Why Do Children in Slums Suffer from Anemia, Iron, Zinc, and Vitamin A Deficiency? Results from a Birth Cohort Study in Dhaka
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Inclusion and Exclusion Criteria
2.3. Ethical Issues
2.4. Data Collection
2.5. Biological Sample Collection
2.6. Measurement of Plasma Micronutrient Status
2.7. Infection Category and Adjustment of Ferritin, Zinc and Retinol for Inflammation
2.8. Definitions and Cut-offs
2.9. Statistical Analysis
3. Results
3.1. Basic Socio-Demographic Characteristics
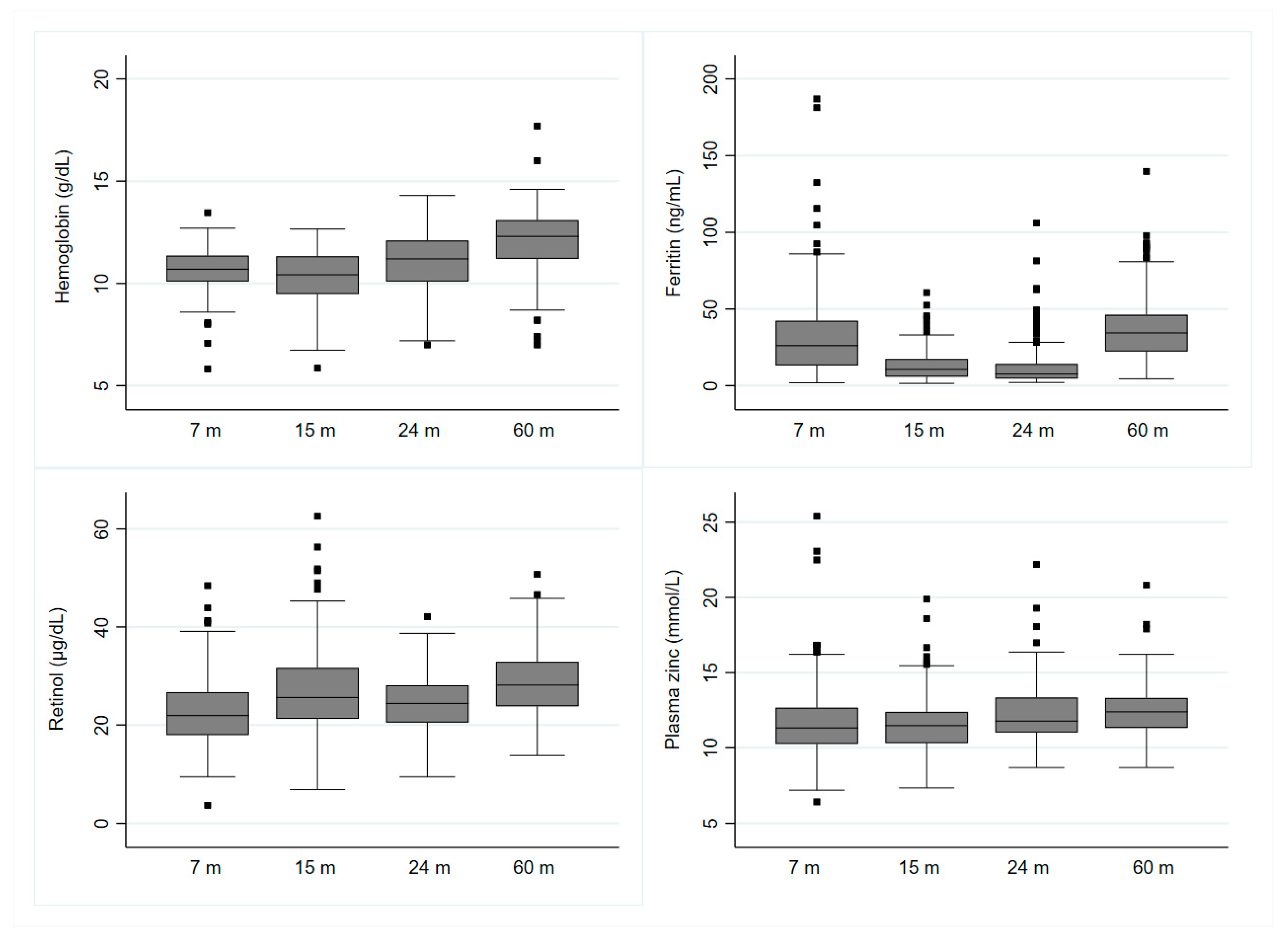
3.2. Plasma Micronutrient Status
3.2.1. Factors Associated with Anemia from 7 to 60 Months
3.2.2. Factors Associated with Iron Deficiency (Low Ferritin) from 7 to 60 Months
3.3.3. Factors Associated with Zinc Deficiency from 7 to 60 Months
3.3.4. Factors Associated with Vitamin A Deficiency from 7 to 60 Months
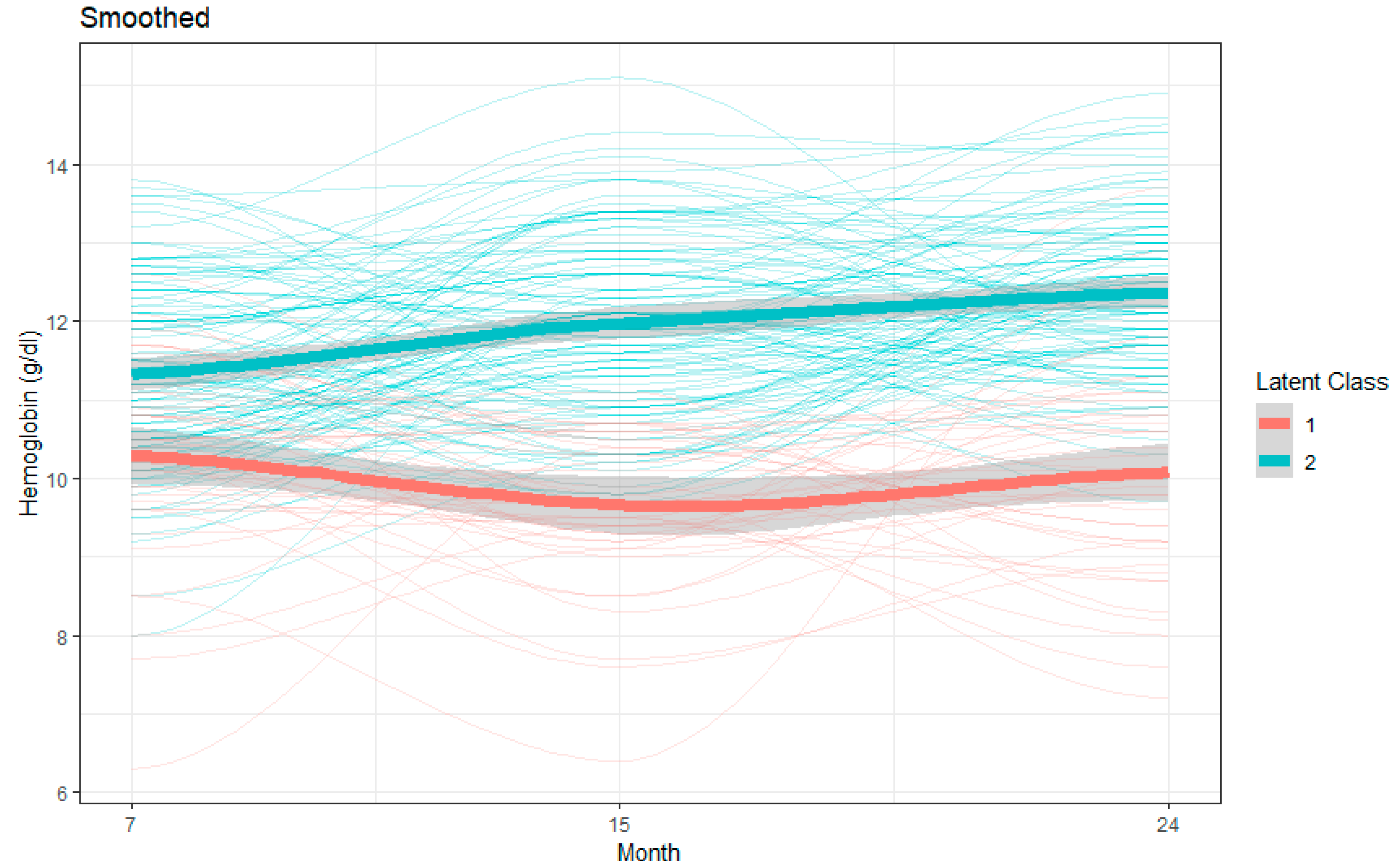
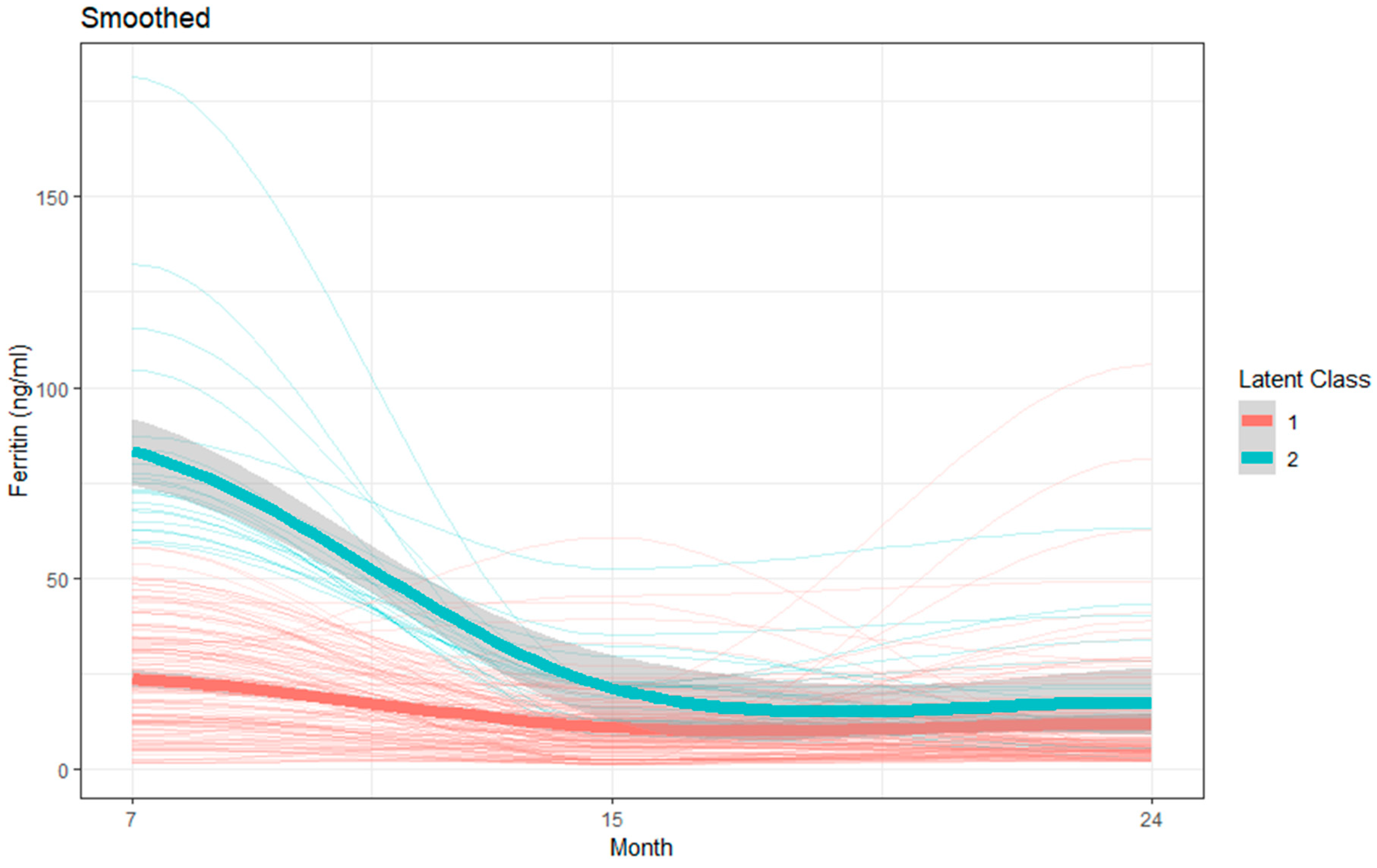
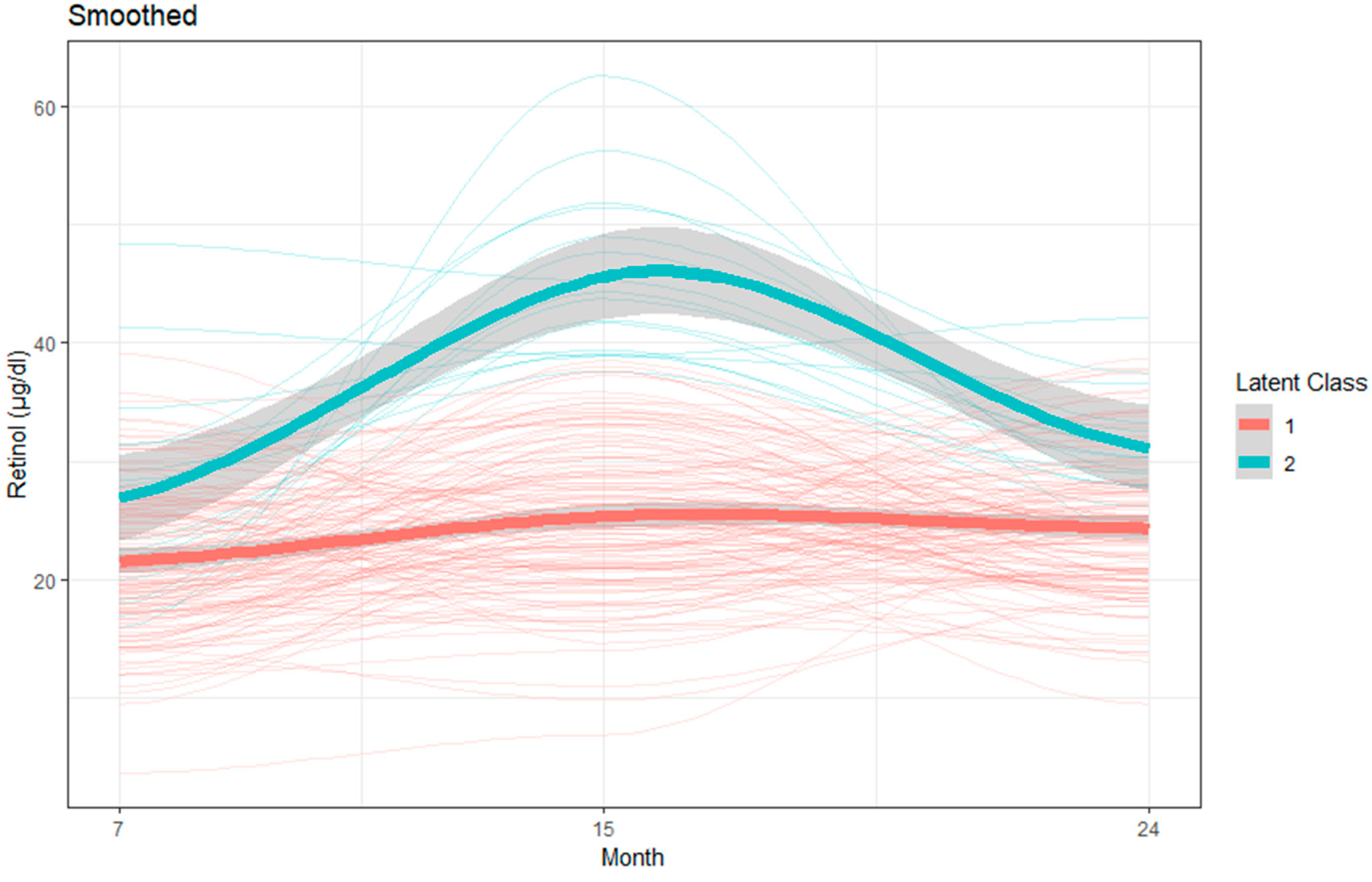
3.3. Latent Class Growth Model to Identify Group Trajectories of Plasma Micronutrients
3.4. Multiple Linear Regression Models to Examine Association of Group Trajectories with Micronutrient Concentrations at 60 Months
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stewart, C.P.; Iannotti, L.; Dewey, K.G.; Michaelsen, K.F.; Onyango, A.W. Contextualising complementary feeding in a broader framework for stunting prevention. Matern. Child Nutr. 2013, 9 (Suppl. S2), 27–45. [Google Scholar] [CrossRef]
- Prentice, A.M.; Ward, K.A.; Goldberg, G.R.; Jarjou, L.M.; Moore, S.E.; Fulford, A.J.; Prentice, A. Critical windows for nutritional interventions against stunting. Am. J. Clin. Nutr. 2013, 97, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Dewey, K.G.; Mayers, D.R. Early child growth: How do nutrition and infection interact? Matern. Child Nutr. 2011, 7 (Suppl. S3), 129–142. [Google Scholar] [CrossRef]
- Sanin, K.I.; Islam, M.M.; Mahfuz, M.; Ahmed, A.M.S.; Mondal, D.; Haque, R.; Ahmed, T. Micronutrient adequacy is poor, but not associated with stunting between 12–24 months of age: A cohort study findings from a slum area of Bangladesh. PLoS ONE 2018, 13, e0195072. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet (Lond. Engl.) 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Rivera, J.A.; Hotz, C.; Gonzalez-Cossio, T.; Neufeld, L.; Garcia-Guerra, A. The effect of micronutrient deficiencies on child growth: A review of results from community-based supplementation trials. J. Nutr. 2003, 133, 4010s–4020s. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.H.; Wuehler, S.E.; Peerson, J.M. The Importance of Zinc in Human Nutrition and Estimation of the Global Prevalence of Zinc Deficiency. Food Nutr. Bull. 2001, 22, 113–125. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Farooq, H.; Maham, S.N.; Qayyum, Z.; Waheed, A.; Nasir, W. Frequency of Anemia and Iron Deficiency among Children Starting First Year of School Life and Their Association with Weight and Height. Anemia 2018, 2018, 8906258. [Google Scholar] [CrossRef]
- Akhtar, S.; Ahmed, A.; Randhawa, M.A.; Atukorala, S.; Arlappa, N.; Ismail, T.; Ali, Z. Prevalence of vitamin A deficiency in South Asia: Causes, outcomes, and possible remedies. J. Health Popul. Nutr. 2013, 31, 413–423. [Google Scholar] [CrossRef]
- Sandstead, H.H. Nutrition and brain function: Trace elements. Nutr. Rev. 1986, 44, 37–41. [Google Scholar] [CrossRef]
- Bailey, R.L.; West, K.P., Jr.; Black, R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015, 66 (Suppl. S2), 22–33. [Google Scholar] [CrossRef]
- Investigators, T.M.-E.N. The MAL-ED Study: A Multinational and Multidisciplinary Approach to Understand the Relationship Between Enteric Pathogens, Malnutrition, Gut Physiology, Physical Growth, Cognitive Development, and Immune Responses in Infants and Children Up to 2 Years of Age in Resource-Poor Environments. J. Clin. Infect. Dis. 2014, 59, S193–S206. [Google Scholar] [CrossRef]
- Ahmed, T.; Mahfuz, M.; Islam, M.M.; Mondal, D.; Hossain, M.I.; Ahmed, A.S.; Tofail, F.; Gaffar, S.A.; Haque, R.; Guerrant, R.L.; et al. The MAL-ED cohort study in Mirpur, Bangladesh. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014, 59 (Suppl. S4), S280–S286. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.A.; Barrett, L.J.; Guerrant, R.L.; Checkley, W.; Miller, M.A. Disease surveillance methods used in the 8-site MAL-ED cohort study. Clin. Infect. Dis. 2014, 59 (Suppl. S4), S220–S224. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, L.E.; Bose, A.; Chandyo, R.K.; Nesamvuni, C.; de Moraes, M.L.; Turab, A.; Patil, C.; Mahfuz, M.; Ambikapathi, R.; Ahmed, T. Infant feeding practices, dietary adequacy, and micronutrient status measures in the MAL-ED study. Clin. Infect. Dis. 2014, 59 (Suppl. S4), S248–S254. [Google Scholar] [CrossRef] [PubMed]
- Helrich, K. AOAC Official Methods of Analysis (1990), 15th ed.; 2nd Supplement; AOAC Inc.: Washington, DC, USA, 1991; Volume 2, p. 81. [Google Scholar]
- Driskell, W.J.; Neese, J.W.; Bryant, C.C.; Bashor, M.M. Measurement of vitamin A and vitamin E in human serum by high-performance liquid chromatography. J. Chromatogr. 1982, 231, 439–444. [Google Scholar] [CrossRef]
- Grant, F.K.; Suchdev, P.S.; Flores-Ayala, R.; Cole, C.R.; Ramakrishnan, U.; Ruth, L.J.; Martorell, R. Correcting for inflammation changes estimates of iron deficiency among rural Kenyan preschool children. J. Nutr. 2012, 142, 105–111. [Google Scholar] [CrossRef]
- Mahfuz, M.; Alam, M.A.; Islam, M.M.; Mondal, D.; Hossain, M.I.; Ahmed, A.M.S.; Choudhury, N.; Raihan, M.J.; Haque, R.; Ahmed, T. Effect of micronutrient powder supplementation for two and four months on hemoglobin level of children 6–23 months old in a slum in Dhaka: A community based observational study. BMC Nutr. 2016, 2, 21. [Google Scholar] [CrossRef]
- WHO UNICEF UNU. Iron Deficiency Anaemia: Assessment, Prevention, and Control; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Kraemer, K.; Zimmermann, M.B. Nutritional Anemia; Sight and Life Press: Waldkirch, Germany, 2007. [Google Scholar]
- Ayoya, M.A.; Spiekermann-Brouwer, G.M.; Stoltzfus, R.J.; Nemeth, E.; Habicht, J.P.; Ganz, T.; Rawat, R.; Traore, A.K.; Garza, C. Alpha 1-acid glycoprotein, hepcidin, C-reactive protein, and serum ferritin are correlated in anemic schoolchildren with Schistosoma haematobium. Am. J. Clin. Nutr. 2010, 91, 1784–1790. [Google Scholar] [CrossRef]
- Psaki, S.R.; Seidman, J.C.; Miller, M.; Gottlieb, M.; Bhutta, Z.A.; Ahmed, T.; Ahmed, A.S.; Bessong, P.; John, S.M.; Kang, G.; et al. Measuring socioeconomic status in multicountry studies: Results from the eight-country MAL-ED study. Popul. Health Metr. 2014, 12, 8. [Google Scholar] [CrossRef]
- World Health Organization. Available online: http://www.who.int/bulletin/volumes/88/3/10-020310/en/ (accessed on 8 November 2018).
- Coates, J.; Swindale, A.; Bilinsky, P. Household Food Insecurity Access Scale (HFIAS) for Measurement of Food Access: Indicator Guide (v. 3). 2007; Food and Nutrition Technical Assistance Project (FANTA), Academy for Educational Development: Washington, DC, USA, 2015. [Google Scholar]
- Nagin, D.S. Analyzing developmental trajectories: A semiparametric, group-based approach. Psychol. Methods 1999, 4, 139. [Google Scholar] [CrossRef]
- Nagin, D.S.; Tremblay, R.E. Analyzing developmental trajectories of distinct but related behaviors: A group-based method. Psychol. Methods 2001, 6, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Nagin, D.S. Group-Based Modeling of Development; Harvard University Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Jones, B.L.; Nagin, D.S.; Roeder, K. A SAS Procedure Based on Mixture Models for Estimating Developmental Trajectories. Sociol. Methods Res. 2001, 29, 374–393. [Google Scholar] [CrossRef]
- Andruff, H.; Carraro, N.; Thompson, A.; Gaudreau, P.; Louvet, B. Latent class growth modelling: A tutorial. Tutor. Quant. Methods Psychol. 2009, 5, 11–24. [Google Scholar] [CrossRef]
- Van de Schoot, R.; Sijbrandij, M.; Winter, S.D.; Depaoli, S.; Vermunt, J.K. The GRoLTS-Checklist: Guidelines for Reporting on Latent Trajectory Studies. Struct. Equ. Modeling A Multidiscip. J. 2017, 24, 451–467. [Google Scholar] [CrossRef]
- Jones, B.L.; Nagin, D.S. A Note on a Stata Plugin for Estimating Group-based Trajectory Models. Sociol. Methods Res. 2013, 42, 608–613. [Google Scholar] [CrossRef]
- National Micronutrients Status Survey 2011-12, Bangladesh. icddr,b UNICEF, Bangladesh, GAIN, Institute of Public Health and Nutrition. Bangladesh 2013. Available online: http://dspace.icddrb.org/jspui/handle/123456789/6450 (accessed on 12 January 2019).
- Rahman, S.; Ahmed, T.; Rahman, A.S.; Alam, N.; Ahmed, A.S.; Ireen, S.; Chowdhury, I.A.; Chowdhury, F.P.; Rahman, S.M. Determinants of iron status and Hb in the Bangladesh population: The role of groundwater iron. Public Health Nutr. 2016, 19, 1862–1874. [Google Scholar] [CrossRef]
- Roos, N.; Ponce, M.C.; Doak, C.M.; Dijkhuizen, M.; Polman, K.; Chamnan, C.; Khov, K.; Chea, M.; Prak, S.; Kounnavong, S.; et al. Micronutrient status of populations and preventive nutrition interventions in South East Asia. Matern. Child Health J. 2019, 23, 29–45. [Google Scholar] [CrossRef]
- Markel, T.A.; Crisostomo, P.R.; Wang, M.; Herring, C.M.; Meldrum, K.K.; Lillemoe, K.D.; Meldrum, D.R. The struggle for iron: Gastrointestinal microbes modulate the host immune response during infection. J. Leukoc. Biol. 2007, 81, 393–400. [Google Scholar] [CrossRef]
- MAL-ED Network Investigators. Early childhood cognitive development is affected by interactions among illness, diet, enteropathogens and the home environment: Findings from the MAL-ED birth cohort study. BMJ Glob. Health 2018, 3, e000752. [Google Scholar]
- Saraiva, B.C.A.; Soares, M.C.C.; Santos, L.C.D.; Pereira, S.C.L.; Horta, P.M. Iron deficiency and anemia are associated with low retinol levels in children aged 1 to 5 years. J. Pediatr. 2014, 90, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Citelli, M.; Bittencourt, L.L.; da Silva, S.V.; Pierucci, A.P.; Pedrosa, C. Vitamin A modulates the expression of genes involved in iron bioavailability. Biol. Trace Elem. Res. 2012, 149, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Mwanri, L.; Worsley, A.; Ryan, P.; Masika, J. Supplemental vitamin A improves anemia and growth in anemic school children in Tanzania. J. Nutr. 2000, 130, 2691–2696. [Google Scholar] [CrossRef] [PubMed]
- Kumwenda, N.; Miotti, P.G.; Taha, T.E.; Broadhead, R.; Biggar, R.J.; Jackson, J.B.; Melikian, G.; Semba, R.D. Antenatal vitamin A supplementation increases birth weight and decreases anemia among infants born to human immunodeficiency virus-infected women in Malawi. Clin. Infect. Dis. 2002, 35, 618–624. [Google Scholar] [CrossRef]
- Gibson, R.S.; Abebe, Y.; Stabler, S.; Allen, R.H.; Westcott, J.E.; Stoecker, B.J.; Krebs, N.F.; Hambidge, K.M. Zinc, gravida, infection, and iron, but not vitamin B-12 or folate status, predict hemoglobin during pregnancy in Southern Ethiopia. J. Nutr. 2008, 138, 581–586. [Google Scholar] [CrossRef]
- Folin, M.; Contiero, E.; Vaselli, G.M. Zinc content of normal human serum and its correlation with some hematic parameters. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 1994, 7, 75–79. [Google Scholar] [CrossRef]
- Solomons, N.W.; Jacob, R.A. Studies on the bioavailability of zinc in humans: Effects of heme and nonheme iron on the absorption of zinc. Am. J. Clin. Nutr. 1981, 34, 475–482. [Google Scholar] [CrossRef]
- De la Cruz-Gongora, V.; Villalpando, S.; Shamah-Levy, T. Prevalence of anemia and consumption of iron-rich food groups in Mexican children and adolescents: Ensanut MC 2016. Salud Publica Mex. 2018, 60, 291–300. [Google Scholar] [CrossRef]
- FAO; WHO. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004; Available online: http://whqlibdoc.who.int/publications/2004/9241546123.pdf (accessed on 20 March 2019).
| Characteristics | N = 212 (%/mean ± sd) |
|---|---|
| Male | 47.6 |
| Birth weight, kg | 2.8 ± 0.4 |
| Low birth weight (<2.5 kg) | 22.17 |
| Total days of exclusive breastfeeding | 106.5 ± 57.4 |
| Maternal age | 24.8 ± 4.9 |
| Maternal education | |
| None | 19.3 |
| Some primary | 25.9 |
| Primary complete | 18.4 |
| Some secondary | 31.6 |
| Secondary complete or higher | 4.7 |
| Treated drinking water | 60.3 |
| Improved toilet a | 76.0 |
| Hand wash after helping the child defecate | 72.7 |
| Hand wash before preparing food | 22.3 |
| Hand wash after using toilet | 75.6 |
| Food insecurity b | |
| Secure | 74.5 |
| Mild insecurity | 6.13 |
| Moderate insecurity | 11.8 |
| Severe insecurity | 7.6 |
| Monthly income (US$), median (IQR) | 97.6 (73.1, 121.9) |
| Asset Index c | |
| Poorest | 20.9 |
| Poor | 19.4 |
| Middle | 20.4 |
| Wealthier | 20.4 |
| Wealthiest | 18.9 |
| Stunted (length-for-age z-score <−2) | 16.9 |
| Underweight (weight-for-age z-score <−2) | 21.2 |
| Wasted (weight-for-length z-score <−2) | 16.9 |
| Cut-Off | Percent (n) | ||||
|---|---|---|---|---|---|
| 7 Months | 15 Months | 24 Months | 60 Months | ||
| Anemia | Hb < 11 g/dL | 64.3 (117) | 63.9 (106) | 41.7 (68) | 18.8 (31) |
| Low Ferritin | <12 ng/mL | 20.3 (42) | 54.1 (105) | 68 (119) | 4.9 (9) |
| Low Zinc | <9.9 mmol/L | 21.5 (44) | 14.9 (29) | 1.1 (2) | 3.8 (7) |
| Low Retinol | <20 µg/dL | 39.0 (80) | 19.2 (37) | 21.7 (38) | 6.7 (12) |
| Anemia | Zinc Deficiency | Vitamin A Deficiency | Iron Deficiency | |||||
|---|---|---|---|---|---|---|---|---|
| AOR (95% CI) | p-Value | AOR (95% CI) | p-Value | AOR (95% CI) | p-Value | AOR (95% CI) | p-Value | |
| Inflammation group | ||||||||
| Non-inflammed | Reference | |||||||
| Incubation | 2.43 (0.74, 7.94) | 0.142 | ||||||
| Early convalescence | 2.21 (1.14, 4.27) | 0.019 | ||||||
| Late convalescence | 1.65 (1.03, 2.64) | 0.037 | ||||||
| Age in months | 0.94 (0.92, 0.96) | <0.0001 | 0.95 (0.93, 0.98) | 0.001 | 0.96 (0.95, 0.98) | <0.0001 | 0.98 (0.97, 0.98) | <0.0001 |
| Female | 0.49 (0.32, 0.77) | 0.002 | 0.65 (0.47, 0.97) | 0.013 | ||||
| Birth weight | 0.67 (0.39, 1.14) | 0.136 | 0.61 (0.36, 1.04) | 0.072 | 0.58 (0.38, 0.88) | 0.010 | ||
| Wasting at birth | 1.86 (1.11, 3.11) | 0.018 | ||||||
| Food insecurity access | ||||||||
| Food secure | Reference | |||||||
| Mild food insecurity | 1.08 (0.42, 2.80) | 0.87 | ||||||
| Moderate food insecurity | 0.58 (0.29, 1.12) | 0.105 | ||||||
| Severe food insecurity | 1.49 (0.69, 3.19) | 0.302 | ||||||
| Monthly income | 0.99 (0.99, 1.00) | 0.545 | 1.00 (0.99, 1.00) | 0.079 | 0.99 (0.99, 1.00) | 0.168 | ||
| Maternal education | ||||||||
| No | Reference | Reference | Reference | |||||
| Some primary | 1.14 (0.63, 2.09) | 0.661 | 0.69 (0.35, 1.37) | 0.29 | 1.11 (0.71, 1.73) | 0.65 | ||
| Primary complete | 1.17 (0.59, 2.30) | 0.660 | 0.78 (0.36, 1.69) | 0.53 | 1.29 (0.77, 2.16) | 0.33 | ||
| Some secondary | 0.71 (0.39, 1.28) | 0.250 | 0.53 (0.27, 1.0) | 0.072 | 0.95 (0.62, 1.46) | 0.814 | ||
| Secondary complete or higher | 0.38 (0.15, 0.92) | 0.032 | 0.28 (0.06, 1.25) | 0.096 | 2.40 (1.08, 5.34) | 0.031 | ||
| Treated drinking water | 0.57 (0.36, 0.89) | 0.016 | ||||||
| Improved toilet | 0.67 (0.39, 1.16) | 0.15 | 1.63 (0.98, 2.70) | 0.061 | ||||
| Hand wash after helping the child defecate | 1.63 (0.93, 2.86) | 0.089 | 1.33 (0.65, 2.75) | 0.44 | ||||
| Hand washing before food preparation | 1.11 (0.66, 1.85) | 0.700 | 1.47 (0.99, 2.17) | 0.052 | ||||
| Hand washing after using toilet | 1.44 (0.82, 2.52) | 0.200 | 1.54 (0.79, 3.00) | 0.21 | 0.79 (0.46, 1.38) | 0.421 | ||
| Anemia | 0.81 (0.49, 1.31) | 0.39 | 1.69 (1.06, 2.69) | 0.025 | 2.75 (1.87, 4.05) | <0.0001 | ||
| Low zinc | 0.79 (0.46, 1.38) | 0.420 | 1.28 (0.71, 2.32) | 0.406 | ||||
| Low ferritin | 3.04 (2.08, 4.44) | 0.0001 | 1.16 (0.77, 1.75) | 0.476 | ||||
| Low retinol | 2.07 (1.31, 3.28) | 0.002 | 1.36 (0.78, 2.38) | 0.27 | 0.81 (0.56, 1.19) | 0.288 | ||
| Trajectory Group | Age = 7 Months | Age = 15 Months | Age = 24 Months |
|---|---|---|---|
| Hemoglobin (g/dL) | |||
| Group 1 (Decreasing) | Mean = 10.31; 95% CI = 9.87, 10.75 | Mean = 9.78; 95% CI = 9.29, 10.27 | Mean = 10.16; 95% CI = 9.56, 10.77 |
| Group 2 (Increasing) | Mean = 11.36; 95% CI = 11.12, 11.60 | Mean = 11.83; 95% CI = 11.64, 12.02 | Mean = 12.36; 95% CI = 12.07, 12.65 |
| Ferritin (ng/mL) | |||
| Group 1 (Lower) | Mean = 24.20; 95% CI = 20.76, 27.65 | Mean = 13.19; 95% CI = 10.31, 16.07 | Mean = 13.99; 95% CI = 10.99, 16.99 |
| Group 2 (Higher) | Mean = 82.97; 95% CI = 75.01, 90.93 | Mean = 21.18; 95% CI = 12.85, 29.51 | Mean = 18.21; 95% CI = 9.64, 26.78 |
| Retinol (µg/dL) | |||
| Group 1 (Lower) | Mean = 21.47; 95% CI = 20.32, 22.62 | Mean = 25.27; 95% CI = 24.0, 26.55 | Mean = 24.33; 95% CI = 23.25, 25.41 |
| Group 2 (Higher) | Mean = 26.80; 95% CI = 23.58, 30.02 | Mean = 44.01; 95% CI = 38.54, 49.48 | Mean = 30.45; 95% CI = 26.98, 33.93 |
| Zinc (mmol/L) | |||
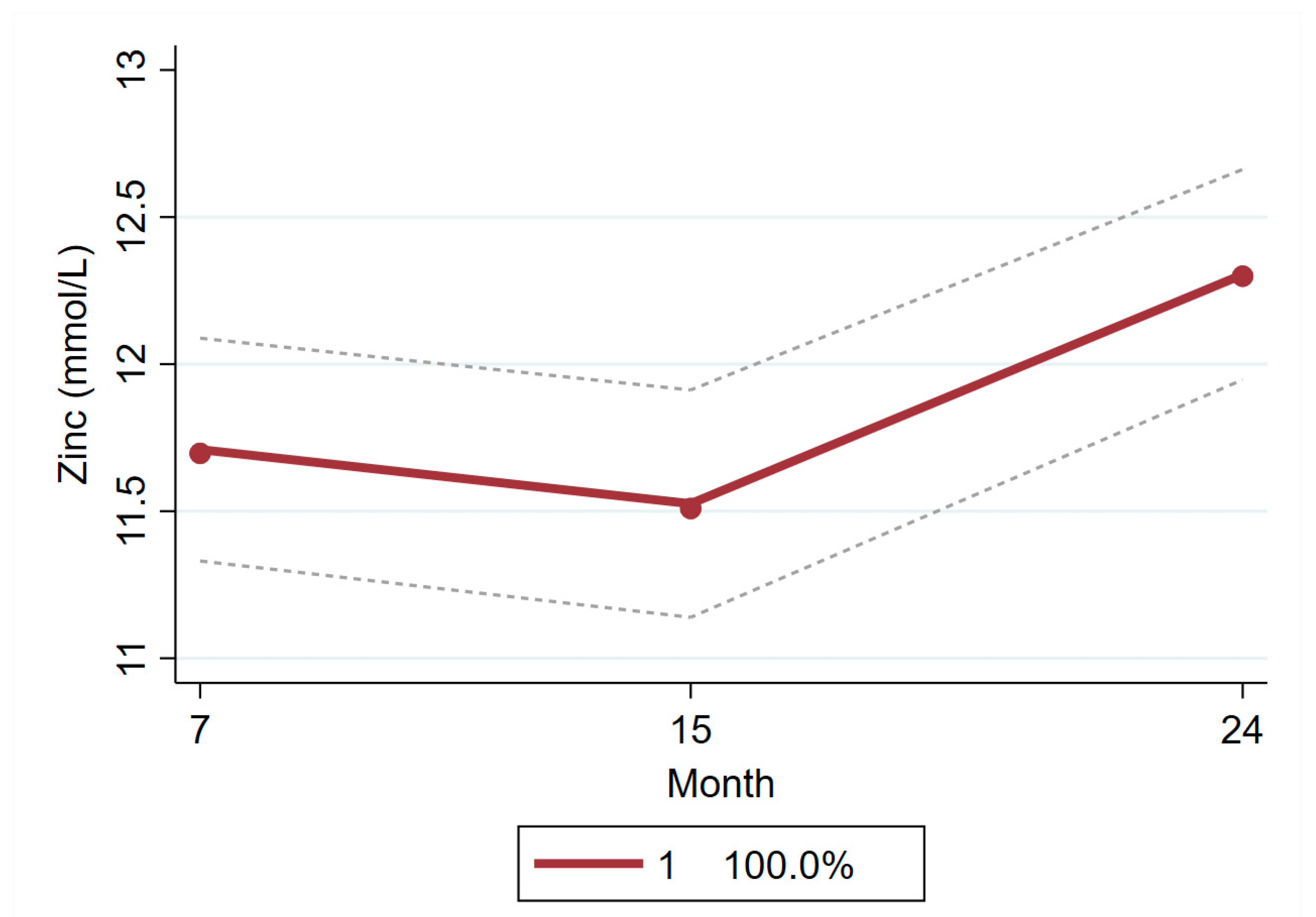
| All children | Mean = 11.71; 95% CI = 11.33, 12.09 | Mean = 11.53; 95% CI = 11.14, 11.91 | Mean = 12.30; 95% CI = 11.95, 12.66 |
| Outcome | Explanatory Variables | Coefficient, 95% CI | p Value |
|---|---|---|---|
| Hemoglobin level at 60 months | Trajectories for Hb, 7 to 24 months | ||
| Group 1 (Decreasing) | Reference | ||
| Group 2 (Increasing) | 0.21 (−0.56, 0.97) | 0.54 | |
| Mean iron intake at 60 mo in mg | 0.29 (0.04, 0.55) | 0.022 | |
| Mean percent energy from protein at 60 mo | 0.45 (0.18, 0.73) | 0.002 | |
| WAMI | −2.63 (−5.52, 0.25) | 0.073 | |
| Adjusted plasma ferritin at 60 months | Trajectories for ferritin, 7 to 24 months | ||
| Group 1 (Lower) | Reference | ||
| Group 2 (Higher) | 13.72 (1.15, 26.28) | 0.033 | |
| Plasma zinc (mmol) at 24 months | 1.98 (0.24, 3.71) | 0.026 | |
| Mean energy intake at 60 mo (kcal) | 0.002 (−0.02, 0.02) | 0.84 | |
| Mean protein intake at 60 mo | 0.09 (−0.48, 0.65) | 0.76 | |
| WAMI | −4.65 (−27.12, 17.82) | 0.68 | |
| Adjusted plasma retinol at 60 months | Trajectories for retinol, 7 to 24 months | ||
| Group 1 (Lower) | Reference | ||
| Group 2 (Higher) | 3.99 (1.04, 6.94) | 0.008 | |
| Mean vitamin A intake at 60 mos in µgRE | 0.003 (0.00007, 0.005) | 0.004 | |
| WAMI | 4.48 (−3.75, 12.73) | 0.28 | |
| Adjusted plasma zinc at 60 months | Zinc (mmol/L), 24 months | ||
| All children | 0.21 (0.02, 0.39) | 0.035 | |
| Mean zinc intake at 60 mo in mg | 0.02 (−0.18, 0.21) | 0.87 | |
| WAMI | −0.17 (−1.60, 1.27) | 0.82 |
| Factors Associated with Micronutrient Deficiencies from 7 Months to 60 Months | |
|---|---|
| Anemia |
|
| Iron deficiency |
|
| Zinc deficiency |
|
| Vitamin A deficiency |
|
| Early life trajectories of micronutrient that can predict 60 months micronutrient concentrations | |
| Plasma ferritin concentration at 60 months |
|
| Plasma zinc concentration at 60 months |
|
| Plasma retinol concentration at 60 months |
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahfuz, M.; Murray-Kolb, L.E.; Hasan, S.M.T.; Das, S.; Fahim, S.M.; Alam, M.A.; Caulfield, L.; Ahmed, T. Why Do Children in Slums Suffer from Anemia, Iron, Zinc, and Vitamin A Deficiency? Results from a Birth Cohort Study in Dhaka. Nutrients 2019, 11, 3025. https://doi.org/10.3390/nu11123025
Mahfuz M, Murray-Kolb LE, Hasan SMT, Das S, Fahim SM, Alam MA, Caulfield L, Ahmed T. Why Do Children in Slums Suffer from Anemia, Iron, Zinc, and Vitamin A Deficiency? Results from a Birth Cohort Study in Dhaka. Nutrients. 2019; 11(12):3025. https://doi.org/10.3390/nu11123025
Chicago/Turabian StyleMahfuz, Mustafa, Laura E. Murray-Kolb, S. M. Tafsir Hasan, Subhasish Das, Shah Mohammad Fahim, Mohammed Ashraful Alam, Laura Caulfield, and Tahmeed Ahmed. 2019. "Why Do Children in Slums Suffer from Anemia, Iron, Zinc, and Vitamin A Deficiency? Results from a Birth Cohort Study in Dhaka" Nutrients 11, no. 12: 3025. https://doi.org/10.3390/nu11123025
APA StyleMahfuz, M., Murray-Kolb, L. E., Hasan, S. M. T., Das, S., Fahim, S. M., Alam, M. A., Caulfield, L., & Ahmed, T. (2019). Why Do Children in Slums Suffer from Anemia, Iron, Zinc, and Vitamin A Deficiency? Results from a Birth Cohort Study in Dhaka. Nutrients, 11(12), 3025. https://doi.org/10.3390/nu11123025





