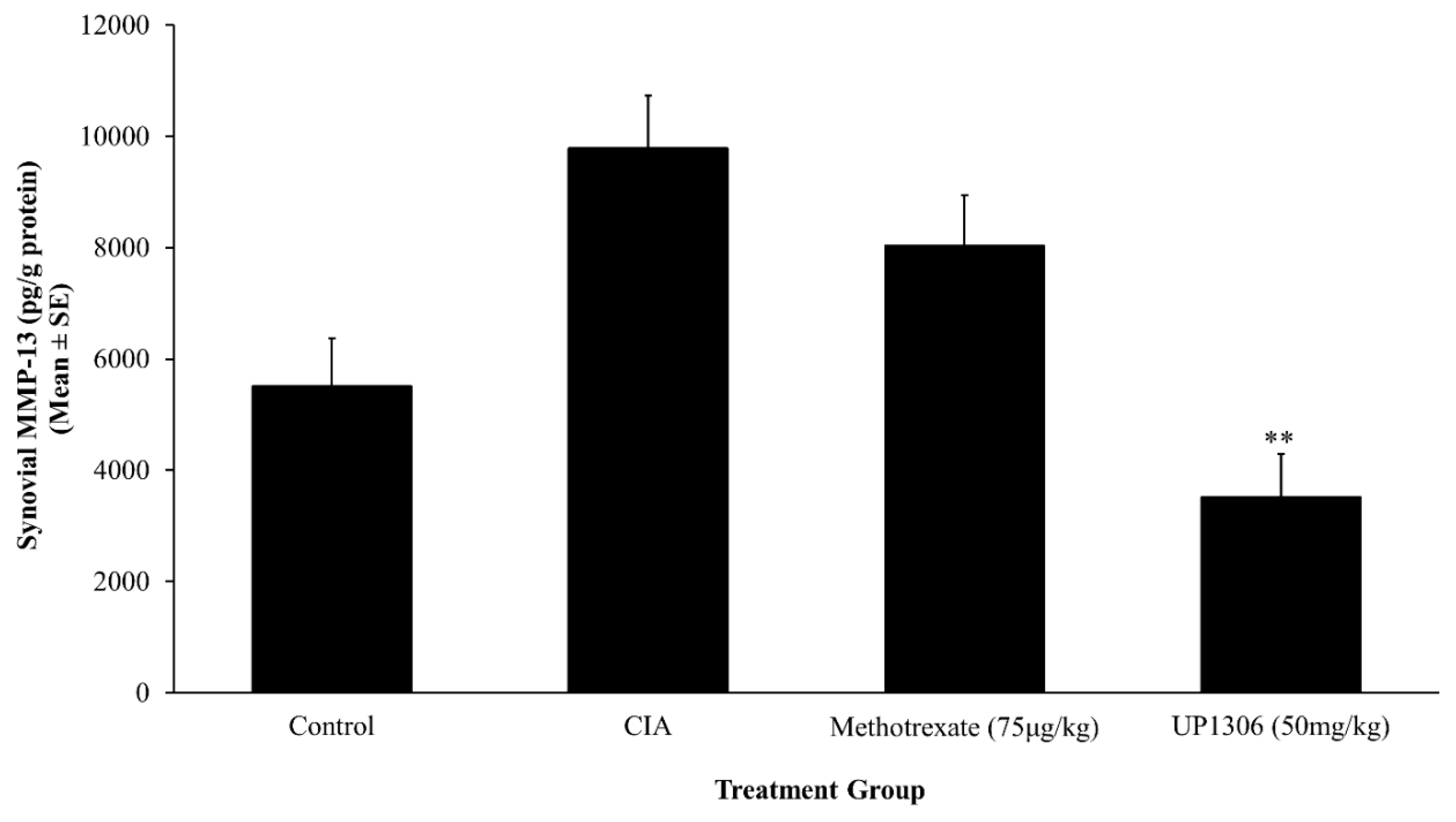
3.6. Synovial MMP-13
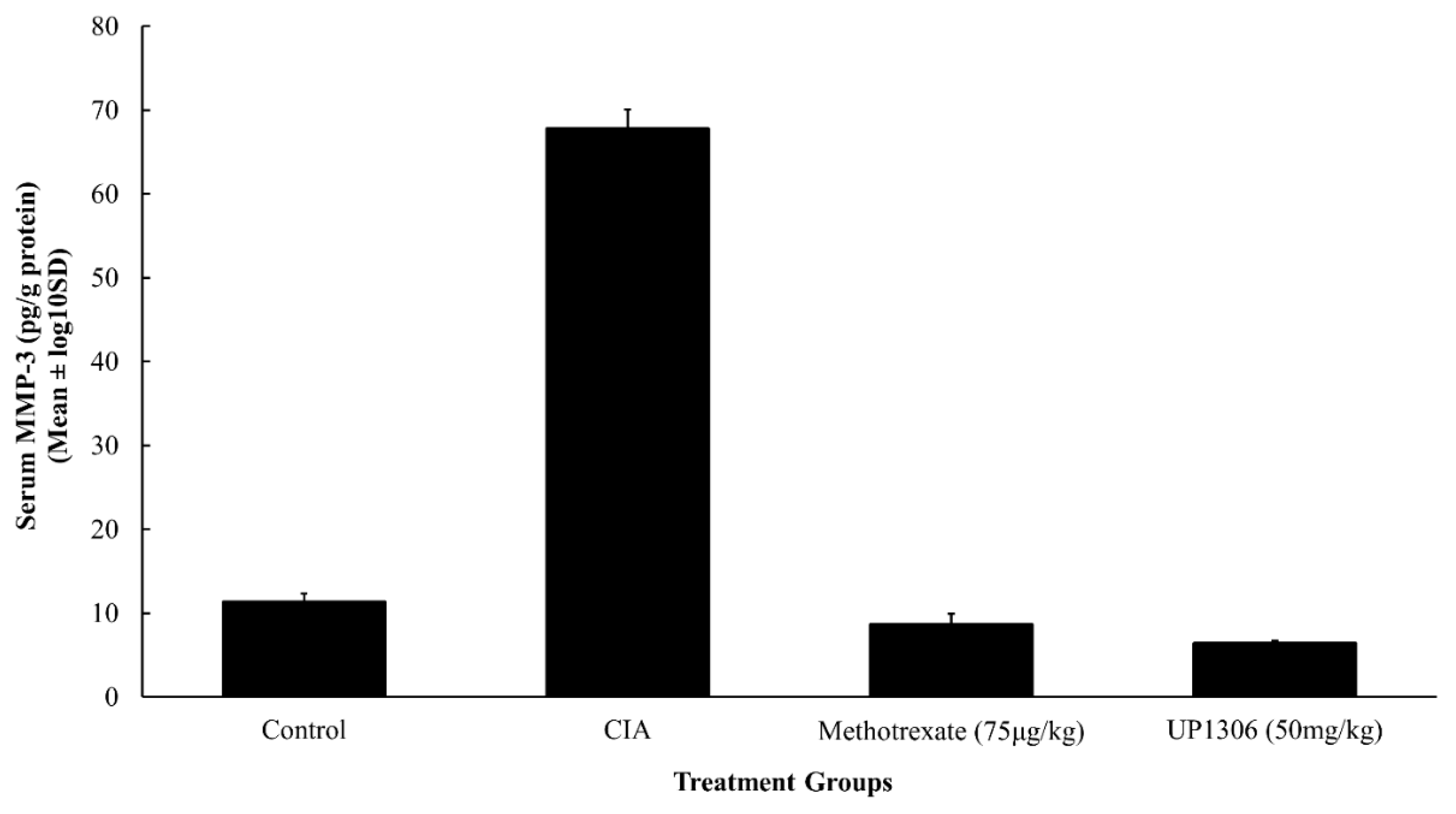
3.7. Serum MMP-3
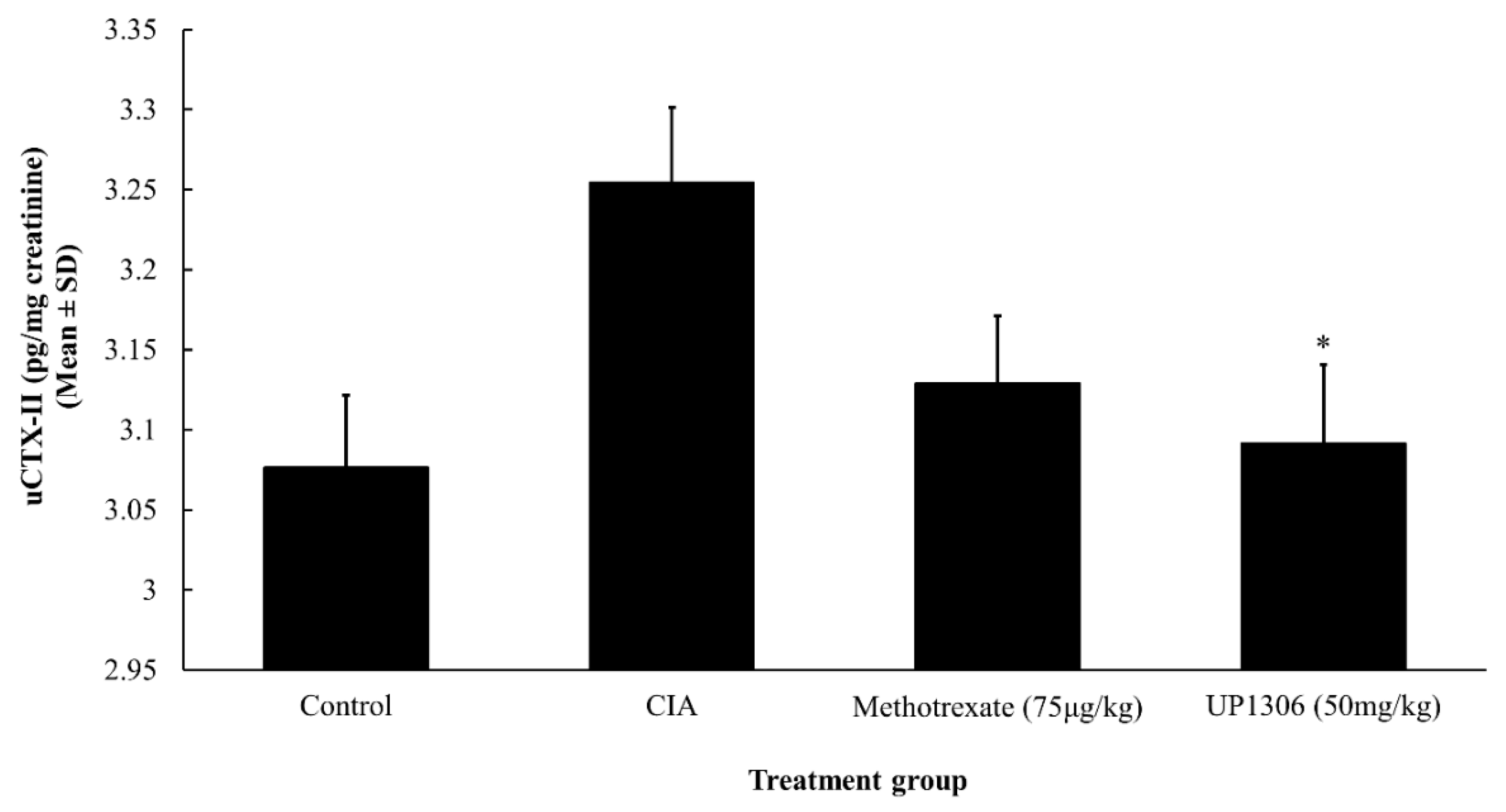
3.8. Urinary CTX-II
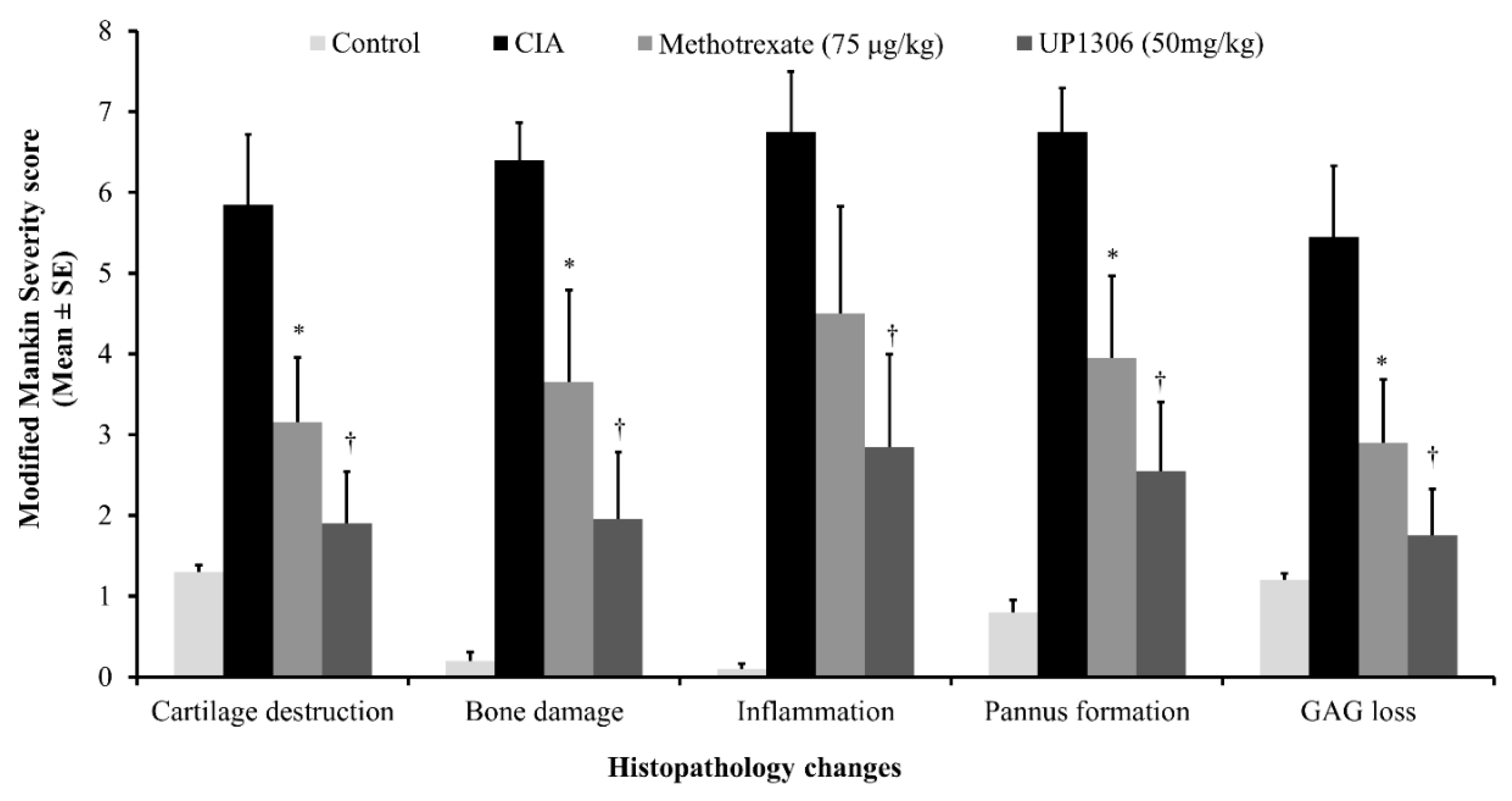
3.9. Histopathology Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ashford, S.; Williard, J. Osteoarthritis: A review. Nurse Pract. 2014, 39, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hootman, J.M.; Helmick, C.G.; Barbour, K.E.; Theis, K.A.; Boring, M.A. Updated Projected Prevalence of Self-Reported Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation Among US Adults, 2015–2040. Arthritis Rheumatol. 2016, 68, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Bozimowski, G. A Review of Nonsteroidal Anti-inflammatory Drugs. AANA J. 2015, 83, 425–433. [Google Scholar] [PubMed]
- Gunjal, S.; Ankola, A.V.; Bhat, K. In vitro antibacterial activity of ethanolic extract of Morus alba leaf against periodontal pathogens. Indian J. Dent. Res. 2015, 26, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.T.; Ganeshan, A.K.; Chen, C.; Jin, C.; Li, S.H.; Chen, H.J.; Gui, Z. In vitro and In vivo Antioxidant Activity of Flavonoid Extracted from Mulberry Fruit (Morus alba L.). Pharmacogn Mag. 2016, 12, 128–133. [Google Scholar] [PubMed]
- Wang, Y.; Xiang, L.; Wang, C.; Tang, C.; He, X. Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (Morus alba L.) polyphenol enhanced extract. PLoS ONE 2013, 8, e71144. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.P.; Kim, J.K.; Lim, Y.H. Antihyperlipidemic effects of stilbenoids isolated from Morus alba in rats fed a high-cholesterol diet. Food Chem. Toxicol. 2014, 65, 213–218. [Google Scholar] [CrossRef] [PubMed]
- El-Beshbishy, H.A.; Singab, A.N.; Sinkkonen, J.; Pihlaja, K. Hypolipidemic and antioxidant effects of Morus alba L. (Egyptian mulberry) root bark fractions supplementation in cholesterol-fed rats. Life Sci. 2006, 78, 2724–2733. [Google Scholar] [CrossRef]
- Eo, H.J.; Park, J.H.; Park, G.H.; Lee, M.H.; Lee, J.R.; Koo, J.S.; Jeong, J.B. Anti-inflammatory and anti-cancer activity of mulberry (Morus alba L.) root bark. BMC Complement. Altern. Med. 2014. [Google Scholar] [CrossRef]
- Chan, E.W.; Lye, P.Y.; Wong, S.K. Phytochemistry, pharmacology, and clinical trials of Morus alba. Chin. J. Nat. Med. 2016, 14, 17–30. [Google Scholar]
- Lee, J.H.; Ko, H.J.; Woo, E.R.; Lee, S.K.; Moon, B.S.; Lee, C.W.; Mandava, S.; Samala, M.; Lee, J.; Kim, H.P. Moracin M inhibits airway inflammation by interrupting the JNK/c-Jun and NF-κB pathways in vitro and in vivo. Eur J. Pharmacol. 2016, 783, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, A.R.; Park, H.J.; Park, D.K.; Kim, D.K.; Ko, N.Y.; Kim, B.; Choi, D.K.; Won, H.S.; Shin, W.S.; et al. Morus bombycis Koidzumi extract suppresses collagen-induced arthritis by inhibiting the activation of nuclear factor-κB and activator protein-1 in mice. J. Ethnopharmacol. 2011, 136, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Jin, H.G.; Woo, E.R.; Lee, S.K.; Kim, H.P. The root barks of Morus alba and the flavonoid constituents inhibit airway inflammation. J. Ethnopharmacol. 2013, 149, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Zelová, H.; Hanáková, Z.; Čermáková, Z.; Šmejkal, K.; Dalĺ Acqua, S.; Babula, P.; Cvačka, J.; Hošek, J. Evaluation of anti-inflammatory activity of prenylated substances isolated from Morus alba and Morus nigra. J. Nat Prod. 2014, 77, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Naik, G.H.; Priyadarsini, K.I.; Satav, J.G.; Banavalikar, M.M.; Sohoni, D.P.; Biyani, M.K.; Mohan, H. Comparative antioxidant activity of individual herbal components used in Ayurvedic medicine. Phytochemistry 2003, 63, 97–104. [Google Scholar] [CrossRef]
- Hazra, B.; Sarkar, R.; Ghate, N.B.; Chaudhuri, D.; Mandal, N. Study of the protective effects of Katha (Heartwood Extract of Acacia catechu) in liver damage induced by iron overload. J. Environ. Pathol. Toxicol. Oncol. 2013, 32, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Guleria, S.; Tiku, A.K.; Singh, G.; Vyas, D.; Bhardwaj, A. Antioxidant activity and protective effect against plasmid DNA strand scission of leaf, bark, and heartwood extracts from Acacia catechu. J. Food Sci. 2011, 76, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Nadumane, V.K.; Nair, S. Evaluation of the anticancer and cytotoxic potentials of Acacia catechu extracts in vitro. J. Nat. Pharm. 2011, 2, 190–195. [Google Scholar] [CrossRef]
- Jarald, E.; Joshi, S.B.; Jain, D.C. Biochemical study on the hypoglycemic effects of extract and fraction of Acacia catechu Willd in alloxan-induced diabetic rats. Int. J. Diabetes Metabol. 2009, 17, 63–69. [Google Scholar]
- Gunindro, N.; Devi, K.P.; Singh, T.I. Effects of Acacia catechu on intestinal absorption of glucose in rats. J. Chem. Pharm. Res. 2013, 5, 78–81. [Google Scholar]
- Ray, D.; Sharatchandra, K.; Thokchom, I.S. Antipyretic, antidiarrheal, hypoglycemic and hepatoprotective activities of ethyl acetate extract of Acacia catechu Willd. in albino rats. Indian J. Pharmacol. 2006, 38, 408–413. [Google Scholar] [CrossRef]
- Kuang, X.; Huang, Y.; Gu, H.F.; Zu, X.Y.; Zou, W.Y.; Song, Z.B.; Guo, Q.L. Effects of intrathecal epigallocatechin gallate, an inhibitor of Toll-like receptor 4, on chronic neuropathic pain in rats. Eur. J. Pharmacol. 2012, 676, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Rahmatullah, M.; Hossain, M.; Mahmud, A.; Sultana, N.; Rahman, S.M.; Islam, M.R.; Khatoon, M.S.; Jahan, S.; Islam, F. Antihyperglycemic and antinociceptive activity evaluation of “khoyer” prepared from boiling the wood of Acacia catechu in water. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Monga, J.; Chauhan, C.S.; Sharma, M. Human breast adenocarcinoma cytotoxicity and modulation of 7,12-dimethylbenz[a]anthracene-induced mammary carcinoma in Balb/c mice by Acacia catechu (L.f.) Wild heartwood. Integr. Cancer Ther. 2013, 12, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.D.; Kumar, V.; Bhatt, S.A. Antimicrobial screening and phytochemical analysis of the resin part of Acacia catechu. Pharmaceut. Biol. 2009, 47, 34–37. [Google Scholar] [CrossRef]
- Zhong, Y.; Chiou, Y.S.; Pan, M.H.; Shahidi, F. Anti-inflammatory activity of lipophilic epigallocatechin gallate (EGCG) derivatives in LPS-stimulated murine macrophages. Food Chem. 2012, 134, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Yimam, M.; Lee, Y.C.; Jiao, P.; Hong, M.; Nam, J.B.; Brownell, L.; Hyun, E.; Jia, Q. UP1306, a Botanical Composition with Analgesic and Anti-inflammatory Effect. Pharmacogn. Res. 2016, 8, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Yimam, M.; Lee, Y.C.; Wright, L.; Jiao, P.; Horm, T.; Hong, M.; Brownell, L.; Jia, Q. A Botanical Composition Mitigates Cartilage Degradations and Pain Sensitivity in Osteoarthritis Disease Model. J. Med. Food 2017, 20, 568–576. [Google Scholar] [CrossRef]
- Kalman, D.S.; Hewlings, S.J. The Effects of Morus alba and Acacia catechu on Quality of Life and Overall Function in Adults with Osteoarthritis of the Knee. J. Nutr. Metab. 2017, 2017, 4893104. [Google Scholar] [CrossRef]
- Cho, Y.G.; Cho, M.L.; Min, S.Y.; Kim, H.Y. Type II collagen autoimmunity in a mouse model of human rheumatoid arthritis. Autoimmun. Rev. 2007, 7, 65–70. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Martinez-Cutillas, J.; Alerany-Pardo, C.; Borrás-Blasco, J.; Broto-Sumalla, A.; Burgos-SanJosé, A.; Climent-Bolta, C.; Fernández-Fuente, M.A.; Ferrit-Martin, M.; Gómez-Germá, P.; Martínez-Sesmero, J.M.; et al. The use of adalimumab, etanercept, golimumab and infliximab in rheumatic pathologies: Variation between label dosage and real-world use. Expert Rev. Pharmacoecon. Outcomes Res. 2015, 15, 851–858. [Google Scholar] [CrossRef]
- Koenders, M.I.; van den Berg, W.B. Novel therapeutic targets in rheumatoid arthritis. Trends Pharmacol. Sci. 2015, 36, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Sandborg, C.; Mellins, E.D. A new era in the treatment of systemic juvenile idiopathic arthritis. N. Engl. J. Med. 2012, 367, 2439–2440. [Google Scholar] [CrossRef] [PubMed]
- Siebuhr, A.S.; Wang, J.; Karsdal, M.; Bay-Jensen, A.C.; Jin, Y.; Zheng, Q. Matrix metalloproteinase-dependent turnover of cartilage, synovial membrane, and connective tissue is elevated in rats with collagen induced arthritis. J. Transl. Med. 2012, 10, 195. [Google Scholar] [CrossRef]
- Sumeet, G.; Rachna, K.; Samrat, C.; Ipshita, C.; Vikas, J.; Manu, S. Anti-Inflammatory and Anti Arthritic Activity of Different Milk Based Formulation of Curcumin in Rat Model. Curr. Drug Deliv. 2018, 15, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; Umar, K.; Sarwar, A.H.; Khan, A.; Ahmad, N.; Ahmad, S.; Katiyar, C.K.; Husain, S.A.; Khan, H.A. Boswellia serrata extract attenuates inflammatory mediators and oxidative stress in collagen induced arthritis. Phytomedicine 2014, 21, 847–856. [Google Scholar] [CrossRef]
- Brand, D.D.; Kang, A.H.; Rosloniec, E.F. Immunopathogenesis of collagen arthritis. Springer Semin. Immunopathol. 2003, 25, 3–18. [Google Scholar] [CrossRef]
- Rosloniec, E.F.; Cremer, M.; Kang, A.H.; Myers, L.K.; Brand, D.D. Collagen-induced arthritis. Curr. Protoc. Immunol. 2001. [Google Scholar] [CrossRef]
- Mankin, H.J.; Dorfman, H.; Lippiello, L.; Zarins, A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J. Bone Jt. Surg. Am. 1971, 53, 523–537. [Google Scholar] [CrossRef]
- Miyoshi, M.; Liu, S. Collagen-Induced Arthritis Models. Methods Mol. Biol. 2018, 1868, 3–7. [Google Scholar] [PubMed]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Saklatvala, J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature 1986, 322, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Fukuo, K.; Birkhead, J.R.; Dudek, E.; Sandell, L.J. Transcriptional suppression by interleukin-1 and interferon-gamma of type II collagen gene expression in human chondrocytes. J. Cell Biochem. 1994, 54, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, V.; Peeters-Joris, C.; Vaes, G. Modulation by interleukin 1 and tumor necrosis factor alpha of production of collagenase, tissue inhibitor of metalloproteinases and collagen types in differentiated and dedifferentiated articular chondrocytes. Biochim. Biophys. Acta 1990, 1052, 366–378. [Google Scholar] [CrossRef]
- Guerne, P.A.; Carson, D.A.; Lotz, M. IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J. Immunol. 1990, 144, 499–505. [Google Scholar] [PubMed]
- Mathy-Hartert, M.; Hogge, L.; Sanchez, C.; Deby-Dupont, G.; Crielaard, J.M.; Henrotin, Y. Interleukin-1beta and interleukin-6 disturb the antioxidant enzyme system in bovine chondrocytes: A possible explanation for oxidative stress generation. Osteoarthr. Cartil. 2008, 16, 756–763. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta 2016, 1862, 576–591. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Harvey, A.K.; Hrubey, P.S. Intra-articular administration of interleukin-1 causes prolonged suppression of cartilage proteoglycan synthesis in rats. Matrix 1992, 12, 1–10. [Google Scholar] [CrossRef]
- Bolon, B.; Campagnuolo, G.; Zhu, L.; Duryea, D.; Zack, D.; Feige, U. Interleukin-1beta and tumor necrosis factor-alpha produce distinct, time-dependent patterns of acute arthritis in the rat knee. Vet. Pathol. 2004, 41, 235–243. [Google Scholar] [CrossRef]
- Joosten, L.A.; Helsen, M.M.; Saxne, T.; van De Loo, F.A.; Heinegard, D.; van Den Berg, W.B. IL-1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammation. J. Immunol. 1999, 163, 5049–5055. [Google Scholar] [PubMed]
- Kobayashi, M.; Squires, G.R.; Mousa, A.; Tanzer, M.; Zukor, D.J.; Antoniou, J.; Feige, U.; Poole, A.R. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005, 52, 128–135. [Google Scholar] [CrossRef] [PubMed]
- van de Loo, F.A.; Arntz, O.J.; Otterness, I.G.; van den Berg, W.B. Protection against cartilage proteoglycan synthesis inhibition by antiinterleukin 1 antibodies in experimental arthritis. J. Rheumatol. 1992, 19, 348–356. [Google Scholar] [PubMed]
- Wei, S.T.; Sun, Y.H.; Zong, S.H.; Xiang, Y.B. Serum Levels of IL-6 and TNF-α May Correlate with Activity and Severity of Rheumatoid Arthritis. Med. Sci. Monit. 2015, 21, 4030–4038. [Google Scholar] [CrossRef] [PubMed]
- Fosang, A.J.; Stanton, H.; Little, C.B.; Atley, L.M. Neoepitopes as biomarkers of cartilage catabolism. Inflamm. Res. 2003, 52, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Rose, B.J.; Kooyman, D.L. A Tale of Two Joints: The Role of Matrix Metalloproteases in Cartilage Biology. Dis. Markers 2016, 2016, 4895050. [Google Scholar] [CrossRef]
- Yamanaka, H.; Matsuda, Y.; Tanaka, M.; Sendo, W.; Nakajima, H.; Taniguchi, A.; Kamatani, N. Serum matrix metalloproteinase 3 as a predictor of the degree of joint destruction during the six months after measurement, in patients with early rheumatoid arthritis. Arthritis Rheum. 2000, 43, 852–858. [Google Scholar] [CrossRef]
- Galil, S.M.; El-Shafey, A.M.; Hagrass, H.A.; Fawzy, F.; Sammak, A.E. Baseline serum level of matrix metalloproteinase-3 as a biomarker of progressive joint damage in rheumatoid arthritis patients. Int. J. Rheum. Dis. 2016, 19, 377–384. [Google Scholar] [CrossRef]
- Ma, M.J.; Liu, H.C.; Qu, X.Q.; Wang, J.L. Matrix metalloproteinase-3 gene polymorphism and its mRNA expression in rheumatoid arthritis. Genet. Mol. Res. 2015, 14, 15652–15659. [Google Scholar] [CrossRef]
- Oestergaard, S.; Chouinard, L.; Doyle, N.; Smith, S.Y.; Tankó, L.B.; Qvist, P. Early elevation in circulating levels of C-telopeptides of type II collagen predicts structural damage in articular cartilage in the rodent model of collagen-induced arthritis. Arthritis Rheum. 2006, 54, 2886–2890. [Google Scholar] [CrossRef]
- Garnero, P.; Piperno, M.; Gineyts, E.; Christgau, S.; Delmas, P.D.; Vignon, E. Cross sectional evaluation of biochemical markers of bone, cartilage, and synovial tissue metabolism in patients with knee osteoarthritis: Relations with disease activity and joint damage. Ann. Rheum. Dis. 2001, 60, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Oestergaard, S.; Chouinard, L.; Doyle, N.; Karsdal, M.A.; Smith, S.Y.; Qvist, P.; Tankó, L.B. The utility of measuring C-terminal telopeptides of collagen type II (CTX-II) in serum and synovial fluid samples for estimation of articular cartilage status in experimental models of destructive joint diseases. Osteoarthr. Cartil. 2006, 14, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef] [PubMed]
- Kalaiselvi, P.; Rajashree, K.; Bharathi Priya, L.; Padma, V.V. Cytoprotective effect of epigallocatechin-3-gallate against deoxynivalenol-induced toxicity through anti-oxidative and anti-inflammatory mechanisms in HT-29 cells. Food Chem. Toxicol. 2013, 56, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.A.; Choi, J.H.; Rhee, S.J. Effects of green tea catechin on phospholipase A2 activity and antithrombus in streptozotocin diabetic rats. J. Nutr. Sci. Vitaminol. (Tokyo) 1999, 45, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Hošek, J.; Bartos, M.; Chudík, S.; Dall’Acqua, S.; Innocenti, G.; Kartal, M.; Kokoška, L.; Kollár, P.; Kutil, Z.; Landa, P.; et al. Natural compound cudraflavone B shows promising anti-inflammatory properties in vitro. J. Nat. Prod. 2011, 74, 614–619. [Google Scholar]
- Chen, Y.C.; Tien, Y.J.; Chen, C.H.; Beltran, F.N.; Amor, E.C.; Wang, R.J.; Wu, D.J.; Mettling, C.; Lin, Y.L.; Yang, W.C. Morus alba and active compound oxyresveratrol exert anti-inflammatory activity via inhibition of leukocyte migration involving MEK/ERK signaling. BMC Complement. Altern. Med. 2013, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.O.; Kim, B.Y.; Lee, M.H.; Kim, Y.R.; Chung, H.Y.; Park, J.H.; Moon, J.O. In vitro and in vivo anti-inflammatory effect of oxyresveratrol from Morus alba L. J. Pharm. Pharmacol. 2003, 55, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Cheon, B.S.; Kim, Y.H.; Son, K.S.; Chang, H.W.; Kang, S.S.; Kim, H.P. Effects of prenylated flavonoids and biflavonoids on lipopolysaccharide-induced nitric oxide production from the mouse macrophage cell line RAW 264.7. Planta Med. 2000, 66, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Ling, S.; Zhang, H.P.; Shi, H.X.; Xue, Y.L.; Yang, X.L.; Xu, J.W.; Bian, K. Effects of total flavones from Morus alba L. On inflammation reaction of macrophages. Shizhen Guoyi Guoyao 2010, 21, 2787–2790. [Google Scholar]
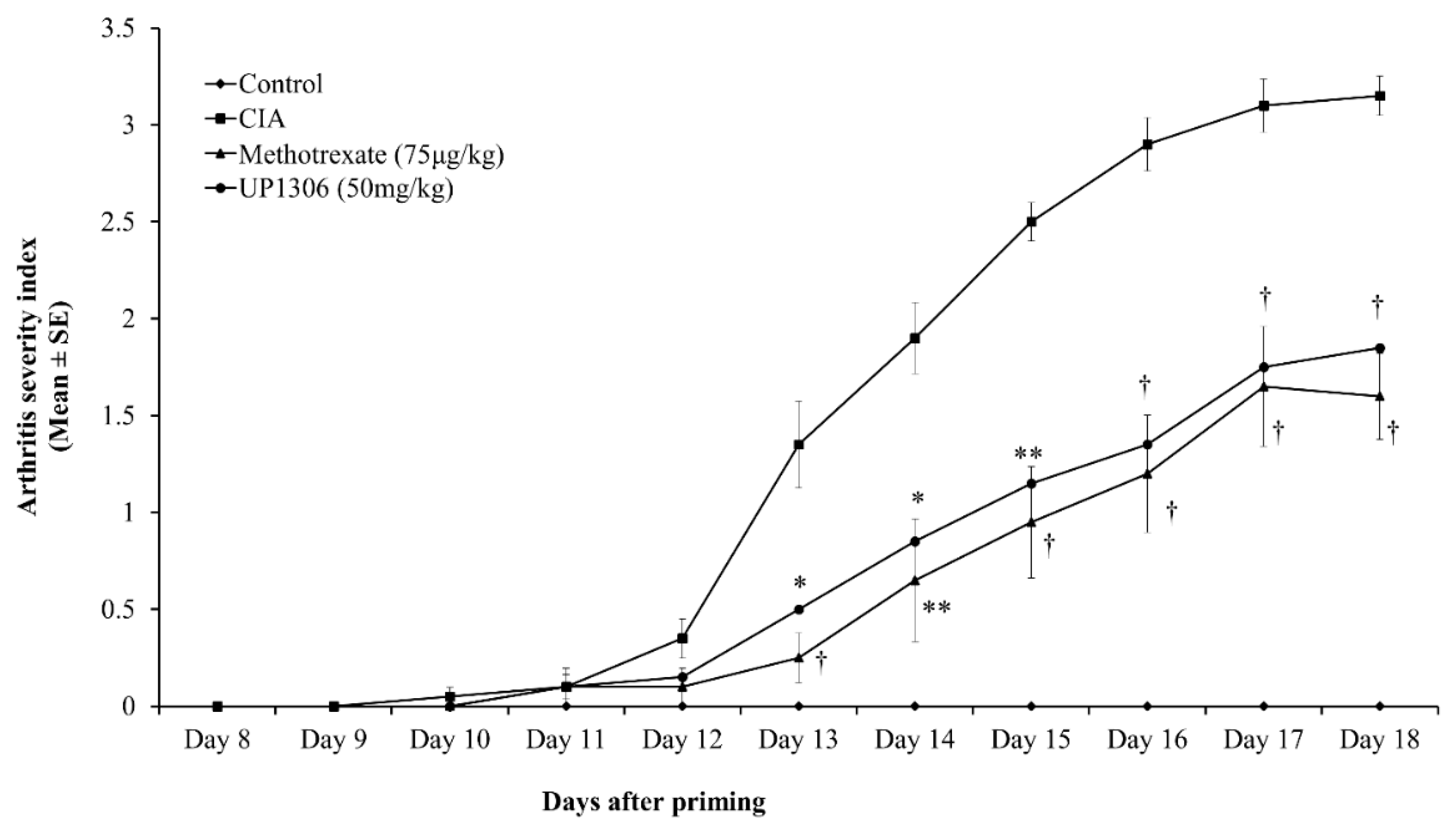
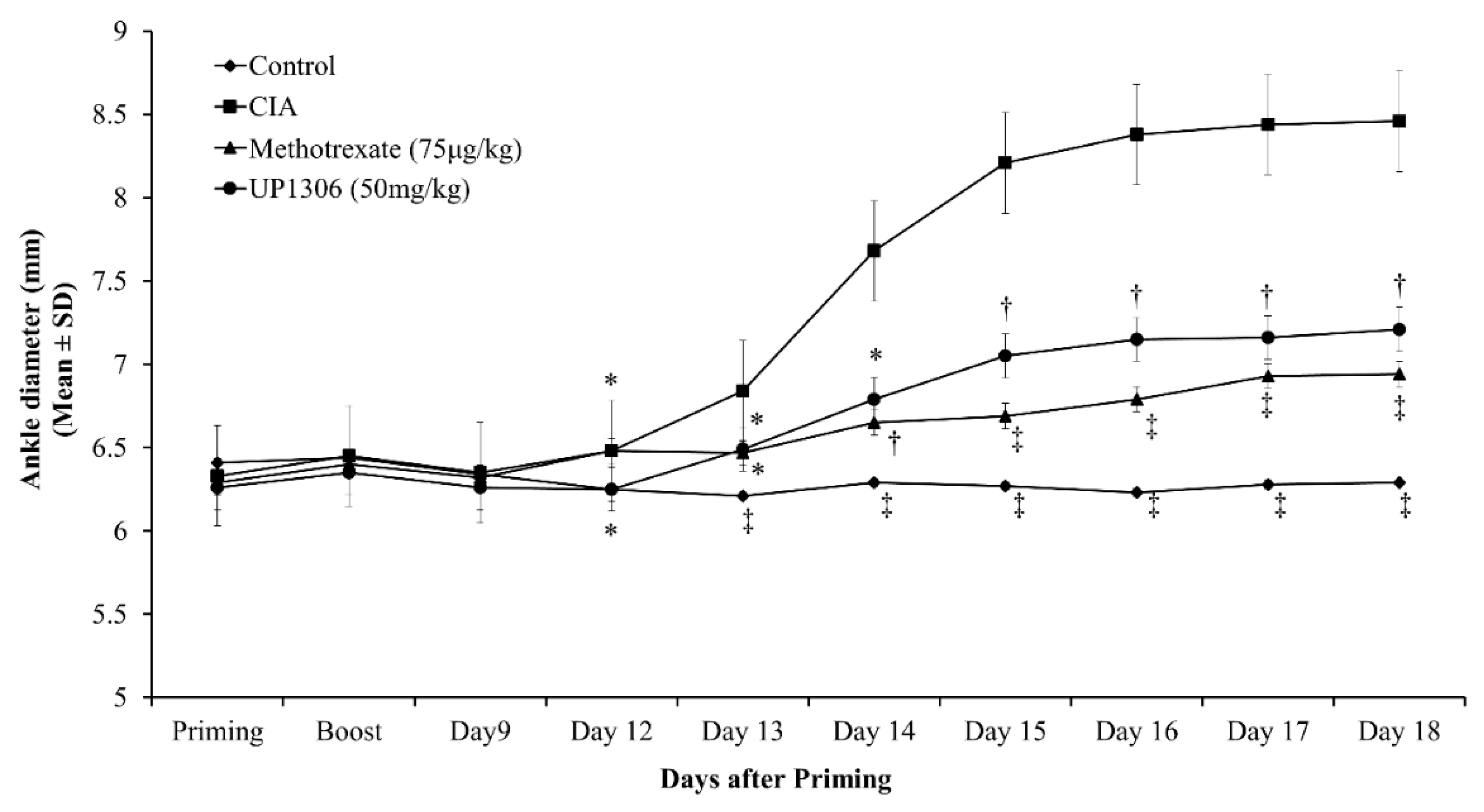
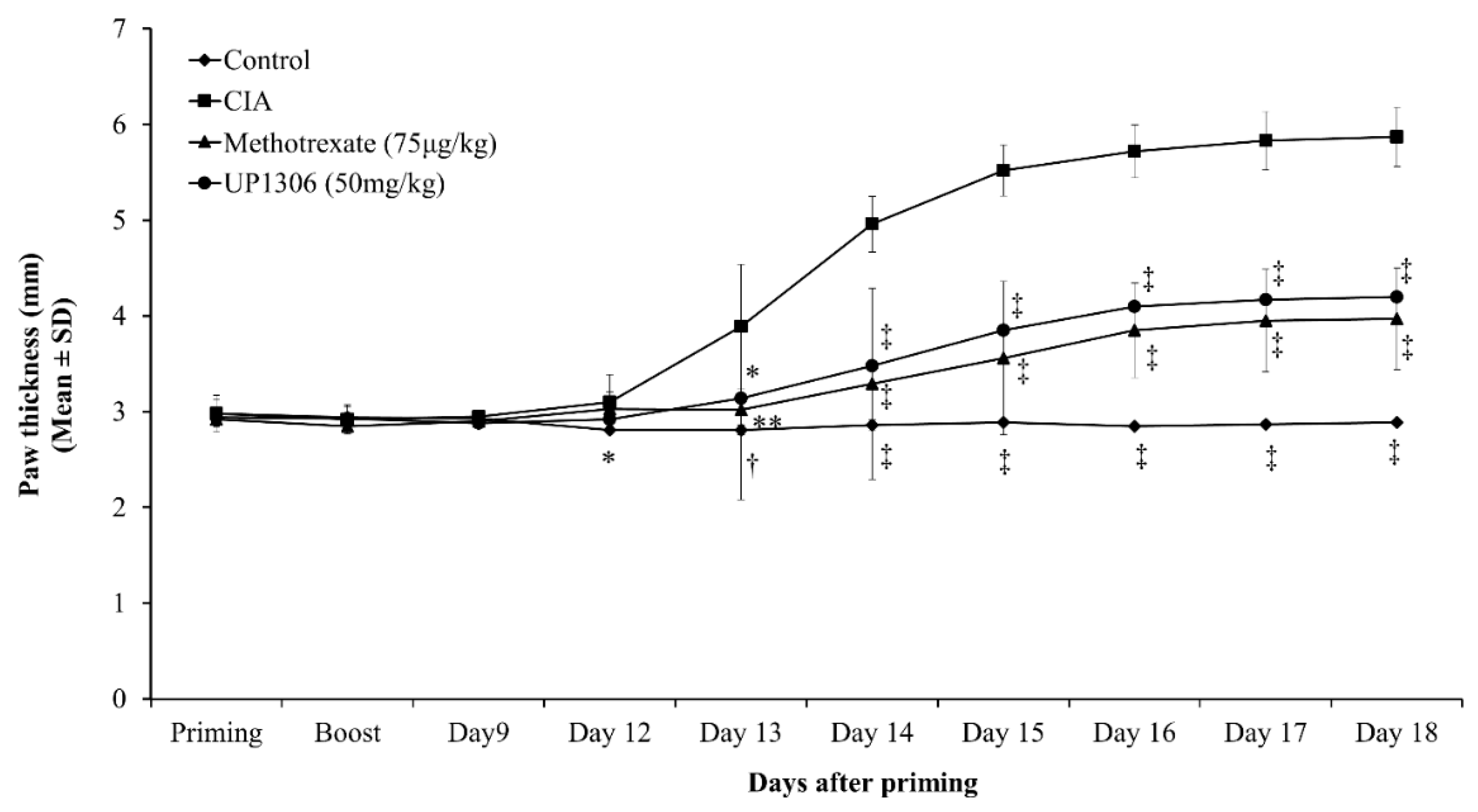
| Parameters | AUC (d12–d18) | |||
|---|---|---|---|---|
| Control | CIA | Methotrexate (75 μg/kg) | UP1306 (50 mg/kg) | |
| Arthritis Index | 0 | 13.50 ± 0.80 | 5.55 ± 0.51 * | 6.60 ± 0.50 * |
| Ankle diameter | 35.55 ± 0.02 † | 47.02 ± 0.66 | 40.24 ± 0.16 ** | 41.37 ± 0.30 * |
| Paw thickness | 17.13 ± 0.02 † | 30.41 ± 0.85 | 21.17 ± 0.36 ** | 22.30 ± 0.43 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yimam, M.; Horm, T.; Wright, L.; Jiao, P.; Hong, M.; Brownell, L.; Jia, Q. UP1306: A Composition Containing Standardized Extracts of Acacia catechu and Morus alba for Arthritis Management. Nutrients 2019, 11, 272. https://doi.org/10.3390/nu11020272
Yimam M, Horm T, Wright L, Jiao P, Hong M, Brownell L, Jia Q. UP1306: A Composition Containing Standardized Extracts of Acacia catechu and Morus alba for Arthritis Management. Nutrients. 2019; 11(2):272. https://doi.org/10.3390/nu11020272
Chicago/Turabian StyleYimam, Mesfin, Teresa Horm, Laura Wright, Ping Jiao, Mei Hong, Lidia Brownell, and Qi Jia. 2019. "UP1306: A Composition Containing Standardized Extracts of Acacia catechu and Morus alba for Arthritis Management" Nutrients 11, no. 2: 272. https://doi.org/10.3390/nu11020272
APA StyleYimam, M., Horm, T., Wright, L., Jiao, P., Hong, M., Brownell, L., & Jia, Q. (2019). UP1306: A Composition Containing Standardized Extracts of Acacia catechu and Morus alba for Arthritis Management. Nutrients, 11(2), 272. https://doi.org/10.3390/nu11020272