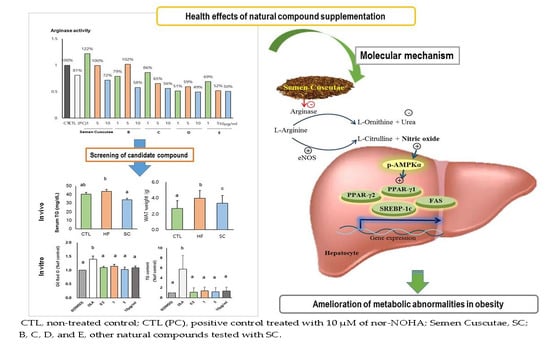
Semen Cuscutae Administration Improves Hepatic Lipid Metabolism and Adiposity in High Fat Diet-Induced Obese Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Arginase Activity Assay by Enzymatic Method
2.2. Cell Culture and Oleic Acid Induction for Steatosis
2.3. Lipid Content by SC Treatment
2.4. High-Fat Diet Induced Obese Animals and Study Design
2.5. Sample Collection of Animals
2.6. Measurement of Blood Biochemical Parameters
2.7. Analysis of Nitric Oxide (NO) and Arginase Activity of the Liver
2.8. Hepatic RNA Extraction and Semi-Quantitative RT-PCR
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Effect of SC on Lipid Accumulation in OLA-Induced Hepatic Steatosis In Vitro
3.2. SC Administration Ameliorates Diet-Induced Obesity
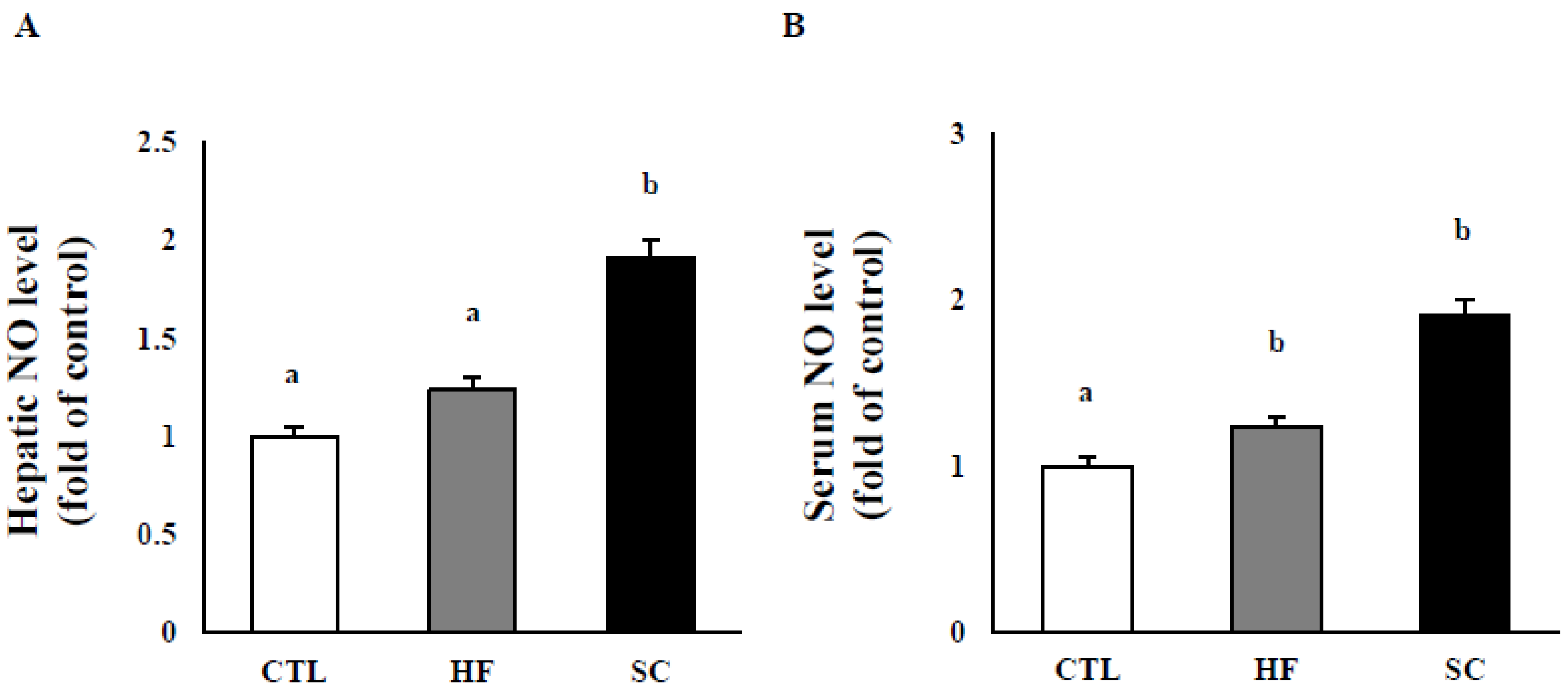
3.3. Effects of SC on Hepatic and Circulating NO, and Circulating Biochemical Parameters
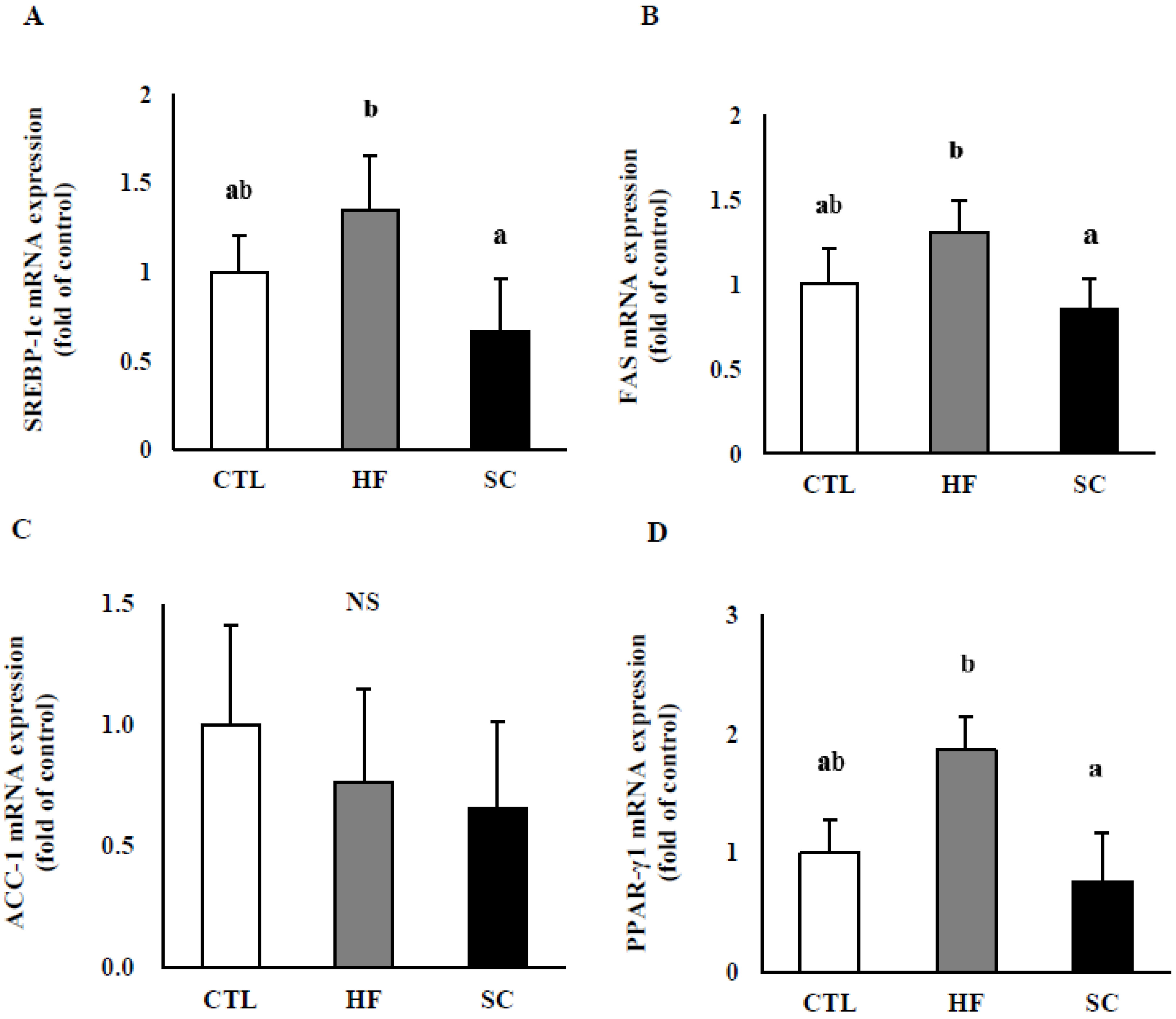
3.4. Effects of SC on Hepatic Lipid Metabolism In Vivo
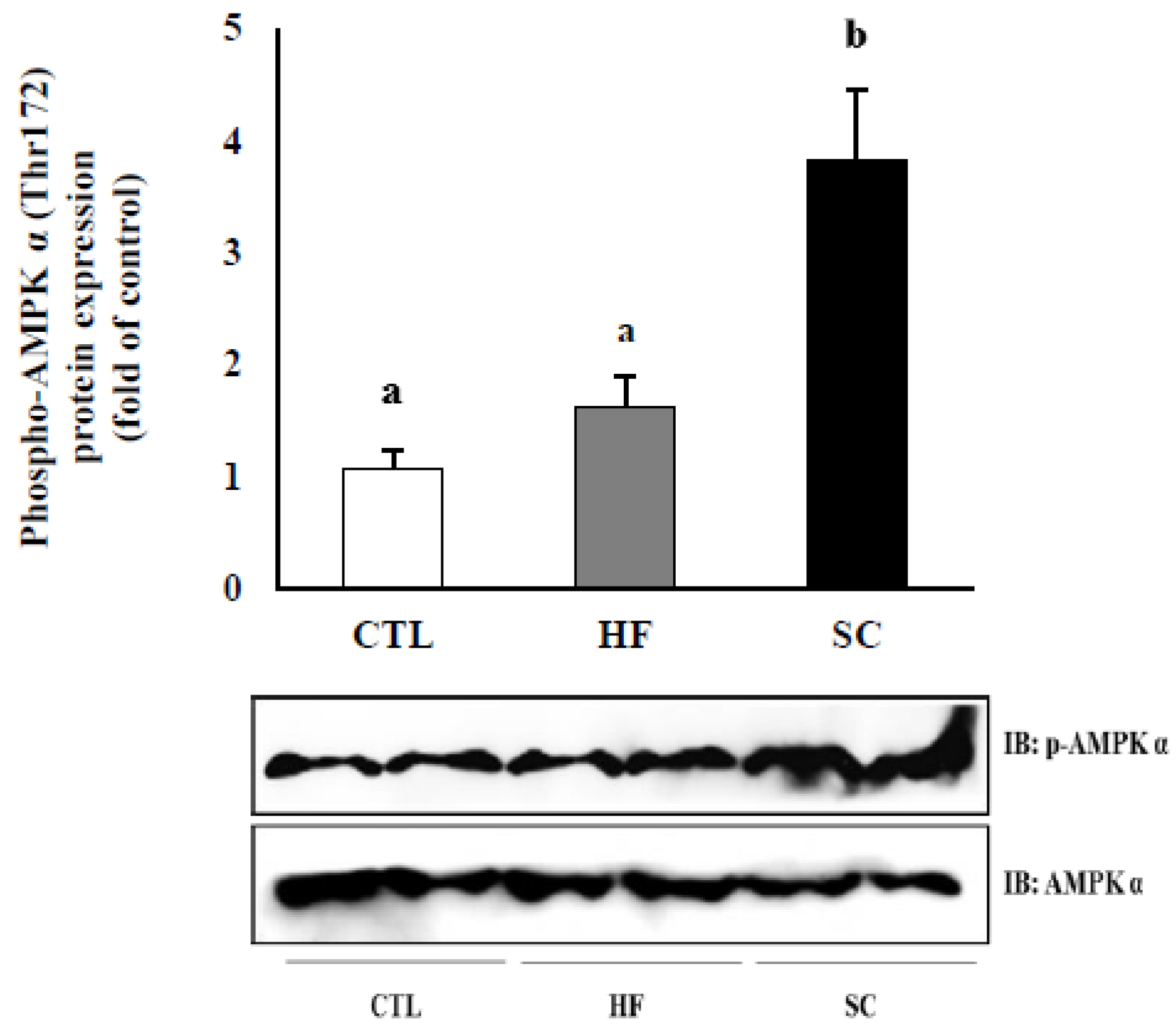
3.5. Effects of SC on Phosphorylation of Hepatic AMPKα In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Nor-NOHA | N(omega)-hydroxy-nor-1-arginine |
| SC | Semen cuscutae |
| TG | triglyceride |
| TC | total cholesterol |
| WAT | white adipose tissue |
| SREBP-1c | sterol regulatory element-binding protein 1 |
| FAS | fatty-acid synthase |
| PPAR-γ | peroxisome proliferator-activated receptor gamma |
| ADRP | adipose differentiation-related protein |
| eNOS | endothelial nitric oxide synthase |
| ACC-1 | acetyl-coA carboxylase-1 |
| SCD-1 | stearoyl-CoA desaturase-1 |
| p-AMPK | phosphorylated 5′ adenosine monophosphate-activated protein kinase |
References
- Romero, M.J.; Platt, D.H.; Tawfik, H.E.; Labazi, M.; El-Remessy, A.B.; Bartoli, M.; Caldwell, R.B.; Caldwell, R.W. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ. Res. 2008, 102, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Peyton, K.J.; Liu, X.M.; Shebib, A.R.; Johnson, F.K.; Johnson, R.A.; Durante, W. Arginase inhibition prevents the development of hypertension and improves insulin resistance in obese rats. Amino Acids 2018, 50, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Sikka, G.; Pandey, D.; Bhuniya, A.K.; Steppan, J.; Armstrong, D.; Santhanam, L.; Nyhan, D.; Berkowitz, D.E. Contribution of arginase activation to vascular dysfunction in cigarette smoking. Atherosclerosis 2013, 231, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Pernow, J.; Jung, C. Arginase as a potential target in the treatment of cardiovascular disease: Reversal of arginine steal? Cardiovasc. Res. 2013, 98, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Moon, J.; Chung, J.H.; Kim, O.Y.; Yu, R.; Shin, M.J. Arginase inhibition ameliorates adipose tissue inflammation in mice with diet-induced obesity. Biochem. Biophys. Res. Commun. 2015, 464, 840–847. [Google Scholar] [CrossRef]
- Kim, O.Y.; Lee, S.M.; Chung, J.H.; Do, H.J.; Moon, J.; Shin, M.J. Arginase I and the very low-density lipoprotein receptor are associated with phenotypic biomarkers for obesity. Nutrition 2012, 28, 635–639. [Google Scholar] [CrossRef]
- Chung, J.H.; Moon, J.; Lee, Y.S.; Chung, H.K.; Lee, S.M.; Shin, M.J. Arginase inhibition restores endothelial function in diet-induced obesity. Biochem. Biophys. Res. Commun. 2014, 451, 179–183. [Google Scholar] [CrossRef]
- White, A.R.; Ryoo, S.; Li, D.; Champion, H.C.; Steppan, J.; Wang, D.; Nyhan, D.; Shoukas, A.A.; Hare, J.M.; Berkowitz, D.E. Knockdown of arginase I restores NO signaling in the vasculature of old rats. Hypertension 2006, 47, 245–251. [Google Scholar] [CrossRef]
- Kovamees, O.; Shemyakin, A.; Pernow, J. Effect of arginase inhibition on ischemia-reperfusion injury in patients with coronary artery disease with and without diabetes mellitus. PLoS ONE 2014, 9, e103260. [Google Scholar] [CrossRef]
- Mahdi, A.; Pernow, J.; Kovamees, O. Arginase inhibition improves endothelial function in an age-dependent manner in healthy elderly humans. Rejuvenation Res. 2019, 22, 385–389. [Google Scholar] [CrossRef]
- Atawia, R.T.; Toque, H.A.; Meghil, M.M.; Benson, T.W.; Yiew, N.H.; Cutler, C.W.; Weintraub, N.L.; Caldwell, R.B.; Caldwell, R.W. Role of Arginase 2 in Systemic Metabolic Activity and Adipose Tissue Fatty Acid Metabolism in Diet-Induced Obese Mice. Int. J. Mol. Sci. 2019, 20, 1462. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Rajapakse, A.G.; Riedo, E.; Fellay, B.; Bernhard, M.C.; Montani, J.P.; Yang, Z.; Ming, X.F. Targeting arginase-II protects mice from high-fat-diet-induced hepatic steatosis through suppression of macrophage inflammation. Sci. Rep. 2016, 6, 20405. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Kim, O.Y.; Jo, G.; Shin, M.J. Alterations in Circulating Amino Acid Metabolite Ratio Associated with Arginase Activity Are Potential Indicators of Metabolic Syndrome: The Korean Genome and Epidemiology Study. Nutrients 2017, 9, 740. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Do, H.J.; Cho, Y.; Shin, M.J. Arginase inhibition ameliorates hepatic metabolic abnormalities in obese mice. PLoS ONE 2014, 9, e103048. [Google Scholar] [CrossRef]
- Thounaojam, M.C.; Nammi, S.; Jadeja, R. Natural Products for the Treatment of Obesity, Metabolic Syndrome, and Type 2 Diabetes 2016. Evid. Based. Complement. Alternat. Med. 2016, 2016, 9072345. [Google Scholar] [CrossRef]
- Pan, M.H.; Lai, C.S.; Tsai, M.L.; Ho, C.T. Chemoprevention of nonalcoholic fatty liver disease by dietary natural compounds. Mol. Nutr. Food Res. 2014, 58, 147–171. [Google Scholar] [CrossRef]
- Ehsani, N.; Solouk, A.; Mardafkan, N. Evaluation and Comparison of Synthetic and Natural Antioxidants Effectiveness. JBUMS 2018, 20. Available online: jbums.org/article-1-7708-en.pdf (accessed on 17 March 2018).
- Zhang, Y.Y.; Zhang, F.; Thakur, K.; Ci, A.T.; Wang, H.; Zhang, J.G.; Wei, Z.J. Effect of natural polyphenol on the oxidative stability of pecan oil. Food Chem. Toxicol. 2017, 119, 489–495. [Google Scholar] [CrossRef]
- Lin, M.K.; Yu, Y.L.; Chen, K.C.; Chang, W.T.; Lee, M.S.; Yang, M.J.; Cheng, H.C.; Liu, C.H.; Chen, D.C.; Chu, C.L. Kaempferol from Semen cuscutae attenuates the immune function of dendritic cells. Immunobiology 2011, 216, 1103–1109. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, Y.Q.; Liang, Y.Z.; Zou, C.; Liu, H.; Qiu, F.; Liang, C.L.; Jin, X.W.; Su, Z.R.; Dai, Z. Medicinal herbs Fructus corni and Semen cuscutae suppress allograft rejection via distinct immune mechanisms. Oncotarget 2016, 7, 35680–35691. [Google Scholar] [CrossRef]
- Pan, H.J.; Sun, H.X.; Pan, Y.J. Adjuvant effect of ethanol extract of Semen Cuscutae on the immune responses to ovalbumin in mice. J. Ethnopharmacol. 2005, 99, 99–103. [Google Scholar] [CrossRef]
- Yen, F.L.; Wu, T.H.; Lin, L.T.; Lin, C.C. Hepatoprotective and antioxidant effects of Cuscuta chinensis against acetaminophen-induced hepatotoxicity in rats. J. Ethnopharmacol. 2007, 111, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Wang, Z.; Fang, J.; Li, X. Structural features of an immunostimulating and antioxidant acidic polysaccharide from the seeds of Cuscuta chinensis. Planta Med. 2002, 68, 237–243. [Google Scholar] [CrossRef]
- Liu, J.H.; Ho, S.C.; Lai, T.H.; Liu, T.H.; Chi, P.Y.; Wu, R.Y. Protective effects of Chinese herbs on D-galactose-induced oxidative damage. Methods Find Exp. Clin. Pharm. 2003, 25, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Nisa, M.; Akbar, S.; Tariq, M.; Hussain, Z. Effect of Cuscuta chinensis water extract on 7, 12-dimethylbenz[a]anthracene-induced skin papillomas and carcinomas in mice. J. Ethnopharmacol. 1986, 18, 21–31. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, E.K.; Lee, H.S.; Sohn, Y.; Soh, Y.; Jung, H.S.; Sohn, N.W. Protective effects of Cuscutae semen against dimethylnitrosamine-induced acute liver injury in Sprague-Dawley rats. Biol. Pharm Bull. 2007, 30, 1427–1431. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peng, W.H.; Chen, Y.W.; Lee, M.S.; Chang, W.T.; Tsai, J.C.; Lin, Y.C.; Lin, M.K. Hepatoprotective Effect of Cuscuta campestris Yunck. Whole plant on carbon tetrachloride induced chronic liver injury in mice. Int. J. Mol. Sci. 2016, 17, 2056. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Q.; Wang, F.; Zhang, G. Antiosteoporotic compounds from seeds of Cuscuta chinensis. J. Ethnopharmacol. 2011, 135, 553–560. [Google Scholar] [CrossRef]
- Yang, H.M.; Shin, H.K.; Kang, Y.H.; Kim, J.K. Cuscuta chinensis extract promotes osteoblast differentiation and mineralization in human osteoblast-like MG-63 cells. J. Med Food. 2009, 12, 85–92. [Google Scholar] [CrossRef]
- Lai, Y.C.; Gladwin, M.T. Response by Lai and Gladwin to Letter Regarding Article, “SIRT3-AMP-Activated Protein Kinase Activation by Nitrite and Metformin Improves Hyperglycemia and Normalizes Pulmonary Hypertension Associated With Heart Failure With Preserved Ejection Fraction”. Circulation 2016, 134, e79–e80. [Google Scholar] [CrossRef]
- Jiang, H.; Torregrossa, A.C.; Potts, A.; Pierini, D.; Aranke, M.; Garg, H.K.; Bryan, N.S. Dietary nitrite improves insulin signaling through GLUT4 translocation. Free Radic. Biol. Med. 2014, 67, 51–57. [Google Scholar] [CrossRef]
- Joost, H.G.; Tschop, M.H. NO to obesity: Does nitric oxide regulate fat oxidation and insulin sensitivity? Endocrinology 2007, 148, 4545–4547. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sansbury, B.E.; Bhatnagar, A.; Hill, B.G. Impact of nutrient excess and endothelial nitric oxide synthase on the plasma metabolite profile in mice. Front. Physiol. 2014, 5, 453. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, S.; Bhunia, A.; Chang, F.; Shoukas, A.; Berkowitz, D.E.; Romer, L.H. OxLDL-dependent activation of arginase II is dependent on the LOX-1 receptor and downstream RhoA signaling. Atherosclerosis 2011, 214, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.C.; Park, J.T.; Jeon, Y.G.; Jeon, B.H.; Hoe, K.L.; Kim, Y.M.; Lim, H.K.; Ryoo, S. Arginase Inhibition Restores Peroxynitrite-Induced Endothelial Dysfunction via L-Arginine-Dependent Endothelial Nitric Oxide Synthase Phosphorylation. Yonsei. Med. J. 2016, 57, 1329–1338. [Google Scholar] [CrossRef]
- Demougeot, C.; Prigent-Tessier, A.; Marie, C.; Berthelot, A. Arginase inhibition reduces endothelial dysfunction and blood pressure rising in spontaneously hypertensive rats. J. Hypertens 2005, 23, 971–978. [Google Scholar] [CrossRef]
- Bonnard, C.; Durand, A.; Vidal, H.; Rieusset, J. Changes in adiponectin, itsreceptors and AMPK activity in tissues of diet-induced diabetic mice. Diabetes Metab. 2008, 34, 52–61. [Google Scholar] [CrossRef]
- Forstermann, U.; Munzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef]
- O’Neill, H.M. AMPK and exercise: Glucose uptake and insulin sensitivity. Diabetes Metab. J. 2013, 37, 1–21. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Invest. 2013, 123, 2764–2772. [Google Scholar] [CrossRef]
- Grygiel-Gorniak, B. Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications-a review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef]
- Fajas, L.; Schoonjans, K.; Gelman, L.; Kim, J.B.; Najib, J.; Martin, G.; Fruchart, J.C.; Briggs, M.; Spiegelman, B.M.; Auwerx, J. Regulation of peroxisome proliferator-activated receptor gamma expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: Implications for adipocyte differentiation and metabolism. Mol. Cell Biol. 1999, 19, 5495–5503. [Google Scholar] [CrossRef] [PubMed]
- Gilardi, F.; Giudici, M.; Mitro, N.; Maschi, O.; Guerrini, U.; Rando, G.; Maggi, A.; Cermenati, G.; Laghezza, A.; Loiodice, F.; et al. LT175 is a novel PPARalpha/gamma ligand with potent insulin-sensitizing effects and reduced adipogenic properties. J. Biol. Chem. 2014, 289, 6908–6920. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Ko, E.H.; Kim, J.E.; Kim, E.; Lee, H.; Choi, H.; Yu, J.H.; Kim, H.J.; Seong, J.K.; Kim, K.S.; et al. Nuclear receptor PPARgamma-regulated monoacylglycerol O-acyltransferase 1 (MGAT1) expression is responsible for the lipid accumulation in diet-induced hepatic steatosis. Proc. Natl. Acad. Sci. USA 2012, 109, 13656–13661. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, N.; Takei, Y.; Hirose, M.; Konno, A.; Shibuya, T.; Matsuyama, S.; Suzuki, S.; Kitamura, K.I.; Sato, N. Prevention of ethanol-induced liver injury in rats by an agonist of peroxisome proliferator-activated receptor-gamma, pioglitazone. J. Pharmacol. Exp. Ther. 2003, 306, 846–854. [Google Scholar] [CrossRef]
- Ansari, R.A.; Husain, K.; Rizvi, S.A. Role of Transcription Factors in Steatohepatitis and Hypertension after Ethanol: The Epicenter of Metabolism. Biomolecules 2016, 6, 29. [Google Scholar] [CrossRef]
- Zeng, T.; Xie, K.Q. Ethanol and liver: Recent advances in the mechanisms of ethanol-induced hepatosteatosis. Arch. Toxicol. 2009, 83, 1075–1081. [Google Scholar] [CrossRef]
- Bae, J.S.; Oh, A.R.; Lee, H.J.; Ahn, Y.H.; Cha, J.Y. Hepatic Elovl6 gene expression is regulated by the synergistic action of ChREBP and SREBP-1c. Biochem. Biophys. Res. Commun. 2016, 478, 1060–1066. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Ge, C.X.; Yu, R.; Xu, M.X.; Li, P.Q.; Fan, C.Y.; Li, J.M.; Kong, L.D. Betaine prevented fructose-induced NAFLD by regulating LXRalpha/PPARalpha pathway and alleviating ER stress in rats. Eur. J. Pharmacol. 2016, 770, 154–164. [Google Scholar] [CrossRef]
- Eberle, D.; Hegarty, B.; Bossard, P.; Ferre, P.; Foufelle, F. SREBP transcription factors: Master regulators of lipid homeostasis. Biochimie 2004, 86, 839–848. [Google Scholar] [CrossRef]
- Liu, H.; Zhong, H.; Leng, L.; Jiang, Z. Effects of soy isoflavone on hepatic steatosis in high fat-induced rats. J. Clin. Biochem. Nutr. 2017, 61, 85–90. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferre, P.; Foufelle, F. SREBP-1c transcription factor and lipid homeostasis: Clinical perspective. Horm. Res. 2007, 68, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Ferre, P.; Foufelle, F. Hepatic steatosis: A role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes. Metab. 2010, 12, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, I.; Bashmakov, Y.; Horton, J.D. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J. Biol. Chem. 1999, 274, 30028–30032. [Google Scholar] [CrossRef]
- Koo, S.H. Nonalcoholic fatty liver disease: Molecular mechanisms for the hepatic steatosis. Clin. Mol. Hepatol. 2013, 19, 210–215. [Google Scholar] [CrossRef]
- Amemiya-Kudo, M.; Shimano, H.; Hasty, A.H.; Yahagi, N.; Yoshikawa, T.; Matsuzaka, T.; Okazaki, H.; Tamura, Y.; Iizuka, Y.; Ohashi, K.; et al. Transcriptional activities of nuclear SREBP-1a, -1c, and -2 to different target promoters of lipogenic and cholesterogenic genes. J. Lipid Res. 2002, 43, 1220–1235. [Google Scholar]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.; et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef]
- Frederico, M.J.; Vitto, M.F.; Cesconetto, P.A.; Engelmann, J.; De Souza, D.R.; Luz, G.; Pinho, R.A.; Ropelle, E.R.; Cintra, D.E.; De Souza, C.T. Short-term inhibition of SREBP-1c expression reverses diet-induced non-alcoholic fatty liver disease in mice. Scand. J. Gastroenterol. 2011, 46, 1381–1388. [Google Scholar] [CrossRef]
- Sozio, M.S.; Lu, C.; Zeng, Y.; Liangpunsakul, S.; Crabb, D.W. Activated AMPK Inhibits PPAR{alpha} and PPAR{gamma} Transcriptional Activity in Hepatoma Cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G739–G747. [Google Scholar] [CrossRef]
- Villalpando-Arteaga, E.V.; Mendieta-Condado, E.; Esquivel-Solis, H.; Canales-Aguirre, A.A.; Galvez-Gastelum, F.J.; Mateos-Diaz, J.C.; Rodriguez-Gonzalez, J.A.; Marquez-Aguirre, A.L. Hibiscus sabdariffa L. aqueous extract attenuates hepatic steatosis through down-regulation of PPAR-gamma and SREBP-1c in diet-induced obese mice. Food Funct. 2013, 4, 618–626. [Google Scholar] [CrossRef]




| Ingredients | CTL | HFD | HFD with SC |
|---|---|---|---|
| corn starch | 15 | 15 | 15 |
| casein | 20 | 20 | 20 |
| sucrose | 50 | 34 | 34 |
| corn oil | 5 | 3 | 3 |
| mineral mix 1 | 3.5 | 3.5 | 3.5 |
| vitamin mix 2 | 1 | 1 | 1 |
| cellulose | 5 | 5 | 5 |
| DL-methionine | 0.3 | 0.3 | 0.3 |
| choline bitartrate | 0.2 | 0.2 | 0.2 |
| lard | - | 17 | 17 |
| cholesterol | - | 1 | 1 |
| BHT | 0.001 | 0.001 | 0.001 |
| total | 100 | 100 | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, J.; Ha, M.J.; Shin, M.-J.; Kim, O.Y.; Yoo, E.H.; Song, J.; Chung, J.H. Semen Cuscutae Administration Improves Hepatic Lipid Metabolism and Adiposity in High Fat Diet-Induced Obese Mice. Nutrients 2019, 11, 3035. https://doi.org/10.3390/nu11123035
Moon J, Ha MJ, Shin M-J, Kim OY, Yoo EH, Song J, Chung JH. Semen Cuscutae Administration Improves Hepatic Lipid Metabolism and Adiposity in High Fat Diet-Induced Obese Mice. Nutrients. 2019; 11(12):3035. https://doi.org/10.3390/nu11123035
Chicago/Turabian StyleMoon, Jiyoung, Min Jin Ha, Min-Jeong Shin, Oh Yoen Kim, Eun Hye Yoo, Juhyun Song, and Ji Hyung Chung. 2019. "Semen Cuscutae Administration Improves Hepatic Lipid Metabolism and Adiposity in High Fat Diet-Induced Obese Mice" Nutrients 11, no. 12: 3035. https://doi.org/10.3390/nu11123035
APA StyleMoon, J., Ha, M. J., Shin, M.-J., Kim, O. Y., Yoo, E. H., Song, J., & Chung, J. H. (2019). Semen Cuscutae Administration Improves Hepatic Lipid Metabolism and Adiposity in High Fat Diet-Induced Obese Mice. Nutrients, 11(12), 3035. https://doi.org/10.3390/nu11123035