Malnutrition as a Strong Predictor of the Onset of Sarcopenia
Abstract
1. Introduction
2. Materials and Methods
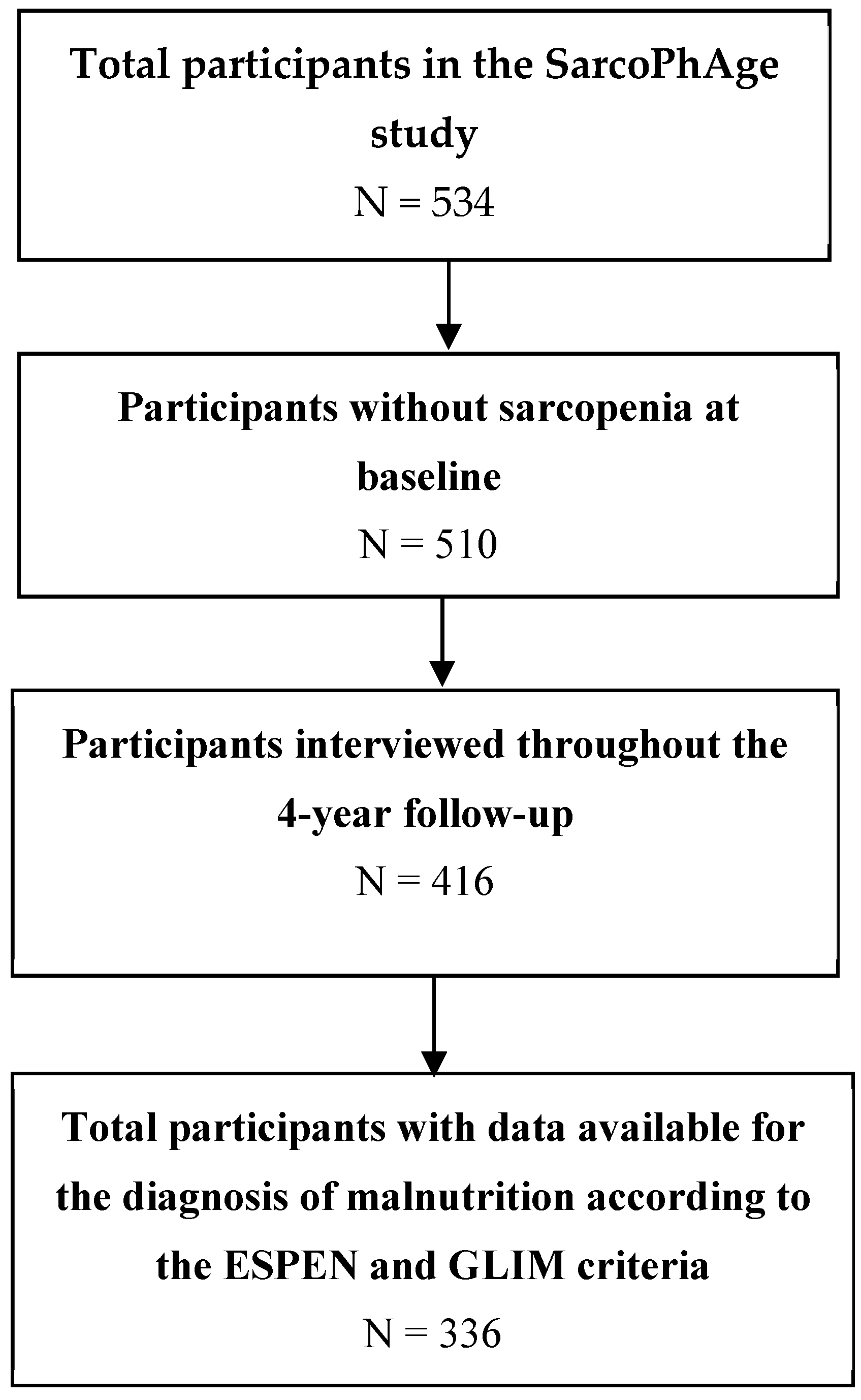
2.1. Population
2.2. Data Collection
2.2.1. Malnutrition Diagnosis
2.2.2. Sarcopenia Diagnosis
- (1)
- Low muscle strength (expressed in kg). Muscle strength was measured with a handgrip hand-held dynamometer (Saehan Corporation, MSD Europe Bvba, Brussels, Belgium) calibrated at the beginning of the study and at each year of follow-up for 10, 40, and 90 kg. We followed standardized procedures by asking participants to squeeze as hard as possible three times per hand. The highest value of the six measurements was considered in our analyses (Southampton protocol) [24]. Low muscle strength is defined as <27 kg in men and <16 kg in women [12].
- (2)
- Low muscle mass. Muscle mass was measured with a dual X-ray absorptiometer (Hologic Discovery A, USA), which was calibrated daily. Fat-free mass and appendicular lean mass, obtained from whole-body DXA scans, were divided by height squared (kg/m2) to obtain the fat-free mass index and appendicular lean mass index (ALMI) values, respectively. A low muscle mass is defined as FFMI <17 kg/m2 in men and <15 kg/m2 in women or ALMI <7 kg/m2 in men and <5.5 kg/m2 in women.
2.3. Covariates
2.4. Statistical Analysis
3. Results
3.1. Population and Diagnosis of Malnutrition
3.2. Incidence of Sarcopenia
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sánchez-Rodríguez, D.; Annweiler, C.; Ronquillo-Moreno, N.; Vázquez-Ibar, O.; Escalada, F.; Duran, X.; Muniesa, J.M.; Marco, E. Prognostic Value of the ESPEN Consensus and Guidelines for Malnutrition: Prediction of Post-Discharge Clinical Outcomes in Older Inpatients. Nutr. Clin. Pract. 2019, 34, 304–312. [Google Scholar] [CrossRef]
- Sanz-París, A.; Gómez-Candela, C.; Martín-Palmero, Á.; García-Almeida, J.M.; Burgos-Pelaez, R.; Matía-Martin, P.; Arbones-Mainar, J.M. Application of the new ESPEN definition of malnutrition in geriatric diabetic patients during hospitalization: A multicentric study. Clin. Nutr. 2016, 35, 1564–1567. [Google Scholar] [CrossRef]
- Jiang, J.; Hu, X.; Chen, J.; Wang, H.; Zhang, L.; Dong, B.; Yang, M. Predicting long-term mortality in hospitalized elderly patients using the new ESPEN definition. Sci. Rep. 2017, 7, 4067. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, D.; Marco, E.; Schott, A.-M.; Rolland, Y.; Blain, H.; Vázquez-Ibar, O.; Escalada, F.; Duran, X.; Muniesa, J.M.; Annweiler, C. Malnutrition according to ESPEN definition predicts long-term mortality in general older population: Findings from the EPIDOS study-Toulouse cohort. Clin. Nutr. 2018, 38, 2652–2658. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Krznarić, Z.; Singer, P.; Barazzoni, R.; Cederholm, T.; Golay, A.; Van Gossum, A.; Kennedy, N.; Kreymann, G.; Laviano, A.; et al. Effectiveness and efficacy of nutritional therapy: A systematic review following Cochrane methodology. Clin. Nutr. 2017, 36, 939–957. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholmm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef]
- Beard, J.R.; Officer, A.; Araujo de Carvalho, I.; Sadana, R.; Margriet Pot, A.; Michel, J.; Lloyd-Sherlock, P.; Epping-Jordan, J.E.; GMEEG, P.; Mahanani, W.R.; et al. The World report on ageing and health: A policy framework for healthy ageing. Lancet 2016, 387, 2145–2154. [Google Scholar] [CrossRef]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L. To create a consensus on malnutrition diagnostic criteria: A report from the Global Leadership Initiative on Malnutrition (GLIM) meeting at the ESPEN Congress 2016. Clin. Nutr. 2017, 36, 7–10. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.; Correia, M.; Gonzalez, M.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachex Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Sieber, C.C. Malnutrition and sarcopenia. Aging Clin. Exp. Res. 2019, 31, 793–798. [Google Scholar] [CrossRef]
- Mohseni, R.; Aliakbar, S.; Abdollahi, A.; Yekaninejad, M.S.; Maghbooli, Z.; Mirzaei, K. Relationship between major dietary patterns and sarcopenia among menopausal women. Aging Clin. Exp. Res. 2017, 29, 1241–1248. [Google Scholar] [CrossRef]
- Beaudart, C.; Locquet, M.; Touvier, M.; Reginster, J.-Y.; Bruyère, O. Association between dietary nutrient intake and sarcopenia in the SarcoPhAge study. Aging Clin. Exp. Res. 2019, 31, 815–824. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet (Lond. Engl.) 2007, 370, 453–457. [Google Scholar] [CrossRef]
- Beaudart, C.; Reginster, J.; Petermans, J.; Gillain, S.; Quabron, A.; Locquet, M.; Slomian, J.; Buckinx, F.; Bruyere, O. Quality of life and physical components linked to sarcopenia: The SarcoPhAge study. Exp. Gerontol. 2015, 69, 103–110. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Guigoz, Y.; Lauque, S.; Vellas, B.J. Identifying the elderly at risk for malnutrition. Clin. Geriatr. Med. 2002, 18, 737–757. [Google Scholar] [CrossRef]
- Justice, J.N.; Ferrucci, L.; Newman, A.B.; Aroda, V.R.; Bahnson, J.L.; Divers, J.; Espeland, M.A.; Marcovina, S.; Pollak, M.N.; Kritchevsky, S.B.; et al. A framework for selection of blood-based biomarkers for geroscience-guided clinical trials: Report from the TAME Biomarkers Workgroup. GeroScience 2018, 40, 419–436. [Google Scholar] [CrossRef]
- Adriaensen, W.; Mathei, C.; Vaes, B.; Van Pottelbergh, G.; Wallemacq, P.; Degryse, J.-M. Interleukin-6 predicts short-term global functional decline in the oldest old: Results from the BELFRAIL study. AGE 2014, 36, 1–14. [Google Scholar] [CrossRef][Green Version]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Ferrucci, L.; Pieper, C.F.; Leveille, S.G.; Markides, K.S.; Ostir, G.V.; Studenski, S.; Berkman, L.F.; Wallace, R.B. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2000, 55, M221–M231. [Google Scholar] [CrossRef]
- Beaudart, C.; Rolland, Y.; Cruz-Jentoft, A.J.; Bauer, J.M.; Sieber, C.; Cooper, C.; Al-Daghri, N.; De Carvalho, I.A.; Bautmans, I.; Bernabei, R.; et al. Assessment of Muscle Function and Physical Performance in Daily Clinical Practice: A position paper endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Calcif. Tissue Int. 2019, 105, 1–14. [Google Scholar] [CrossRef]
- Tombaugh, T.N.; McIntyre, N.J. The Mini-Mental State Examination: A Comprehensive Review. J. Am. Geriatr Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living. Gerontology 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Taylor, H.L.; Jacobs, D.R.; Schucker, B.; Knudsen, J.; Leon, A.S.; Debacker, G.; Jacobs, D.R., Jr. A questionnaire for the assessment of leisure time physical activities. J. Chronic Dis. 1978, 31, 741–755. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef]
- Zadak, Z.; Hyspler, R.; Ticha, A.; Vlcek, J. Polypharmacy and malnutrition. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 50–55. [Google Scholar] [CrossRef]
- Sanders, C.; Behrens, S.; Schwartz, S.; Wengreen, H.; Corcoran, C.D.; Lyketsos, C.G.; Tschanz, J.T. Nutritional Status is Associated with Faster Cognitive Decline and Worse Functional Impairment in the Progression of Dementia: The Cache County Dementia Progression Study1. J. Alzheimer’s Dis. 2016, 52, 33–42. [Google Scholar] [CrossRef]
- Steffl, M.; Bohannon, R.W.; Sontakova, L.; Tufano, J.J.; Shiells, K.; Holmerova, I. Relationship between sarcopenia and physical activity in older people: A systematic review and meta-analysis. Clin. Interv. Aging 2017, 12, 835–845. [Google Scholar] [CrossRef]
- Contreras-Bolívar, V.; Sánchez-Torralvo, F.J.; Ruiz-Vico, M.; González-Almendros, I.; Barrios, M.; Padín, S.; Alba, E.; Olveira, G. GLIM Criteria Using Hand Grip Strength Adequately Predict Six-Month Mortality in Cancer Inpatients. Nutrients 2019, 11, 2043. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Atilla, F.D.; Sahin, F.; Saydam, G. The effect of malnutrition on mortality in hospitalized patients with hematologic malignancy. Support. Care Cancer 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, C.; Slinde, F.; Majeed, A.; Bottai, M.; Wahlin, S. Nutrition impact symptoms are related to malnutrition and quality of life-A cross-sectional study of patients with chronic liver disease. Clin. Nutr. 2019. [Google Scholar] [CrossRef]
- Karahalios, A.; English, D.R.; Simpson, J.A. Change in body size and mortality: A systematic review and meta-analysis. Int. J. Epidemiol. 2017, 46, 526–546. [Google Scholar] [CrossRef]
- Vettoretti, S.; Caldiroli, L.; Armelloni, S.; Ferrari, C.; Cesari, M.; Messa, P. Sarcopenia is Associated with Malnutrition but Not with Systemic Inflammation in Older Persons with Advanced CKD. Nutrition 2019, 11, 1378. [Google Scholar] [CrossRef]
- Marco, E.; Sánchez-Rodríguez, D.; Dávalos-Yerovi, V.N.; Duran, X.; Pascual, E.M.; Muniesa, J.M.; Rodríguez, D.A.; Aguilera-Zubizarreta, A.; Escalada, F.; Duarte, E. Malnutrition according to ESPEN consensus predicts hospitalizations and long-term mortality in rehabilitation patients with stable chronic obstructive pulmonary disease. Clin. Nutr. 2019, 38, 2180–2186. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, D.; Marco, E.; Ronquillo-Moreno, N.; Miralles, R.; Vázquez-Ibar, O.; Escalada, F.; Muniesa, J.M. Prevalence of malnutrition and sarcopenia in a post-acute care geriatric unit: Applying the new ESPEN definition and EWGSOP criteria. Clin. Nutr. 2017, 36, 1339–1344. [Google Scholar] [CrossRef]
- Martone, A.M.; Bianchi, L.; Abete, P.; Bellelli, G.; Bo, M.; Cherubini, A.; Corica, F.; Di Bari, M.; Maggio, M.; Manca, G.M.; et al. The incidence of sarcopenia among hospitalized older patients: Results from the Glisten study. J. Cachex Sarcopenia Muscle 2017, 8, 907–914. [Google Scholar] [CrossRef]
- Mithal, A.; Bonjour, J.P.; Boonen, S.; Burckhardt, P.; Degens, H.; Fuleihan, G.A.E.; Josse, R.; Lips, P.; Torres, J.M.; Rizzoli, R.; et al. Impact of nutrition on muscle mass, strength, and performance in older adults. Osteoporos. Int. 2013, 24, 1555–1566. [Google Scholar] [CrossRef] [PubMed]
- Beckwée, D.; Delaere, A.; Aelbrecht, S.; Baert, V.; Beaudart, C.; Bruyere, O.; de Saint-Hubert, M.; Bautmans, I. Exercise Interventions for the Prevention and Treatment of Sarcopenia. A Systematic Umbrella Review. J. Nutr. Health Aging 2019, 23, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.M.; Reginster, J.Y.; Rizzoli, R.; Shaw, S.C.; Kanis, J.A.; Bautmans, I.; Bischoff-Ferrariet, H.; Bruyère, O.; Cesari, M.; Dawson-Hughes, B.; et al. Does nutrition play a role in the prevention and management of sarcopenia? Clin. Nutr. 2018, 37, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Vellas, B.; Fielding, R.A.; Bens, C.; Bernabei, R.; Cawthon, P.M.; Cederholm, T.; Cruz-Jentoft, A.J.; Del Signore, S.; Donahue, S.; Morley, J.; et al. Implications of ICD-10 for Sarcopenia Clinical Practice and Clinical Trials: Report by the International Conference on Frailty and Sarcopenia Research Task Force. J. Frailty Aging 2018, 7, 2–9. [Google Scholar] [PubMed]
- Dubard, C.A.; Jackson, C.T. Active Redesign of a Medicaid Care Management Strategy for Greater Return on Investment: Predicting Impactability. Popul. Health Manag. 2018, 21, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Juby, A.G.; Mager, D.R. A review of nutrition screening tools used to assess the malnutrition-sarcopenia syndrome (MSS) in the older adult. Clin. Nutr. ESPEN 2019, 32, 8–15. [Google Scholar] [CrossRef] [PubMed]

| Phenotypic | |
|---|---|
| Weight loss | A weight loss >4.5 kg in the past year was reported and used as a threshold [20]. Unintentional weight loss was obtained by clinical interview at baseline. |
| BMI | BMI (kg/m2) was considered reduced if <20 kg/m2 or <22 kg/m2 in participants younger and older than 70 years, respectively [19]. |
| Reduced muscle mass | FFMI <17 kg/m² in men and <15 kg/m2 in women or ALMI <7 kg/m2 in men and <5.5 kg/m2 in women was used as a threshold [12,19]. |
| Etiological | |
| Reduced food intake or assimilation | The first Mini-nutritional Assessment- Short Form (MNA-SF) [21] item was used to determine reduced food intake: “Has food intake declined over the past 3 months due to loss of appetite, digestive problems, chewing or swallowing difficulties?” Severe and moderate decreases were considered positive answers [21]. Chronic gastrointestinal conditions that adversely impact food assimilation or absorption of nutrients were also considered. |
| Disease burden and inflammation | Interleukin-6 (IL-6) and insulin-like growth factor 1 (IGF-1) were selected as biomarkers to assess inflammation, following recommendations by the Targeting Aging Biomarkers Workgroup for the selection of blood-based biomarkers for geroscience-guided clinical trials [22]. Quartiles for IGF-1 and IL-6 in our own data were calculated in both sexes, and the lowest quartile was considered as a sex-specific threshold: IGF-1 ≤88 ng/mL in men and ≤82 ng/mL in women and IL-6 >3.84 pg/mL in men and >2.99 pg/mL in women [23]. The number of diseases was recorded; disease burden was not assessed. |
| Studied Sample (n = 336) | Malnutrition According to the ESPEN Criteria | Malnutrition According to the GLIM Criteria | |||||
|---|---|---|---|---|---|---|---|
| Yes (n = 19) | No (n = 317) | p-Value | Yes (n = 59) | No (n = 277) | p-Value | ||
| Age, years | 72.5 ± 5.8 | 71.9 ± 7.1 | 72.6 ± 5.7 | 0.62 | 72.0 ± 6.3 | 72.6 ± 5.7 | 0.44 |
| Sex, women | 186 (55.4%) | 13 (68.4%) | 173 (54.6%) | 0.24 | 39 (66.1%) | 147 (53.1%) | 0.07 |
| Number of concomitant diseases per participant | 4.1 ± 2.4 | 5.0 ± 2.8 | 4.1 ± 2.4 | 0.09 | 4.9 ± 2.4 | 3.9 ± 2.4 | 0.005 |
| Number of drugs per participant | 5.6 ± 3.4 | 5.9 ± 3.5 | 5.6 ± 3.4 | 0.64 | 6.0 ± 3.3 | 5.6 ± 3.4 | 0.29 |
| MMSE, /30 points | 28.3 ± 1.8 | 27.9 ± 1.4 | 28.3 ± 1.9 | 0.41 | 28.0 ± 2.1 | 28.3 ± 1.7 | 0.14 |
| Body mass index, kg/m2 | 27.1 ± 4.6 | 20.9 ± 0.7 | 27.4 ± 0.2 | <0.001 | 24.0 ± 4.0 | 27.7 ± 4.5 | <0.001 |
| Lean mass total, kg | |||||||
| Men | 56.5 ± 8.7 | 44.8 ± 4.0 | 57.0 ± 8.6 | 0.001 | 48.7 ± 8.1 | 57.7 ± 8.2 | <0.001 |
| Women | 39.0 ± 5.8 | 35.1 ± 3.5 | 39.3 ± 5.9 | 0.013 | 36.1 ± 4.8 | 39.7 ± 5.8 | 0.001 |
| ALMI, kg/m2 | |||||||
| Men | 8.1 ± 1.0 | 6.6 ± 0.7 | 8.1 ± 1.0 | 0.001 | 7.1 ± 1.0 | 8.2 ± 1.0 | <0.001 |
| Women | 6.1 ± 1.0 | 5.3 ± 0.5 | 6.2 ± 1.0 | 0.003 | 5.6 ± 0.7 | 6.3 ± 1.0 | <0.001 |
| Muscle strength (kg) | |||||||
| Men | 40.4 ± 8.3 | 25.8 ± 7.9 | 41.0 ± 7.7 | <0.001 | 35.7 ± 11.3 | 41.1 ± 7.5 | 0.006 |
| Women | 22.6 ± 6.9 | 23.3 ± 6.3 | 22.5 ± 7.0 | 0.69 | 21.7 ± 4.9 | 22.8 ± 7.3 | 0.36 |
| Gait speed, m/s | 1.02 ± 0.27 | 1.10 ± 0.30 | 1.01 ± 0.27 | 0.21 | 1.00 ± 0.30 | 1.03 ± 0.26 | 0.53 |
| SPPB, /12 points | 9.7 ± 2.0 | 10.2 ± 2.3 | 9.7 ± 1.9 | 0.27 | 9.3 ± 2.5 | 9.8 ± 1.8 | 0.08 |
| Chair stand test, s | 13.7 ± 5.2 | 13.6 ± 7.3 | 13.7 ± 5.0 | 0.95 | 14.4 ± 6.1 | 13.5 ± 5.0 | 0.26 |
| IADL Lawton | |||||||
| /5 for men | 4.6 ± 1.2 | 3.8 ± 1.8 | 4.6 ± 1.2 | 0.09 | 4.2 ± 1.7 | 4.7 ± 1.1 | 0.08 |
| /8 for women | 7.6 ± 1.0 | 7.3 ± 1.4 | 7.6 ± 1.0 | 0.32 | 7.4 ± 1.3 | 7.6 ± 0.9 | 0.24 |
| Level of physical activity, kcal/day | 745.7 (270–1523.2) | 840 (106–1470) | 742 (270–1554) | 0.57 | 935 (150–1470) | 735 (270–1568) | 0.53 |
| Analysis Performed According to the ESPEN Criteria | |||||
| Occurrence of Sarcopenia | p-Value | Crude HR (95% CI) | Adjusted HR (95% CI) * | ||
| Malnutrition status | No incident sarcopenia (n = 290) | Incident sarcopenia (n = 46) | |||
| Well nourished | 278 (95.9%) | 39 (84.8%) | 0.005 | 3.91(1.73–8.81) | 4.28 (1.86–9.86) |
| Malnourished | 12 (4.1%) | 7 (15.2%) | |||
| Occurrence of severe sarcopenia | p-Value | Crude HR (95% CI) | Adjusted HR (95% CI) * | ||
| Malnutrition status | No incident severe sarcopenia (n = 310) | Incident severe sarcopenia (n = 26) | |||
| Well nourished | 295 (95.2%) | 22 (84.6%) | 0.035 | 3.54 (1.21–10.34) | 3.86 (1.29–11.54) |
| Malnourished | 15 (4.8%) | 4 (15.4%) | |||
| Analysis performed according to the GLIM criteria | |||||
| Occurrence of sarcopenia | p-Value | Crude HR (95% CI) | Adjusted HR (95% CI) * | ||
| Malnutrition status | No incident sarcopenia (n = 290) | Incident sarcopenia (n = 46) | |||
| Well nourished | 247 (85.2%) | 30 (65.2%) | 0.001 | 3.22 (1.74–5.94) | 3.23 (1.73–6.05) |
| Malnourished | 43 (14.8%) | 16 (34.8%) | |||
| Occurrence of severe sarcopenia | p-Value | Crude HR (95% CI) | Adjusted HR (95% CI) * | ||
| Malnutrition status | No incident severe sarcopenia (n = 310) | Incident severe sarcopenia (n = 26) | |||
| Well nourished | 260 (83.9) | 17 (65.4) | 0.021 | 2.90 (1.29–6.53) | 2.87 (1.25–6.56) |
| Malnourished | 50 (16.1) | 9 (34.6) | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beaudart, C.; Sanchez-Rodriguez, D.; Locquet, M.; Reginster, J.-Y.; Lengelé, L.; Bruyère, O. Malnutrition as a Strong Predictor of the Onset of Sarcopenia. Nutrients 2019, 11, 2883. https://doi.org/10.3390/nu11122883
Beaudart C, Sanchez-Rodriguez D, Locquet M, Reginster J-Y, Lengelé L, Bruyère O. Malnutrition as a Strong Predictor of the Onset of Sarcopenia. Nutrients. 2019; 11(12):2883. https://doi.org/10.3390/nu11122883
Chicago/Turabian StyleBeaudart, Charlotte, Dolores Sanchez-Rodriguez, Médéa Locquet, Jean-Yves Reginster, Laetitia Lengelé, and Olivier Bruyère. 2019. "Malnutrition as a Strong Predictor of the Onset of Sarcopenia" Nutrients 11, no. 12: 2883. https://doi.org/10.3390/nu11122883
APA StyleBeaudart, C., Sanchez-Rodriguez, D., Locquet, M., Reginster, J.-Y., Lengelé, L., & Bruyère, O. (2019). Malnutrition as a Strong Predictor of the Onset of Sarcopenia. Nutrients, 11(12), 2883. https://doi.org/10.3390/nu11122883







