Genetic Deletion of Syndecan-4 Alters Body Composition, Metabolic Phenotypes, and the Function of Metabolic Tissues in Female Mice Fed A High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Husbandry
2.2. Body Composition, Activity, and Indirect Calorimetry
2.3. Plasma Analyses and Glucose Tolerance Test
2.4. Histological Analyses
2.5. Hepatic TG Levels
2.6. RNA Isolation and Gene Expression
2.7. Western Blotting Analysis
2.8. Statistical Analysis
2.9. Ethics Approval
3. Results
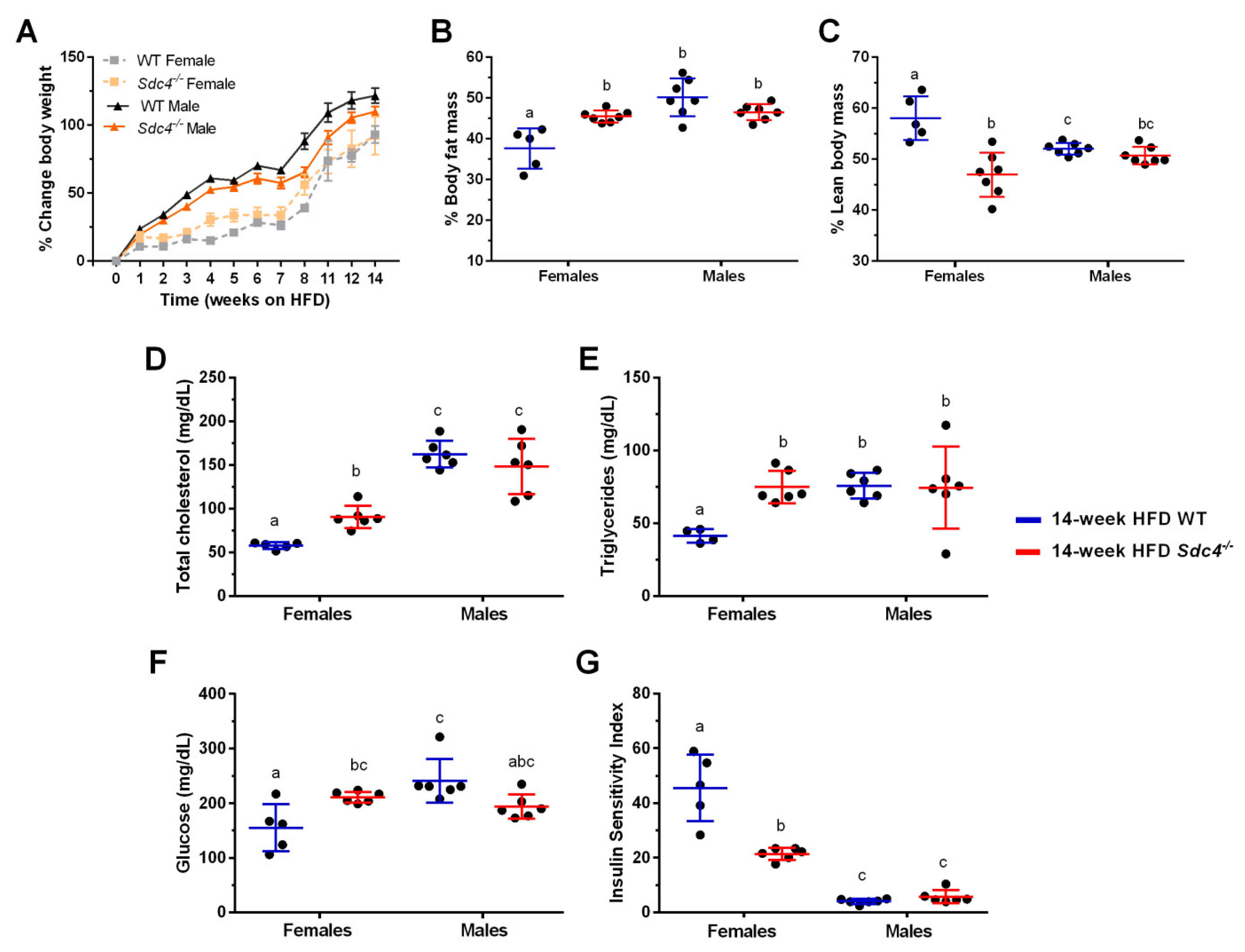
3.1. Sdc4 Deficiency Increases Percent Body Fat Mass and Exacerbates Metabolic Complications in Obese Female Mice but Not in Males
3.2. Sdc4 Deficiency Reduces Food Intake in Obese Mice, Independent of Sex
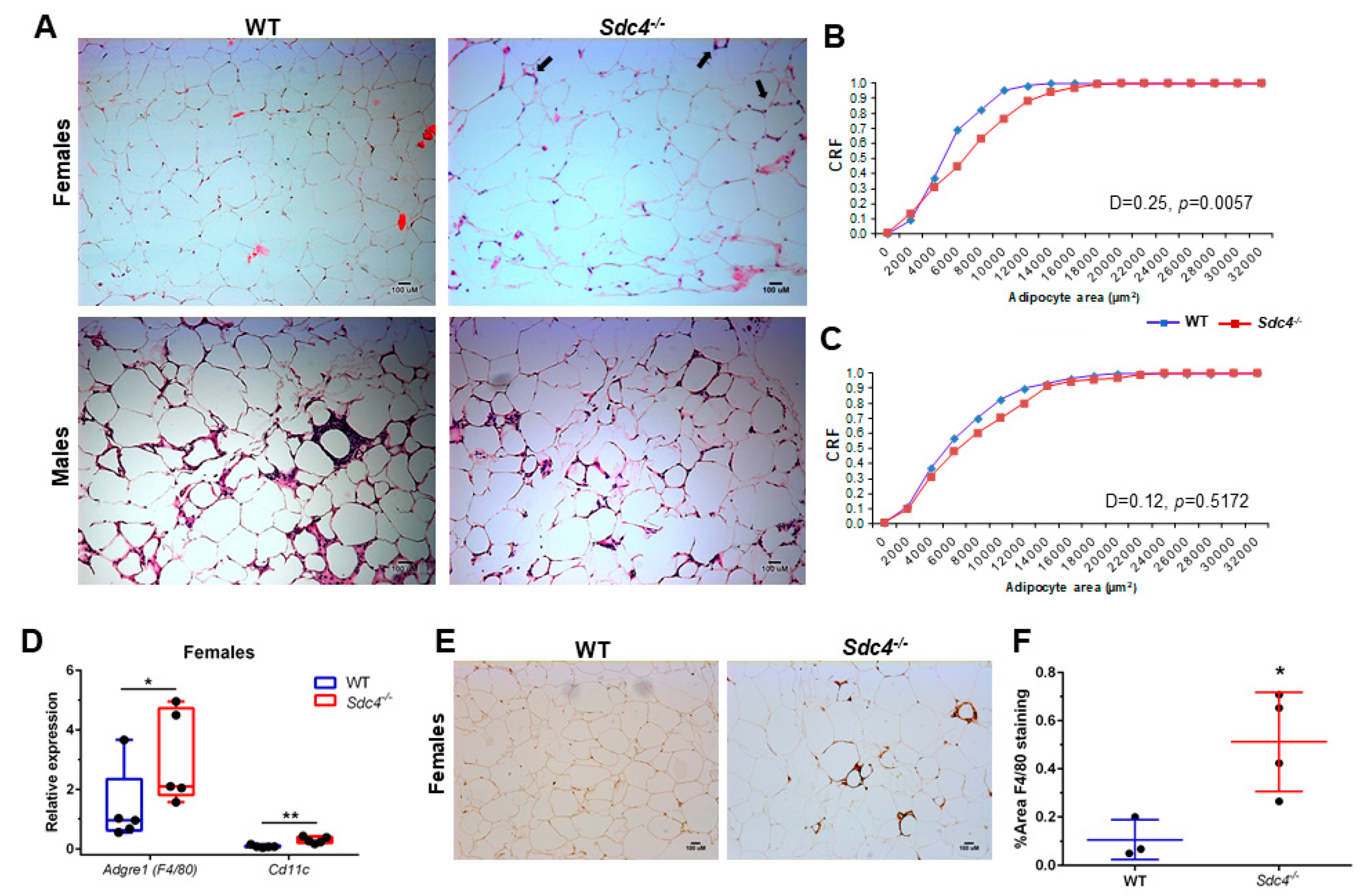
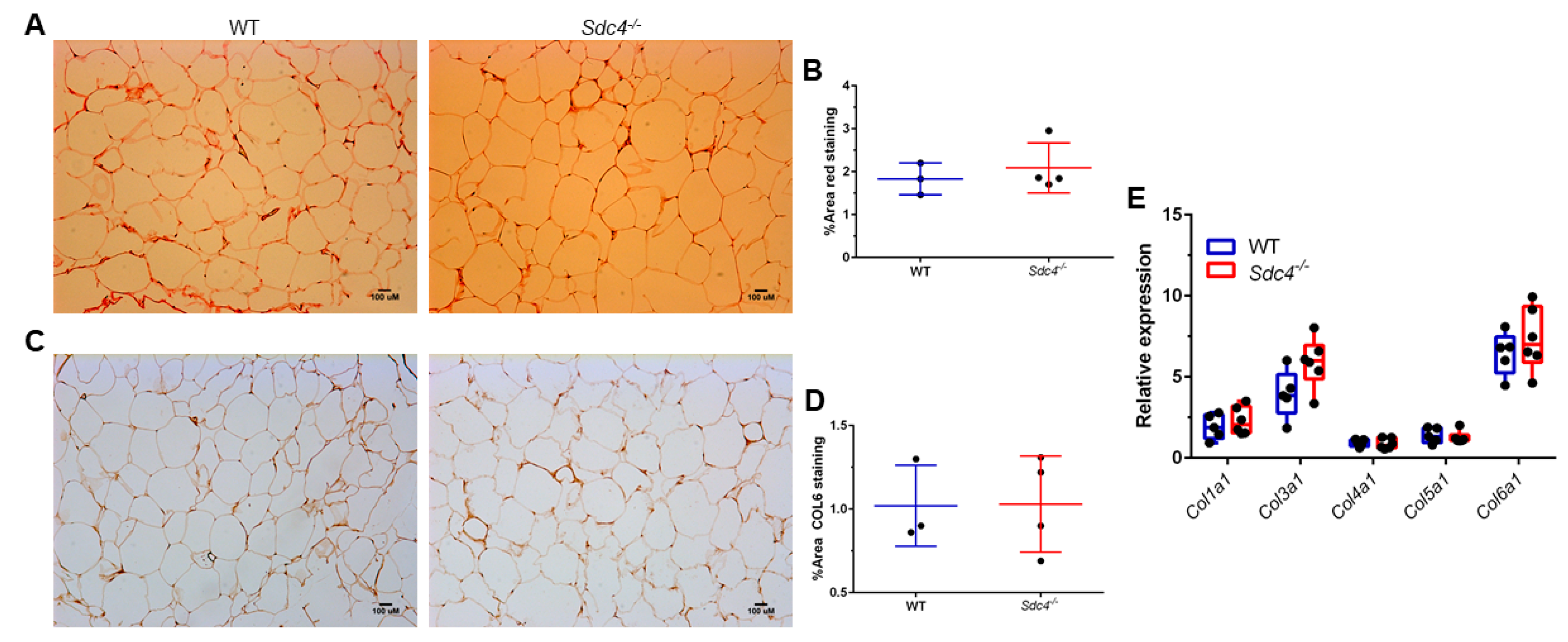
3.3. Sdc4 Deficiency Induces Adipocyte Hypertrophy and Macrophage Infiltration into Visceral Adipose Tissue of Obese Female Mice, but Not Fibrosis
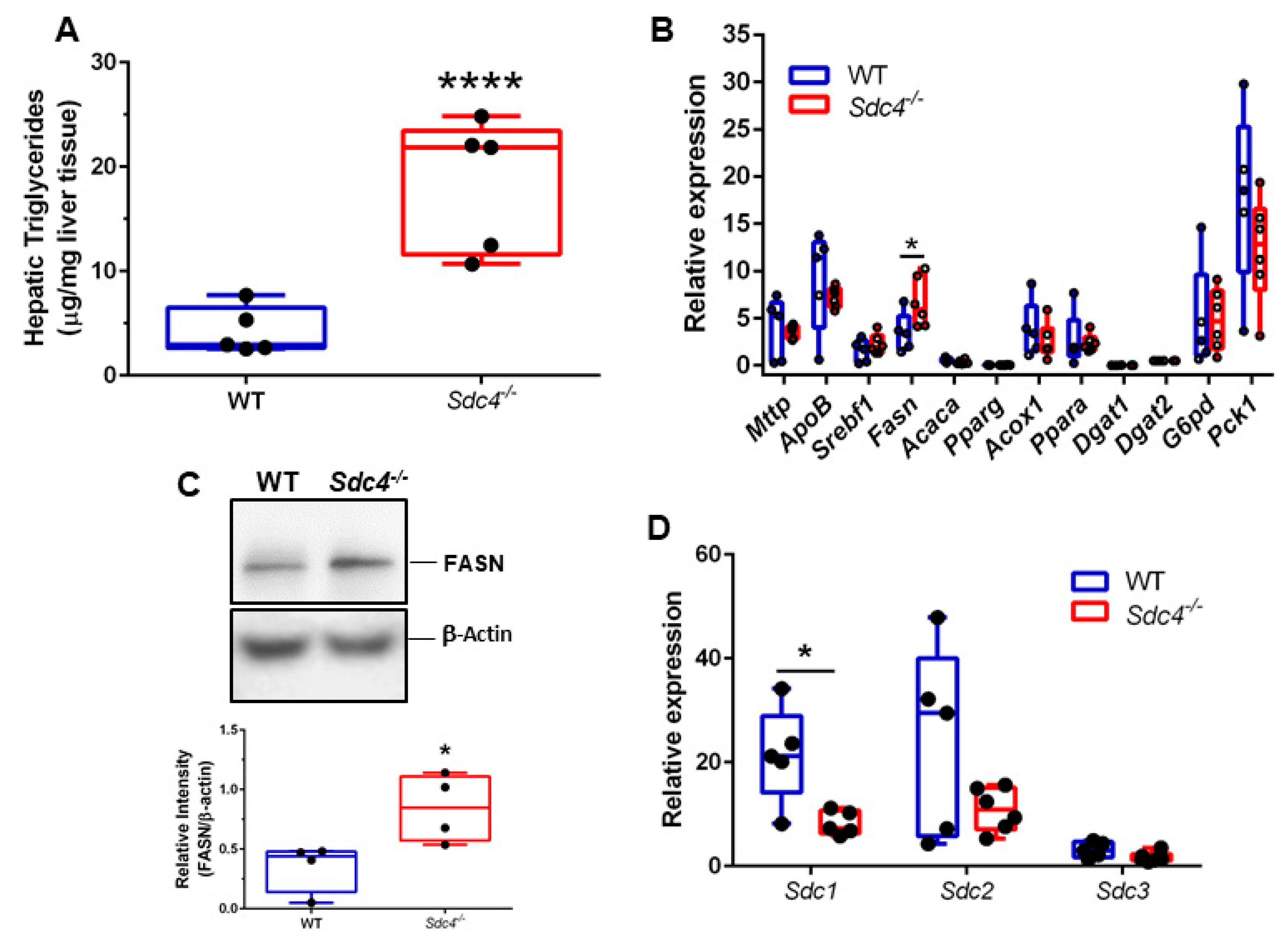
3.4. Sdc4 Deficiency Leads to Increased TG Content and FASN Levels in the Liver of Obese Female Mice
3.5. Sdc4 Deficiency Leads to Decreased Sdc1 Transcript Levels in the Liver of Obese Female Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Collaborators, G.B.D.O.; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Mariman, E.C.M.; Wang, P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell. Mol. Life Sci. 2010, 67, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Rockey, D.C.; Bell, P.D.; Hill, J.A.; Ben Salem, C.; Slim, R.; Fathallah, N.; Urban, M.L.; Manenti, L.; Vaglio, A. Fibrosis—A Common Pathway to Organ Injury and Failure. N. Engl. J. Med. 2015, 372, 1138–1149. [Google Scholar] [CrossRef]
- Lunde, I.G.; Herum, K.M.; Carlson, C.C.; Christensen, G. Syndecans in heart fibrosis. Cell Tissue Res. 2016, 365, 539–552. [Google Scholar] [CrossRef]
- Herum, K.M.; Lunde, I.G.; Škrbić, B.; Florholmen, G.; Behmen, D.; Sjaastad, I.; Carlson, C.R.; Gomez, M.F.; Christensen, G. Syndecan-4 signaling via NFAT regulates extracellular matrix production and cardiac myofibroblast differentiation in response to mechanical stress. J. Mol. Cell. Cardiol. 2013, 54, 73–81. [Google Scholar] [CrossRef]
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 2011, 3, a004952. [Google Scholar] [CrossRef]
- Couchman, J.R. Transmembrane Signaling Proteoglycans. Annu. Rev. Cell Dev. Biol. 2010, 26, 89–114. [Google Scholar] [CrossRef]
- Chakravarti, R.; Adams, J.C. Comparative genomics of the syndecans defines an ancestral genomic context associated with matrilins in vertebrates. BMC Genom. 2006, 7, 83. [Google Scholar] [CrossRef]
- Bellin, R.; Capila, I.; Lincecum, J.; Park, P.W.; Reizes, O.; Bernfield, M.R. Unlocking the secrets of syndecans: Transgenic organisms as a potential key. Glycoconj. J. 2002, 19, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jin, H.; Beauvais, D.M.; Rapraeger, A.C. Cytoplasmic domain interactions of syndecan-1 and syndecan-4 with alpha6beta4 integrin mediate human epidermal growth factor receptor (HER1 and HER2)-dependent motility and survival. J. Biol. Chem. 2014, 289, 30318–30332. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.R.; Humphries, M.J.; Bass, M.D. Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell Biol. 2007, 8, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Bellin, R.M.; Kubicek, J.D.; Frigault, M.J.; Kamien, A.J.; Steward, R.L.; Barnes, H.M.; Digiacomo, M.B.; Duncan, L.J.; Edgerly, C.K.; Morse, E.M.; et al. Defining the role of syndecan-4 in mechanotransduction using surface-modification approaches. Proc. Natl. Acad. Sci. USA 2009, 106, 22102–22107. [Google Scholar] [CrossRef]
- Rønning, S.B.; Carlson, C.R.; Stang, E.; Kolset, S.O.; Hollung, K.; Pedersen, M.E. Syndecan-4 Regulates Muscle Differentiation and Is Internalized from the Plasma Membrane during Myogenesis. PLoS ONE 2015, 10, e0129288. [Google Scholar] [CrossRef]
- Reizes, O.; Lincecum, J.; Wang, Z.; Goldberger, O.; Huang, L.; Kaksonen, M.; Ahima, R.; Hinkes, M.T.; Barsh, G.S.; Rauvala, H.; et al. Transgenic expression of syndecan-1 uncovers a physiological control of feeding behavior by syndecan-3. Cell 2001, 106, 105–116. [Google Scholar] [CrossRef]
- Reizes, O.; Benoit, S.C.; Strader, A.D.; Clegg, D.J.; Akunuru, S.; Seeley, R.J. Syndecan-3 modulates food intake by interacting with the melanocortin/AgRP pathway. Ann. N. Y. Acad. Sci. 2003, 994, 66–73. [Google Scholar] [CrossRef]
- Strader, A.D.; Reizes, O.; Woods, S.C.; Benoit, S.C.; Seeley, R.J. Mice lacking the syndecan-3 gene are resistant to diet-induced obesity. J. Clin. Investig. 2004, 114, 1686–1687. [Google Scholar]
- De Luca, M.; Klimentidis, Y.C.; Casazza, K.; Chambers, M.M.; Cho, R.; Harbison, S.T.; Jumbo-Lucioni, P.; Zhang, S.; Leips, J.; Fernández, J.R. A Conserved Role for Syndecan Family Members in the Regulation of Whole-Body Energy Metabolism. PLoS ONE 2010, 5, e11286. [Google Scholar] [CrossRef]
- Azeez, O.I.; Meintjes, R.; Chamunorwa, J.P. Fat body, fat pad and adipose tissues in invertebrates and vertebrates: The nexus. Lipids Health Dis. 2014, 13, 71. [Google Scholar] [CrossRef]
- Eveland, M.; Brokamp, G.A.; Lue, C.H.; Harbison, S.T.; Leips, J.; De Luca, M. Knockdown expression of Syndecan in the fat body impacts nutrient metabolism and the organismal response to environmental stresses in Drosophila melanogaster. Biochem. Biophys. Res. Commun. 2016, 477, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Rose, G.; Crocco, P.; De Rango, F.; Corsonello, A.; Lattanzio, F.; De Luca, M.; Passarino, G. Metabolism and successful aging: Polymorphic variation of syndecan-4 (SDC4) gene associate with longevity and lipid profile in healthy elderly Italian subjects. Mech. Ageing Dev. 2015, 150, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Kunnas, T.; Nikkari, S.T. Contribution of syndecan-4 genetic variants to hypertension, the TAMRISK study. BMC Res. Notes 2014, 7, 815. [Google Scholar] [CrossRef] [PubMed]
- Tchernof, A.; Després, J.P. Pathophysiology of Human Visceral Obesity: An Update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Smith, D.L.; Keating, K.D.; Allison, D.B.; Nagy, T.R. Variations in body weight, food intake and body composition after long-term high-fat diet feeding in C57BL/6J mice. Obesity 2014, 22, 2147–2155. [Google Scholar] [CrossRef] [PubMed]
- Stubbins, R.E.; Najjar, K.; Holcomb, V.B.; Hong, J.; Nunez, N.P. Oestrogen alters adipocyte biology and protects female mice from adipocyte inflammation and insulin resistance. Diabetes Obes. Metab. 2012, 14, 58–66. [Google Scholar] [CrossRef]
- Echtermeyer, F.; Streit, M.; Wilcox-Adelman, S.; Saoncella, S.; Denhez, F.; Detmar, M.; Goetinck, P.F. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J. Clin. Investig. 2001, 107, R9–R14. [Google Scholar] [CrossRef]
- Jones, A.; Johnson, M.; Nagy, T. Validation of quantitative magnetic resonance for the determination of body composition of mice. Int. J. Body Compos. Res. 2009, 7, 67–72. [Google Scholar]
- Davis, R.A.H.; Halbrooks, J.E.; Watkins, E.E.; Fisher, G.; Hunter, G.R.; Nagy, T.R.; Plaisance, E.P. High-intensity interval training and calorie restriction promote remodeling of glucose and lipid metabolism in diet-induced obesity. Am. J. Physiol. Metab. 2017, 313, E243–E256. [Google Scholar] [CrossRef]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Berry, R.; Church, C.D.; Gericke, M.T.; Jeffery, E.; Colman, L.; Rodeheffer, M.S. Imaging of adipose tissue. Methods Enzymol. 2014, 537, 47–73. [Google Scholar] [PubMed]
- Di Girolamo, M.; Mendlinger, S.; Fertig, J. A simple method to determine fat cell size and number in four mammalian species. Am. J. Physiol. Leg. Content 1971, 221, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Filiano, A.N.; Millender-Swain, T.; Johnson, R.; Young, M.E.; Gamble, K.L.; Bailey, S.M. Chronic Ethanol Consumption Disrupts the Core Molecular Clock and Diurnal Rhythms of Metabolic Genes in the Liver without Affecting the Suprachiasmatic Nucleus. PLoS ONE 2013, 8, e71684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derveaux, S.; Vandesompele, J.; Hellemans, J. How to do successful gene expression analysis using real-time PCR. Methods 2010, 50, 227–230. [Google Scholar] [CrossRef]
- Hwang, L.L.; Wang, C.H.; Li, T.L.; Chang, S.D.; Lin, L.C.; Chen, C.P.; Chen, C.T.; Liang, K.C.; Ho, I.K.; Yang, W.S.; et al. Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obesity 2010, 18, 463–469. [Google Scholar] [CrossRef]
- Pisconti, A.; Bernet, J.D.; Olwin, B.B. Syndecans in skeletal muscle development, regeneration and homeostasis. Muscle Ligaments Tendons J. 2012, 2, 1–9. [Google Scholar]
- Pang, Q.; Zhang, J.Y.; Song, S.D.; Qu, K.; Xu, X.S.; Liu, S.S.; Liu, C. Central obesity and nonalcoholic fatty liver disease risk after adjusting for body mass index. World J. Gastroenterol. 2015, 21, 1650–1662. [Google Scholar] [CrossRef]
- Wang, Z.; Telci, D.; Griffin, M. Importance of syndecan-4 and syndecan-2 in osteoblast cell adhesion and survival mediated by a tissue transglutaminase−fibronectin complex. Exp. Cell Res. 2011, 317, 367–381. [Google Scholar] [CrossRef]
- Deng, Y.; Foley, E.M.; Gonzales, J.C.; Gordts, P.L.; Li, Y.; Esko, J.D. Shedding of syndecan-1 from human hepatocytes alters very low density lipoprotein clearance. Hepatology 2012, 55, 277–286. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Pandya, S.K.; Varshney, S.; Shankar, K.; Rajan, S.; Srivastava, A.; Gupta, A.; Gupta, S.; Vishwakarma, A.L.; Misra, A.; et al. Temporal immmunometabolic profiling of adipose tissue in HFD-induced obesity: Manifestations of mast cells in fibrosis and senescence. Int. J. Obes. 2019, 43, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Podolsky, M.J.; Atabai, K. Fat fibrosis: Friend or foe? JCI Insight 2018, 3, e122289. [Google Scholar] [CrossRef] [PubMed]
- Reizes, O.; Clegg, D.J.; Strader, A.D.; Benoit, S.C. A role for syndecan-3 in the melanocortin regulation of energy balance. Peptides 2006, 27, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Kasza, I.; Suh, Y.; Wollny, D.; Clark, R.J.; Roopra, A.; Colman, R.J.; MacDougald, O.A.; Shedd, T.A.; Nelson, D.W.; Yen, M.I.; et al. Syndecan-1 Is Required to Maintain Intradermal Fat and Prevent Cold Stress. PLoS Genet. 2014, 10, e1004514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palomino, R.; Lee, H.W.; Millhauser, G.L. The agouti-related peptide binds heparan sulfate through segments critical for its orexigenic effects. J. Biol. Chem. 2017, 292, 7651–7661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Schadt, E.E.; Wang, S.; Wang, H.; Arnold, A.P.; Ingram-Drake, L.; Drake, T.A.; Lusis, A.J. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006, 16, 995–1004. [Google Scholar] [CrossRef] [Green Version]
- Gershoni, M.; Pietrokovski, S. The landscape of sex-differential transcriptome and its consequent selection in human adults. BMC Biol. 2017, 15, 7. [Google Scholar] [CrossRef] [Green Version]
- Gopal, S.; Multhaupt, H.A.; Pocock, R.; Couchman, J.R. Cell-extracellular matrix and cell-cell adhesion are linked by syndecan-4. Matrix Biol. 2017, 60, 57–69. [Google Scholar] [CrossRef]
- Crewe, C.; Joffin, N.; Rutkowski, J.M.; Kim, M.; Zhang, F.; Towler, D.A.; Gordillo, R.; Scherer, P.E. An Endothelial-to-Adipocyte Extracellular Vesicle Axis Governed by Metabolic State. Cell 2018, 175, 695–708. [Google Scholar] [CrossRef] [Green Version]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; DeGeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan–syntenin–ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef]
- Corti, F.; Finetti, F.; Ziche, M.; Simons, M. The syndecan-4/protein kinase Calpha pathway mediates prostaglandin E2-induced extracellular regulated kinase (ERK) activation in endothelial cells and angiogenesis in vivo. J. Biol. Chem. 2013, 288, 12712–12721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kangas, R.; Törmäkangas, T.; Fey, V.; Pursiheimo, J.; Miinalainen, I.; Alen, M.; Kaprio, J.; Sipilä, S.; Säämänen, A.M.; Kovanen, V.; et al. Aging and serum exomiR content in women-effects of estrogenic hormone replacement therapy. Sci. Rep. 2017, 7, 42702. [Google Scholar] [CrossRef] [PubMed]
- Voshol, P.J.; Rensen, P.C.; van Dijk, K.W.; Romijn, J.A.; Havekes, L.M. Effect of plasma triglyceride metabolism on lipid storage in adipose tissue: Studies using genetically engineered mouse models. Biochim. Biophys. Acta 2009, 1791, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Dorn, C.; Riener, M.O.; Kirovski, G.; Saugspier, M.; Steib, K.; Weiss, T.S.; Gäbele, E.; Kristiansen, G.; Hartmann, A.; Hellerbrand, C. Expression of fatty acid synthase in nonalcoholic fatty liver disease. Int. J. Clin. Exp. Pathol. 2010, 3, 505–514. [Google Scholar] [PubMed]
- Shimano, H. Sterol regulatory element-binding proteins (SREBPs): Transcriptional regulators of lipid synthetic genes. Prog. Lipid Res. 2001, 40, 439–452. [Google Scholar] [CrossRef]
- Ulven, S.M.; Dalen, K.T.; Gustafsson, J.A.; Nebb, H.I. LXR is crucial in lipid metabolism. Prostaglandins Leukot. Essent. Fatty Acids 2005, 73, 59–63. [Google Scholar] [CrossRef]
- Joseph, S.B.; Laffitte, B.A.; Patel, P.H.; Watson, M.A.; Matsukuma, K.E.; Walczak, R.; Collins, J.L.; Osborne, T.F.; Tontonoz, P. Direct and Indirect Mechanisms for Regulation of Fatty Acid Synthase Gene Expression by Liver X Receptors. J. Biol. Chem. 2002, 277, 11019–11025. [Google Scholar] [CrossRef] [Green Version]
- Anisfeld, A.M.; Kast-Woelbern, H.R.; Jones, S.A.; Williams, K.J.; Meyer, M.E.; Zhang, Y.; Willson, T.; Edwards, P.A. Syndecan-1 Expression Is Regulated in an Isoform-specific Manner by the Farnesoid-X Receptor. J. Biol. Chem. 2003, 278, 20420–20428. [Google Scholar] [CrossRef] [Green Version]
- Schadt, E.E.; Molony, C.; Chudin, E.; Hao, K.; Yang, X.; Lum, P.Y.; Kasarskis, A.; Zhang, B.; Wang, S.; Suver, C.; et al. Mapping the genetic architecture of gene expression in human liver. PLoS Biol. 2008, 6, e107. [Google Scholar] [CrossRef] [Green Version]
- Schorr, M.; Dichtel, L.E.; Gerweck, A.V.; Valera, R.D.; Torriani, M.; Miller, K.K.; Bredella, M.A. Sex differences in body composition and association with cardiometabolic risk. Biol. Sex Differ. 2018, 9, 28. [Google Scholar] [CrossRef]
- Solbu, M.D.; Kolset, S.O.; Jenssen, T.G.; Wilsgaard, T.; Løchen, M.L.; Mathiesen, E.B.; Melsom, T.; Eriksen, B.O.; Reine, T.M. Gender differences in the association of syndecan-4 with myocardial infarction: The population-based Tromsø Study. Atherosclerosis 2018, 278, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Lendorf, M.E.; Manon-Jensen, T.; Kronqvist, P.; Multhaupt, H.A.B.; Couchman, J.R. Syndecan-1 and Syndecan-4 Are Independent Indicators in Breast Carcinoma. J. Histochem. Cytochem. 2011, 59, 615–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Source a | Dfb | MSc | Fd | P-Value |
|---|---|---|---|---|
| Time | 3.758 | 7,6926.32 | 157.99 | <0.0001 |
| Time x Genotype | 3.758 | 279.68 | 0.57 | 0.6714 |
| Time x Sex | 3.758 | 2172.03 | 4.46 | 0.0031 |
| Time x Genotype x Sex | 3.758 | 419.45 | 0.86 | 0.4851 |
| Error(Time) | 82.672 | 486.92 |
| Phenotype | Source a | Df b | MS c | F d | P-Value | Means ± SE |
|---|---|---|---|---|---|---|
| %BFM | Sex | 1 | 28.27 | 2.37 | 0.1377 | F: 44.91 ± 2.04; M: 45.98 ± 0.41 |
| Genotype | 1 | 290.98 | 24.43 | <0.0001 | WT: 42.20 ± 1.48; KO: 48.31 ± 0.60 | |
| Genotype x Sex | 1 | 210.86 | 17.70 | 0.0004 | WTF: 37.62 ± 2.22; KOF: 50.13 ± 1.75 WTM: 45.48 ± 0.54; KOM: 46.48 ± 0.74 | |
| Error | 22 | 4.05 | ||||
| %LBM | Sex | 1 | 8.06 | 0.84 | 0.3691 | F: 51.57 ± 2.04; M: 51.37 ± 0.41 |
| Genotype | 1 | 246.52 | 25.72 | <0.0001 | WT: 54.55 ± 1.19; KO: 48.82 ± 0.57 | |
| Genotype x Sex | 1 | 152.68 | 15.93 | 0.0006 | WTF: 58.06 ± 1.91; KOF: 46.94 ± 1.64 WTM: 52.03 ± 0.44; KOM: 50.71 ± 0.64 | |
| Error | 22 | 9.59 | ||||
| TC (mg/dL) | Sex | 1 | 37,771.05 | 100.23 | <0.0001 | F: 74.54 ± 5.91; M: 155.35 ± 7.21 |
| Genotype | 1 | 497.05 | 1.32 | 0.2650 | WT: 114.80 ± 15.85; KO: 119.36 ± 10.97 | |
| Genotype x Sex | 1 | 3165.53 | 8.40 | 0.0092 | WTF: 57.61 ± 1.69; KOF: 90.48 ± 5.29 WTM: 162.45 ± 6.33; KOM: 148.24 ± 12.99 | |
| Error | 19 | 376.86 | ||||
| TG (mg/dL) | Sex | 1 | 1374.67 | 4.89 | 0.0401 | F: 61.98 ± 5.78; M: 74.60 ± 5.90 |
| Genotype | 1 | 1534.92 | 5.46 | 0.0312 | WT: 61.44. ± 5.85; KO:75.05 ± 5.75 | |
| Genotype x Sex | 1 | 1627.66 | 5.80 | 0.0270 | WTF: 41.32 ± 2.09; KOF: 75.76 ± 3.62 WTM: 74.85 ± 4.56; KOM: 74.34 ± 11.51 | |
| Error | 18 | 280.87 | ||||
| Insulin (ng/dL) | Sex | 1 | 15.21 | 188.34 | <0.0001 | F: 1.39 ± 0.07; M: 7.39 ± 0.87 |
| Genotype | 1 | 0.01 | 0.09 | 0.7648 | WT: 4.94 ± 1.33; KO:4.13 ± 0.91 | |
| Genotype x Sex | 1 | 0.23 | 2.81 | 0.1101 | WTF: 1.22 ± 0.09; KOF: 1.53 ± 0.07 WTM: 8.05 ± 1.49; KOM: 6.73 ± 0.97 | |
| Error | 19 | 0.08 | ||||
| Glucose(mg/dL) | Sex | 1 | 0.20 | 7.61 | 0.0125 | F:185.82 ± 12.24; M: 217.75 ± 11.43 |
| Genotype | 1 | 0.02 | 0.87 | 0.3636 | WT: 202.18 ± 18.00; KO: 202.75 ± 5.46 | |
| Genotype x Sex | 1 | 0.43 | 16.61 | 0.0006 | WTF: 155.2 ± 19.23; KOF: 211.33 ± 4.07 WTM: 241.33 ± 16.36; KOM: 194.34 ± 9.23 | |
| Error | 19 | 0.026 | ||||
| ISI | Sex | 1 | 20.60 | 294.96 | <0.0001 | F: 32.40 ± 4.49; M: 4.86 ± 0.55 |
| Genotype | 1 | 0.24 | 3.40 | 0.0810 | WT: 22.90 ± 6.95; KO: 13.57 ± 2.45 | |
| Genotype x Sex | 1 | 1.57 | 22.41 | 0.0001 | WTF: 45.57 ± 5.47; KOF: 21.43 ± 0.91 WTM: 4.01 ± 0.36; KOM: 5.71 ± 0.97 | |
| Error | 19 | 0.07 |
| Phenotype | Source a | Dfb | MSc | Fd | P-Value | Means ± SE |
|---|---|---|---|---|---|---|
| Food intake (g/day) | Sex | 1 | 1.094 | 4.60 | 0.0438 | F: 2.43 ± 0.15; M: 2.86 ± 0.10 |
| Genotype | 1 | 1.615 | 6.79 | 0.0165 | WT: 2.91 ± 0.14; KO: 2.39 ± 0.14 | |
| Genotype x Sex | 1 | 0.646 | 2.72 | 0.1141 | WTF: 2.86 ± 0.27; KOF: 2.02 ± 0.20 WTM: 2.96 ± 0.08; KOM: 2.77 ± 0.19 | |
| Error | 21 | 0.238 | ||||
| REE (kcal/24 h) | Sex | 1 | 3.763 | 5.97 | 0.0230 | F: 10.48 ± 0.25; M: 11.31 ± 0.20 |
| Genotype | 1 | 0.518 | 0.82 | 0.3745 | WT: 11.10 ± 0.24; KO: 10.78 ± 0.28 | |
| Genotype x Sex | 1 | 1.327 | 2.11 | 0.1608 | WTF: 10.91 ± 0.39; KOF: 10.17 ± 0.29 WTM: 11.23 ± 0.31; KOM: 11.40 ± 0.27 | |
| Error | 22 | 0.630 | ||||
| Locomotor activity (counts/24 h) | Sex | 1 | 1.592 | 11.40 | 0.0027 | F: 39419.64 ± 5330.50; M: 23350.49 ± 1654.00 |
| Genotype | 1 | 0.329 | 2.36 | 0.1391 | WT: 28073.67 ± 3668.21; KO: 33909.27 ± 5220.80 | |
| Genotype x Sex | 1 | 0.493 | 3.53 | 0.0736 | WTF: 49817.96 ± 7849.61; KOF: 31992.27 ± 6155.85 WTM: 22545.92 ± 2161.63; KOM: 24155.06 ± 2639.25 | |
| Error | 22 | 0.140 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, M.; Vecchie’, D.; Athmanathan, B.; Gopalkrishna, S.; Valcin, J.A.; Swain, T.M.; Sertie, R.; Wekesa, K.; Rowe, G.C.; Bailey, S.M.; et al. Genetic Deletion of Syndecan-4 Alters Body Composition, Metabolic Phenotypes, and the Function of Metabolic Tissues in Female Mice Fed A High-Fat Diet. Nutrients 2019, 11, 2810. https://doi.org/10.3390/nu11112810
De Luca M, Vecchie’ D, Athmanathan B, Gopalkrishna S, Valcin JA, Swain TM, Sertie R, Wekesa K, Rowe GC, Bailey SM, et al. Genetic Deletion of Syndecan-4 Alters Body Composition, Metabolic Phenotypes, and the Function of Metabolic Tissues in Female Mice Fed A High-Fat Diet. Nutrients. 2019; 11(11):2810. https://doi.org/10.3390/nu11112810
Chicago/Turabian StyleDe Luca, Maria, Denise Vecchie’, Baskaran Athmanathan, Sreejit Gopalkrishna, Jennifer A. Valcin, Telisha M. Swain, Rogerio Sertie, Kennedy Wekesa, Glenn C. Rowe, Shannon M. Bailey, and et al. 2019. "Genetic Deletion of Syndecan-4 Alters Body Composition, Metabolic Phenotypes, and the Function of Metabolic Tissues in Female Mice Fed A High-Fat Diet" Nutrients 11, no. 11: 2810. https://doi.org/10.3390/nu11112810
APA StyleDe Luca, M., Vecchie’, D., Athmanathan, B., Gopalkrishna, S., Valcin, J. A., Swain, T. M., Sertie, R., Wekesa, K., Rowe, G. C., Bailey, S. M., & Nagareddy, P. R. (2019). Genetic Deletion of Syndecan-4 Alters Body Composition, Metabolic Phenotypes, and the Function of Metabolic Tissues in Female Mice Fed A High-Fat Diet. Nutrients, 11(11), 2810. https://doi.org/10.3390/nu11112810





