Precision Nutrition and Cancer Relapse Prevention: A Systematic Literature Review
Abstract
1. Introduction
1.1. Breast, Colon, Lung, Prostate and Leukemia: The Deadliest Cancers
1.1.1. Lung Cancer
1.1.2. Breast Cancer
1.1.3. Prostate Cancer
1.1.4. Colorectal Cancer
1.1.5. Blood Malignancies
1.2. Cancer Relapse
1.3. Precision Nutrition and Cancer Therapy
2. Material and Methods
2.1. Systematic Search
2.2. Inclusion Criteria
3. Results
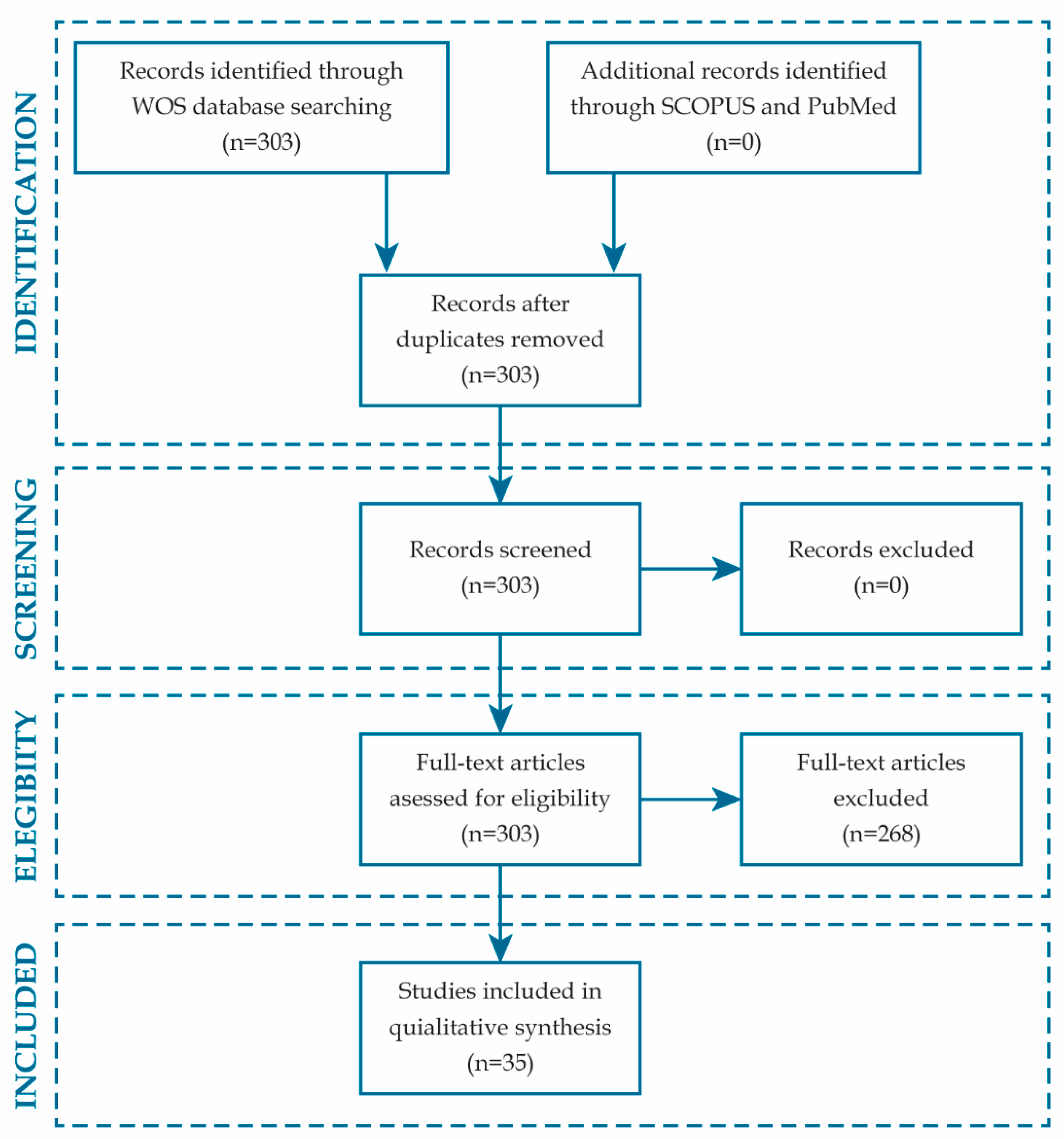
3.1. Literature Search
3.2. Characteristics of Included Studies
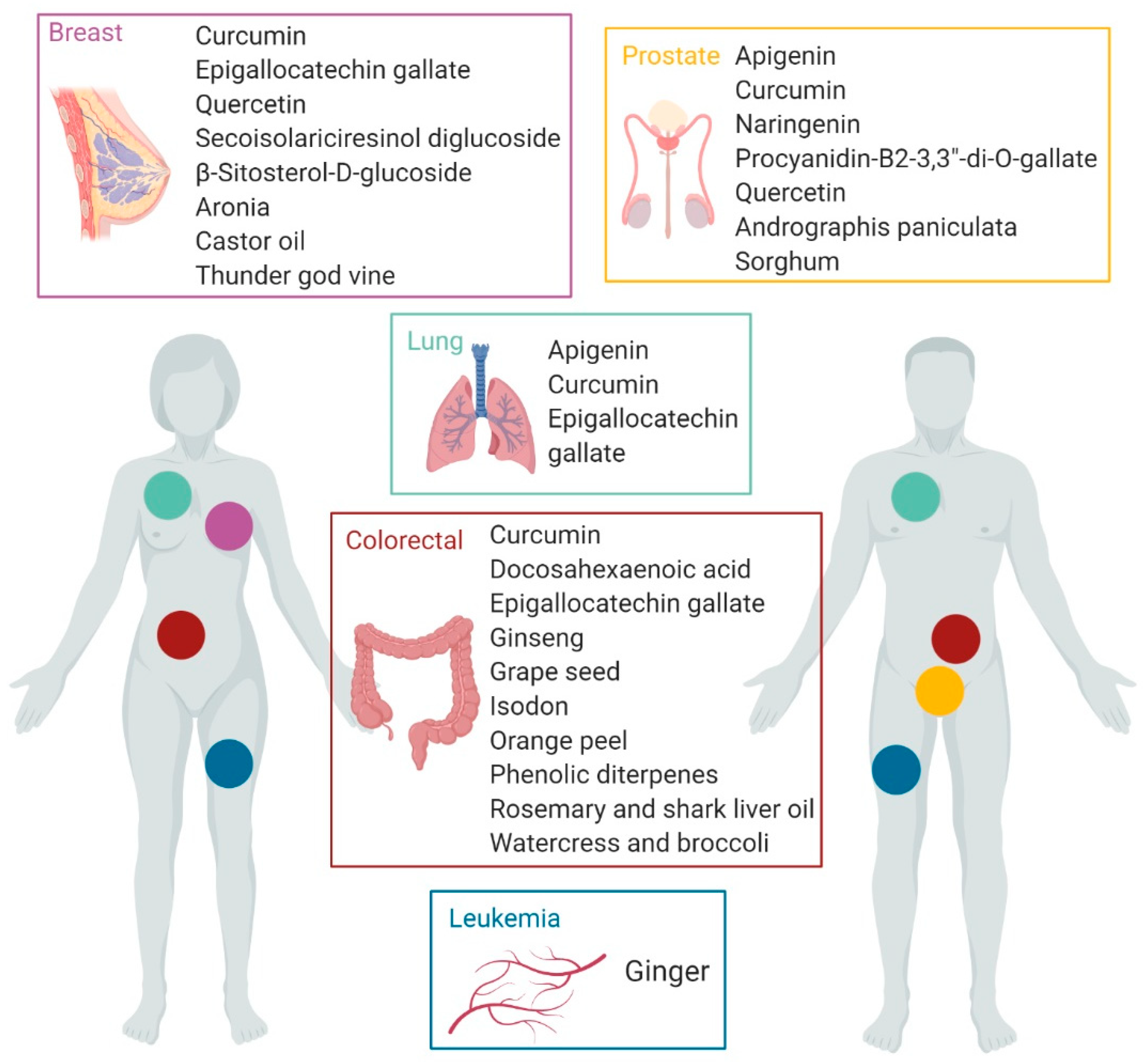
3.3. Outcome
3.4. Nanotechnology and Precision Nutrition for Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Wang, J.; Chen, N.; Fang, W.; Zhong, J.; Liu, Y.; Qin, R.; Yu, X.; Sun, Z.; et al. A 17 gene panel for non-small-cell lung cancer prognosis identified through integrative epigenomic-transcriptomic analyses of hypoxia-induced epithelial–mesenchymal transition. Mol. Oncol. 2019, 13, 1490–1502. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Qiu, M.; Mi, Z.; Meng, M.; Guo, Y.; Jiang, X.; Fang, J.; Wang, H.; Zhao, J.; Liu, Z.; et al. WT1-interacting protein inhibits cell proliferation and tumorigenicity in non-small-cell lung cancer via the AKT/FOXO1 axis. Mol. Oncol. 2019, 13, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Deng, J.; Li, S.; Silk, T.; Dong, L.; Brea, E.J.; Houghton, S.; Redmond, D.; Zhong, H.; Boiarsky, J.; et al. Pulsatile MEK Inhibition Improves Anti-tumor Immunity and T Cell Function in Murine Kras Mutant Lung Cancer. Cell Rep. 2019, 27, 806–819. [Google Scholar] [CrossRef]
- Hamdan, D.; Nguyen, T.T.; Leboeuf, C.; Meles, S.; Janin, A.; Bousquet, G. Genomics applied to the treatment of breast cancer. Oncotarget 2019, 10, 4786–4801. [Google Scholar]
- Bouchal, P.; Schubert, O.T.; Faktor, J.; Capkova, L.; Imrichova, H.; Zoufalova, K.; Paralova, V.; Hrstka, R.; Liu, Y.; Ebhardt, H.A.; et al. Breast Cancer Classification Based on Proteotypes Obtained by SWATH Mass Spectrometry. Cell Rep. 2019, 28, 832–843. [Google Scholar] [CrossRef]
- Kido, T.; Li, Y.; Tanaka, Y.; Dahiya, R.; Lau, Y.-F.C. The X-linked tumor suppressor TSPX downregulates cancer-drivers/oncogenes in prostate cancer in a C-terminal acidic domain dependent manner. Oncotarget 2019, 10, 1491–1506. [Google Scholar] [CrossRef]
- Wang, D.; Wan, X.; Zhang, Y.; Kong, Z.; Lu, Y.; Sun, X.; Huang, Y.; Ji, C.; Li, D.; Luo, J.; et al. A novel androgen-reduced prostate-specific lncRNA, PSLNR, inhibits prostate-cancer progression in part by regulating the p53-dependent pathway. Prostate 2019, 79, 1379–1393. [Google Scholar] [CrossRef]
- Kumar, P.; Chakraborty, J.; Sukumar, G.; Dalgard, C.; Chatterjee, R.; Biswas, R. Comparative RNA-seq analysis reveals dys-regulation of major canonical pathways in ERG-inducible LNCaP cell progression model of prostate cancer. Oncotarget 2019, 10, 4290–4306. [Google Scholar]
- Cruz-Gil, S.; Sanchez-Martinez, R.; Gomez de Cedron, M.; Martin-Hernandez, R.; Vargas, T.; Molina, S.; Herranz, J.; Davalos, A.; Reglero, G.; Ramirez de Molina, A. Targeting the lipid metabolic axis ACSL/SCD in colorectal cancer progression by therapeutic miRNAs: miR-19b-1 role. J. Lipid Res. 2018, 59, 14–24. [Google Scholar] [CrossRef]
- Ohata, H.; Shiokawa, D.; Obata, Y.; Sato, A.; Sakai, H.; Fukami, M.; Hara, W.; Taniguchi, H.; Ono, M.; Nakagama, H.; et al. NOX1-Dependent mTORC1 Activation via S100A9 Oxidation in Cancer Stem-like Cells Leads to Colon Cancer Progression. Cell Rep. 2019, 28, 1282–1295. [Google Scholar] [CrossRef] [PubMed]
- Vargas, T.; Moreno-Rubio, J.; Herranz, J.; Cejas, P.; Molina, S.; Mendiola, M.; Burgos, E.; Custodio, A.B.; De Miguel, M.; Martín-Hernández, R.; et al. 3’UTR Polymorphism in ACSL1 Gene Correlates with Expression Levels and Poor Clinical Outcome in Colon Cancer Patients. PLoS ONE 2016, 11, e0168423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, J.; Lu, X.; Liu, T.; Jin, X.; Xiao, Y.; He, X. PVT1 (rs13281615) and miR-146a (rs2910164) polymorphisms affect the prognosis of colon cancer by regulating COX2 expression and cell apoptosis: ZHANG et al. J. Cell Physiol. 2019, 234, 17538–17548. [Google Scholar] [CrossRef] [PubMed]
- Garrett, W.S. The gut microbiota and colon cancer. Science 2019, 364, 1133–1135. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, A.A.; López-Otín, C. Clonal evolution in leukemia. Nat. Med. 2017, 23, 1135–1145. [Google Scholar] [CrossRef]
- Speedy, H.E.; Beekman, R.; Chapaprieta, V.; Orlando, G.; Law, P.J.; Martín-García, D.; Gutiérrez-Abril, J.; Catovsky, D.; Beà, S.; Clot, G.; et al. Insight into genetic predisposition to chronic lymphocytic leukemia from integrative epigenomics. Nat. Commun. 2019, 10, 3615. [Google Scholar] [CrossRef]
- Hutmacher, C.; Volta, L.; Rinaldi, F.; Murer, P.; Myburgh, R.; Manz, M.G.; Neri, D. Development of a novel fully-human anti-CD123 antibody to target acute myeloid leukemia. Leuk. Res. 2019, 84, 106178. [Google Scholar] [CrossRef]
- Galletti, G.; Scielzo, C.; Barbaglio, F.; Rodriguez, T.V.; Riba, M.; Lazarevic, D.; Cittaro, D.; Simonetti, G.; Ranghetti, P.; Scarfò, L.; et al. Targeting Macrophages Sensitizes Chronic Lymphocytic Leukemia to Apoptosis and Inhibits Disease Progression. Cell Rep. 2016, 14, 1748–1760. [Google Scholar] [CrossRef]
- Blundon, M.A.; Dasgupta, S. Metabolic Dysregulation Controls Endocrine Therapy–Resistant Cancer Recurrence and Metastasis. Endocrinology 2019, 160, 1811–1820. [Google Scholar] [CrossRef]
- Drouillard, A.; Bouvier, A.-M.; Boussari, O.; Romain, G.; Manfredi, S.; Lepage, C.; Faivre, J.; Jooste, V. Net survival in recurrence-free colon cancer patients. Cancer Epidemiol. 2019, 61, 124–128. [Google Scholar] [CrossRef]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Lim, J.S.; Jang, S.J.; Cun, Y.; Ozretić, L.; Kong, G.; Leenders, F.; Lu, X.; Fernández-Cuesta, L.; Bosco, G.; et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015, 524, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wang, T.; Zhang, Y.; Zhang, J.; Zhao, H.; Wang, H.; Wu, Y.; Liu, K. Long Non-Coding RNA AWPPH Promotes Postoperative Distant Recurrence in Resected Non-Small Cell Lung Cancer by Upregulating Transforming Growth Factor beta 1 (TGF-β1). Med. Sci. Monit. 2019, 25, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- Oshima, K.; Khiabanian, H.; da Silva-Almeida, A.C.; Tzoneva, G.; Abate, F.; Ambesi-Impiombato, A.; Sanchez-Martin, M.; Carpenter, Z.; Penson, A.; Perez-Garcia, A.; et al. Mutational landscape, clonal evolution patterns, and role of RAS mutations in relapsed acute lymphoblastic leukemia. Proc. Natl. Acad. Sci. USA 2016, 113, 11306–11311. [Google Scholar] [CrossRef] [PubMed]
- Paganin, M.; Buldini, B.; Germano, G.; Seganfreddo, E.; di Meglio, A.; Magrin, E.; Grillo, F.; Pigazzi, M.; Rizzari, C.; Cazzaniga, G.; et al. A Case of T-cell Acute Lymphoblastic Leukemia Relapsed As Myeloid Acute Leukemia: Lineage Switch Leukemia. Pediatr. Blood Cancer 2016, 63, 1660–1663. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, H.; Bai, Y.; Kirschner-Schwabe, R.; Yang, J.J.; Chen, Y.; Lu, G.; Tzoneva, G.; Ma, X.; Wu, T.; et al. Negative feedback–defective PRPS1 mutants drive thiopurine resistance in relapsed childhood ALL. Nat. Med. 2015, 21, 563–571. [Google Scholar] [CrossRef]
- Tzoneva, G.; Perez-Garcia, A.; Carpenter, Z.; Khiabanian, H.; Tosello, V.; Allegretta, M.; Paietta, E.; Racevskis, J.; Rowe, J.M.; Tallman, M.S.; et al. Activating mutations in the NT5C2 nucleotidase gene drive chemotherapy resistance in relapsed ALL. Nat. Med. 2013, 19, 368–371. [Google Scholar] [CrossRef]
- Dieck, C.L.; Tzoneva, G.; Forouhar, F.; Carpenter, Z.; Ambesi-Impiombato, A.; Sánchez-Martín, M.; Kirschner-Schwabe, R.; Lew, S.; Seetharaman, J.; Tong, L.; et al. Structure and Mechanisms of NT5C2 Mutations Driving Thiopurine Resistance in Relapsed Lymphoblastic Leukemia. Cancer Cell 2018, 34, 136–147. [Google Scholar] [CrossRef]
- Ferguson, L.R.; De Caterina, R.; Görman, U.; Allayee, H.; Kohlmeier, M.; Prasad, C.; Choi, M.S.; Curi, R.; de Luis, D.A.; Gil, Á.; et al. Guide and Position of the International Society of Nutrigenetics/Nutrigenomics on Personalised Nutrition: Part 1—Fields of Precision Nutrition. J. Nutrigenet. Nutrigenom. 2016, 9, 12–27. [Google Scholar] [CrossRef]
- Zeisel, S.H. A Conceptual Framework for Studying and Investing in Precision Nutrition. Front. Genet. 2019, 10, 200. [Google Scholar] [CrossRef]
- Mills, S.; Stanton, C.; Lane, J.A.; Smith, G.J.; Ross, R.P. Precision Nutrition and the Microbiome, Part I: Current State of the Science. Nutrients 2019, 11, 923. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hernández, R.; Reglero, G.; Dávalos, A. Data mining of nutrigenomics experiments: Identification of a cancer protective gene signature. J. Funct. Foods 2018, 42, 380–386. [Google Scholar] [CrossRef]
- Wanchai, A.; Armer, J.M.; Stewart, B.R. Complementary and Alternative Medicine Use among Women with Breast Cancer: A Systematic Review. Clin. J. Oncol. Nurs. 2010, 14, E45–E55. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.M.; Dourado, A.; Oliveira, R. Phytotherapy and Nutritional Supplements on Breast Cancer. BioMed Res. Int. 2017, 2017, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Hauner, H.; Weigl, J.; Hauner, D. Can Nutrition Lower the Risk of Recurrence in Breast Cancer? Breast Care 2018, 13, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Younas, M.; Hano, C.; Giglioli-Guivarc’h, N.; Abbasi, B.H. Mechanistic evaluation of phytochemicals in breast cancer remedy: Current understanding and future perspectives. RSC Adv. 2018, 8, 29714–29744. [Google Scholar] [CrossRef]
- De Cicco, P.; Catani, M.V.; Gasperi, V.; Sibilano, M.; Quaglietta, M.; Savini, I. Nutrition and Breast Cancer: A Literature Review on Prevention, Treatment and Recurrence. Nutrients 2019, 11, 1514. [Google Scholar] [CrossRef]
- Sayeed, M.d.A.; Bracci, M.; Lazzarini, R.; Tomasetti, M.; Amati, M.; Lucarini, G.; Di Primio, R.; Santarelli, L. Use of potential dietary phytochemicals to target miRNA: Promising option for breast cancer prevention and treatment? J. Funct. Foods 2017, 28, 177–193. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Chen, H.; Collins, A.R.; Connell, M.; Damia, G.; Dasgupta, S.; Malhotra, M.; Meeker, A.K.; Amedei, A.; Amin, A.; et al. Genomic instability in human cancer: Molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Semin. Cancer Biol. 2015, 35, S5–S24. [Google Scholar] [CrossRef]
- Braakhuis, A.; Campion, P.; Bishop, K. Reducing Breast Cancer Recurrence: The Role of Dietary Polyphenolics. Nutrients 2016, 8, 547. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Barupal, D.K.; Rothwell, J.A.; Jenab, M.; Fedirko, V.; Romieu, I.; Aleksandrova, K.; Overvad, K.; Kyrø, C.; Tjønneland, A.; et al. Dietary flavonoid intake and colorectal cancer risk in the European prospective investigation into cancer and nutrition (EPIC) cohort. Int. J. Cancer 2017, 140, 1836–1844. [Google Scholar] [CrossRef] [PubMed]
- Ziaei, S.; Halaby, R. Dietary Isoflavones and Breast Cancer Risk. Medicines 2017, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Abbasi, B.A.; Ahmad, R.; Mahmood, T.; Kanwal, S.; Ali, B.; Khalil, A.T.; Shah, S.A.; Alam, M.M.; Badshah, H. Ursolic acid a promising candidate in the therapeutics of breast cancer: Current status and future implications. Biomed. Pharmacother. 2018, 108, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Jaman, M.d.S.; Sayeed, M.d.A. Ellagic acid, sulforaphane, and ursolic acid in the prevention and therapy of breast cancer: Current evidence and future perspectives. Breast Cancer 2018, 25, 517–528. [Google Scholar] [CrossRef]
- Gianfredi, V.; Nucci, D.; Abalsamo, A.; Acito, M.; Villarini, M.; Moretti, M.; Realdon, S. Green Tea Consumption and Risk of Breast Cancer and Recurrence—A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2018, 10, 1886. [Google Scholar] [CrossRef] [PubMed]
- Darband, S.G.; Kaviani, M.; Yousefi, B.; Sadighparvar, S.; Pakdel, F.G.; Attari, J.A.; Mohebbi, I.; Naderi, S.; Majidinia, M. Quercetin: A functional dietary flavonoid with potential chemo-preventive properties in colorectal cancer. J. Cell Physiol. 2018, 233, 6544–6560. [Google Scholar] [CrossRef]
- Paller, C.J.; Pantuck, A.; Carducci, M.A. A review of pomegranate in prostate cancer. Prostate Cancer Prostatic Dis. 2017, 20, 265–270. [Google Scholar] [CrossRef]
- Paller, C.J.; Zhou, X.C.; Heath, E.I.; Taplin, M.-E.; Mayer, T.; Stein, M.N.; Bubley, G.J.; Pili, R.; Hudson, T.; Kakarla, R.; et al. Muscadine Grape Skin Extract (MPX) in Men with Biochemically Recurrent Prostate Cancer: A Randomized, Multicenter, Placebo-Controlled Clinical Trial. Clin. Cancer Res. 2018, 24, 306–315. [Google Scholar] [CrossRef]
- Gómez de Cedrón, M.; Vargas, T.; Madrona, A.; Jiménez, A.; Pérez-Pérez, M.-J.; Quintela, J.-C.; Reglero, G.; San-Félix, A.; Ramírez de Molina, A. Novel Polyphenols That Inhibit Colon Cancer Cell Growth Affecting Cancer Cell Metabolism. J. Pharmacol. Exp. Ther. 2018, 366, 377–389. [Google Scholar] [CrossRef]
- Kouhpeikar, H.; Butler, A.E.; Bamian, F.; Barreto, G.E.; Majeed, M.; Sahebkar, A. Curcumin as a therapeutic agent in leukemia. J. Cell Physiol. 2019, 234, 12404–12414. [Google Scholar]
- González-Vallinas, M.; Reglero, G.; Ramírez de Molina, A. Rosemary (Rosmarinus officinalis L.) Extract as a Potential Complementary Agent in Anticancer Therapy. Nutr. Cancer 2015, 67, 1223–1231. [Google Scholar]
- Aguirre-Portolés, C.; Fernández, L.; Ramírez de Molina, A. Precision Nutrition for Targeting Lipid Metabolism in Colorectal Cancer. Nutrients 2017, 9, 1076. [Google Scholar]
- Carayol, M.; Ninot, G.; Senesse, P.; Bleuse, J.-P.; Gourgou, S.; Sancho-Garnier, H.; Sari, C.; Romieu, I.; Romieu, G.; Jacot, W. Short- and long-term impact of adapted physical activity and diet counseling during adjuvant breast cancer therapy: The “APAD1” randomized controlled trial. BMC Cancer 2019, 19, 737. [Google Scholar]
- Maruca, A.; Catalano, R.; Bagetta, D.; Mesiti, F.; Ambrosio, F.A.; Romeo, I.; Moraca, F.; Rocca, R.; Ortuso, F.; Artese, A.; et al. The Mediterranean Diet as source of bioactive compounds with multi-targeting anti-cancer profile. Eur. J. Med. Chem. 2019, 181, 111579. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.I.; Othman, I.; Abas, F.; Lajis, N.H.; Naidu, R. Mechanism of Apoptosis Induced by Curcumin in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 2454. [Google Scholar] [CrossRef]
- Martínez, N.; Herrera, M.; Frías, L.; Provencio, M.; Pérez-Carrión, R.; Díaz, V.; Morse, M.; Crespo, M.C. A combination of hydroxytyrosol, omega-3 fatty acids and curcumin improves pain and inflammation among early stage breast cancer patients receiving adjuvant hormonal therapy: Results of a pilot study. Clin. Transl. Oncol. 2019, 21, 489–498. [Google Scholar] [CrossRef]
- Erdogan, S.; Turkekul, K.; Serttas, R.; Erdogan, Z. The natural flavonoid apigenin sensitizes human CD44 + prostate cancer stem cells to cisplatin therapy. Biomed. Pharmacother. 2017, 88, 210–217. [Google Scholar] [CrossRef]
- Chang, J.-H.; Cheng, C.-W.; Yang, Y.-C.; Chen, W.-S.; Hung, W.-Y.; Chow, J.-M.; Chen, P.-S.; Hsiao, M.; Lee, W.-J.; Chien, M.-H. Downregulating CD26/DPPIV by apigenin modulates the interplay between Akt and Snail/Slug signaling to restrain metastasis of lung cancer with multiple EGFR statuses. J. Exp. Clin. Cancer Res. 2018, 37, 199. [Google Scholar]
- Fehl, D.J.; Ahmed, M. Curcumin promotes the oncoltyic capacity of vesicular stomatitis virus for the treatment of prostate cancers. Virus Res. 2017, 228, 14–23. [Google Scholar] [CrossRef]
- Ravindranathan, P.; Pasham, D.; Balaji, U.; Cardenas, J.; Gu, J.; Toden, S.; Goel, A. A combination of curcumin and oligomeric proanthocyanidins offer superior anti-tumorigenic properties in colorectal cancer. Sci. Rep. 2018, 8, 13869. [Google Scholar]
- Hu, C.; Li, M.; Guo, T.; Wang, S.; Huang, W.; Yang, K.; Liao, Z.; Wang, J.; Zhang, F.; Wang, H. Anti-metastasis activity of curcumin against breast cancer via the inhibition of stem cell-like properties and EMT. Phytomedicine 2019, 58, 152740. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Huang, H.-P.; Wang, Y.; Jin, J.; Long, W.-G.; Chen, K.; Zhao, X.-H.; Chen, C.-G.; Li, J. Curcumin overcome primary gefitinib resistance in non-small-cell lung cancer cells through inducing autophagy-related cell death. J. Exp. Clin. Cancer Res. 2019, 38, 254. [Google Scholar] [CrossRef] [PubMed]
- Ortea, I.; González-Fernández, M.J.; Ramos-Bueno, R.P.; Guil-Guerrero, J.L. Proteomics Study Reveals That Docosahexaenoic and Arachidonic Acids Exert Different In Vitro Anticancer Activities in Colorectal Cancer Cells. J. Agric. Food Chem. 2018, 66, 6003–6012. [Google Scholar] [CrossRef] [PubMed]
- Dumont, A.; de Rosny, C.; Kieu, T.-L.-V.; Perrey, S.; Berger, H.; Fluckiger, A.; Muller, T.; Pais de Barros, J.-P.; Pichon, L.; Hichami, A.; et al. Docosahexaenoic acid inhibits both NLRP3 inflammasome assembly and JNK-mediated mature IL-1β secretion in 5-fluorouracil-treated MDSC: Implication in cancer treatment. Cell Death Dis. 2019, 10, 485. [Google Scholar] [CrossRef]
- Oya, Y.; Mondal, A.; Rawangkan, A.; Umsumarng, S.; Iida, K.; Watanabe, T.; Kanno, M.; Suzuki, K.; Li, Z.; Kagechika, H.; et al. Down-regulation of histone deacetylase 4, −5 and −6 as a mechanism of synergistic enhancement of apoptosis in human lung cancer cells treated with the combination of a synthetic retinoid, Am80 and green tea catechin. J. Nutr. Biochem. 2017, 42, 7–16. [Google Scholar] [CrossRef]
- Jiang, P.; Xu, C.; Chen, L.; Chen, A.; Wu, X.; Zhou, M.; Haq, I.U.; Mariyam, Z.; Feng, Q. Epigallocatechin-3-gallate inhibited cancer stem cell-like properties by targeting hsa-mir-485-5p/RXRα in lung cancer. J. Cell. Biochem. 2018, 119, 8623–8635. [Google Scholar] [CrossRef]
- Song, X.; Zhang, M.; Chen, L.; Lin, Q. Bioinformatic Prediction of Possible Targets and Mechanisms of Action of the Green Tea Compound Epigallocatechin-3-Gallate Against Breast Cancer. Front. Mol. Biosci. 2017, 4, 43. [Google Scholar] [CrossRef]
- La, X.; Zhang, L.; Li, Z.; Li, H.; Yang, Y. (−)-Epigallocatechin Gallate (EGCG) Enhances the Sensitivity of Colorectal Cancer Cells to 5-FU by Inhibiting GRP78/NF-κB/miR-155-5p/MDR1 Pathway. J. Agric. Food Chem. 2019, 67, 2510–2518. [Google Scholar] [CrossRef]
- Lim, W.; Park, S.; Bazer, F.W.; Song, G. Naringenin-Induced Apoptotic Cell Death in Prostate Cancer Cells Is Mediated via the PI3K/AKT and MAPK Signaling Pathways: Effects of Naringenin on Prostate Cancer Cells. J. Cell. Biochem. 2017, 118, 1118–1131. [Google Scholar] [CrossRef]
- Tyagi, A.; Kumar, S.; Raina, K.; Wempe, M.F.; Maroni, P.D.; Agarwal, R.; Agarwal, C. Differential effect of grape seed extract and its active constituent procyanidin B2 3,3″-di-O-gallate against prostate cancer stem cells. Mol. Carcinog. 2019, 58, 1105–1117. [Google Scholar] [CrossRef]
- Erdogan, S.; Turkekul, K.; Dibirdik, I.; Doganlar, O.; Doganlar, Z.B.; Bilir, A.; Oktem, G. Midkine downregulation increases the efficacy of quercetin on prostate cancer stem cell survival and migration through PI3K/AKT and MAPK/ERK pathway. Biomed. Pharmacother. 2018, 107, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, N.; Wang, J.; Liu, Z.; Wang, X.; Zhang, Q.; Liu, Q.; Gao, L.; Wang, R. Quercetin suppresses breast cancer stem cells (CD44 + /CD24 − ) by inhibiting the PI3K/Akt/mTOR-signaling pathway. Life Sci. 2018, 196, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Bowers, L.W.; Lineberger, C.G.; Ford, N.A.; Rossi, E.L.; Punjala, A.; Camp, K.K.; Kimler, B.K.; Fabian, C.J.; Hursting, S.D. The flaxseed lignan secoisolariciresinol diglucoside decreases local inflammation, suppresses NFκB signaling, and inhibits mammary tumor growth. Breast Cancer Res. Treat. 2019, 173, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, Y.; Han, B.; Li, Z.; Wang, B.; Jiang, P.; Zhang, J.; Ma, W.; Zhou, D.; Li, X.; et al. Anti-breast-Cancer Activity Exerted by β-Sitosterol- d -glucoside from Sweet Potato via Upregulation of MicroRNA-10a and via the PI3K–Akt Signaling Pathway. J. Agric. Food Chem. 2018, 66, 9704–9718. [Google Scholar] [CrossRef]
- Forestier-Román, I.S.; López-Rivas, A.; Sánchez-Vázquez, M.M.; Rohena-Rivera, K.; Nieves-Burgos, G.; Ortiz-Zuazaga, H.; Torres-Ramos, C.A.; Martínez-Ferrer, M. Andrographolide induces DNA damage in prostate cancer cells. Oncotarget 2019, 10, 1085–1101. [Google Scholar] [CrossRef]
- Choi, H.; Kim, S.-L.; Kim, J.-H.; Deng, H.-Y.; Yun, B.-S.; Lee, D.-S. Triterpene Acid (3-O-p-Coumaroyltormentic Acid) Isolated From Aronia Extracts Inhibits Breast Cancer Stem Cell Formation through Downregulation of c-Myc Protein. IJMS 2018, 19, 2528. [Google Scholar] [CrossRef]
- Ahn, J.; Chung, Y.W.; Park, J.-B.; Yang, K.M. ω-hydroxyundec-9-enoic acid induces apoptosis by ROS mediated JNK and p38 phosphorylation in breast cancer cell lines. J. Cell. Biochem. 2018, 119, 998–1007. [Google Scholar] [CrossRef]
- Rahimi Babasheikhali, S.; Rahgozar, S.; Mohammadi, M. Ginger extract has anti-leukemia and anti-drug resistant effects on malignant cells. J. Cancer Res. Clin. Oncol. 2019, 145, 1987–1998. [Google Scholar] [CrossRef]
- Liu, T.; Duo, L.; Duan, P. Ginsenoside Rg3 Sensitizes Colorectal Cancer to Radiotherapy through Downregulation of Proliferative and Angiogenic Biomarkers. Evid. Based Complement. Altern. Med. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Ravindranathan, P.; Pasham, D.; Balaji, U.; Cardenas, J.; Gu, J.; Toden, S.; Goel, A. Mechanistic insights into anticancer properties of oligomeric proanthocyanidins from grape seeds in colorectal cancer. Carcinogenesis 2018, 39, 767–777. [Google Scholar] [CrossRef]
- Xia, Y.; Lam, C.S.; Li, W.; Sarwar, M.d.S.; Liu, K.; Lee, K.M.; Zhang, H.-J.; Tsang, S.W. Flexicaulin A, An ent-Kaurane Diterpenoid, Activates p21 and Inhibits the Proliferation of Colorectal Carcinoma Cells through a Non-Apoptotic Mechanism. IJMS 2019, 20, 1917. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Duarte, M.; Silva, P.; Bento da Silva, A.; Duarte, C.; Cifuentes, A.; García-Cañas, V.; Bronze, M.; Albuquerque, C.; Serra, A. Polymethoxylated Flavones Target Cancer Stemness and Improve the Antiproliferative Effect of 5-Fluorouracil in a 3D Cell Model of Colorectal Cancer. Nutrients 2019, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.-M.; Jang, G.Y.; Woo, K.S.; Kim, T.M.; Jeong, H.S.; Kim, D.J. Effects of sorghum ethyl-acetate extract on PC3M prostate cancer cell tumorigenicity. J. Funct. Foods 2017, 37, 449–459. [Google Scholar] [CrossRef]
- Gómez de Cedrón, M.; Laparra, J.M.; Loria-Kohen, V.; Molina, S.; Moreno-Rubio, J.; Montoya, J.J.; Torres, C.; Casado, E.; Reglero, G.; Ramírez de Molina, A. Tolerability and Safety of a Nutritional Supplement with Potential as Adjuvant in Colorectal Cancer Therapy: A Randomized Trial in Healthy Volunteers. Nutrients 2019, 11, 2001. [Google Scholar] [CrossRef] [PubMed]
- Varghese, E.; Samuel, S.; Varghese, S.; Cheema, S.; Mamtani, R.; Büsselberg, D. Triptolide Decreases Cell Proliferation and Induces Cell Death in Triple Negative MDA-MB-231 Breast Cancer Cells. Biomolecules 2018, 8, 163. [Google Scholar] [CrossRef]
- Pereira, L.; Silva, P.; Duarte, M.; Rodrigues, L.; Duarte, C.; Albuquerque, C.; Serra, A. Targeting Colorectal Cancer Proliferation, Stemness and Metastatic Potential Using Brassicaceae Extracts Enriched in Isothiocyanates: A 3D Cell Model-Based Study. Nutrients 2017, 9, 368. [Google Scholar] [CrossRef]
- Pandolfi, L.; Bellini, M.; Vanna, R.; Morasso, C.; Zago, A.; Carcano, S.; Avvakumova, S.; Bertolini, J.A.; Rizzuto, M.A.; Colombo, M.; et al. H-Ferritin Enriches the Curcumin Uptake and Improves the Therapeutic Efficacy in Triple Negative Breast Cancer Cells. Biomacromolecules 2017, 18, 3318–3330. [Google Scholar] [CrossRef]
- Greish, K.; Pittalà, V.; Taurin, S.; Taha, S.; Bahman, F.; Mathur, A.; Jasim, A.; Mohammed, F.; El-Deeb, I.; Fredericks, S.; et al. Curcumin–Copper Complex Nanoparticles for the Management of Triple-Negative Breast Cancer. Nanomaterials 2018, 8, 884. [Google Scholar] [CrossRef]
- Bessone, F.; Argenziano, M.; Grillo, G.; Ferrara, B.; Pizzimenti, S.; Barrera, G.; Cravotto, G.; Guiot, C.; Stura, I.; Cavalli, R.; et al. Low-dose curcuminoid-loaded in dextran nanobubbles can prevent metastatic spreading in prostate cancer cells. Nanotechnology 2019, 30, 214004. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, N.; Cheng, R.; Zhao, C.; Liu, J.; Tian, Z. Hybrid nanoparticles coated with hyaluronic acid lipoid for targeted co-delivery of paclitaxel and curcumin to synergistically eliminate breast cancer stem cells. J. Mater. Chem. B 2017, 5, 6762–6775. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, N.; Cheng, R.; Zhao, C.; Liu, Z.; Li, X.; Liu, J.; Tian, Z. pH multistage responsive micellar system with charge-switch and PEG layer detachment for co-delivery of paclitaxel and curcumin to synergistically eliminate breast cancer stem cells. Biomaterials 2017, 147, 53–67. [Google Scholar] [CrossRef] [PubMed]
| Field | Keywords | Boolean Operator |
|---|---|---|
| Title | ((cancer* OR carcino* OR tumor* OR tumour* OR onco*) AND (lung* OR breast* OR mammar* OR colon* OR colorect* OR prostat* OR leuk*)) | AND |
| Topic (title, keywords, abstract) | ((phytochem* OR polyphen* OR flavon* OR gallate* OR catechin* OR omega* OR DHA OR docosahexaenoic* OR terpen* OR curcum* OR extract*) AND (gen* OR genetic* OR genomic* OR microbio*)) | AND |
| Topic (title, keywords, abstract) | (relapse* OR recurrence* OR reappearance* OR replication* OR repetition* OR return* OR reemergence*) |
| Bioactive Foodstuff | Source | Cancer Type | Molecular Mechanism | Anticancer Effect | Reference |
|---|---|---|---|---|---|
| Apigenin | Fruits Vegetables Food herbs | Prostate | Apoptosis ↓ Bcl-2, sharpin and survivin ↑ caspase-8, Apaf-1, p21, p53 Signaling pathways inhibition ↓ PI3K/Akt, NF-κB Cell cycle inhibition ↑ p21, CDK-2, -4, -6 Migration inhibition ↓ Snail | Apigenin synergizes with cisplatin significantly increasing its effects on prostate cancer stem cells (CSCs) | [57] |
| Apigenin | Fruits Vegetables Food herbs | Lung | Migration/invasion inhibition ↓ CD26/DPPIV ↓ Akt, Snail/Slug EMT Cell growth and metastasis inhibition ↓ CD26 | CD26high/Akthigh Tumors show the shortest recurrence times of non-small cell lung cancer. apigenin inhibits the migration/invasion of non-small cell lung cancer by targeting CD26 | [58] |
| Curcumin | Turmeric | Prostate | Apoptosis ↓ Bcl-xl, NF-κB Virus infection increase ↓ STAT1 | Curcumin synergizes with vesicular stomatitis virus modulating antiviral responses and potentiating components of the intrinsic apoptotic pathway. | [59] |
| Curcumin | Turmeric | Colorectal | Gene expression regulation in pathways related with DNA replication, cell cycle, protein export, glutathione metabolism and porphyrin metabolism HSPA5, SEC61B, G6PD, HMOX1, PDE3B | Cooperative mechanisms of action of curcumin and oligomeric proanthocyanidins show enhanced anti-tumoral properties, opening up new effective therapies. | [60] |
| Curcumin | Turmeric | Breast | Cell proliferation, migration, invasion suppression ↑ E-cadherin ↓ Vimentin, Fibronectin, β-catenin Decreased stem cell features ↓ Oct4, Nanog, Sox2 | Anti-metastasis activity of curcumin via the inhibition stem cell-like features and epithelial-mesenchymal transition. | [61] |
| Curcumin | Turmeric | Lung | Downregulated EGFR activity (growth inhibition) ↓ Sp1-HADC1 interaction Signaling pathways inhibition ↓ RTKs, ERK/MEK, AKT/S6K Autophagy induction | Combination of curcumin and gefitinib sensitizes EGFR-TKI resistance in wild-type EGFR and/or KRAS mutant cell lines promoting autophagy -mediated cell apoptosis. | [62] |
| Docosahexaenoic acid (DHA) | Fish or algae oils | Colorectal | Induced expression of genes related to apoptosis. Proteasome inhibition in favor of proapoptotic proteins resulting in an accumulation of tumor-suppressor proteins and induction of apoptosis. | DHA have chemopreventive effect significantly inhibiting the growth of cancer cells. | [63] |
| Docosahexaenoic acid (DHA) | Fish or algae oils | Colorectal | Inhibition of 5-FU-induced IL-1β secretion, caspase-1 activity, JNK activation | DHA enriched diet reduces circulating IL-1β concentration and recurrence in 5-FU-treated tumors | [64] |
| Epigallocatechin gallate (EGCG) | Green tea | Lung | Apoptosis ↑ GADD153, death receptor 5, and p21waf1 Protein acetylation inhibition ↓ HDAC4, -5, -6 | In combination with the synthetic retinoid Am80, EGCG or HDAC inhibitor celecoxib, enhances cell apoptosis and increases drug sensitivity in resistant cells. | [65] |
| Epigallocatechin gallate (EGCG) | Green tea | Lung | CSCs growth inhibition and apoptosis ↑ has-mir-485-5p ↓ RXRα | EGCG inhibits non–small-cell lung cancer cell growth and induces cell-apoptosis. | [66] |
| Epigallocatechin gallate (EGCG) | Green tea | Breast | Bioinformatic prediction: disruption of signaling proteins involved in cell death and survival, DNA replication, recombination and repair; and cell cycle JUN, FADD, NFKB1, Bcl-2, GNAO1, MMP14 | EGCG is predicted to affect several molecular pathways that appear altered in breast cancer. | [67] |
| Epigallocatechin gallate (EGCG) | Green tea | Colorectal | Apoptosis and DNA damage ↓ GRP78, MDR1 ↑ NF-κB, miR-155-5p | EGCG acts as a chemo-sensitizer to 5-fluorouracil in colon cancer cell lines. | [68] |
| Naringenin | Citrus fruits | Prostate | Apoptosis ↑ PI3K/AKT ↓ ERK1/2, p38, JNK Loss of MMP ROS generation Loss of mitochondrial membrane potential | Naringenin suppresses cell proliferation and migration, and induces apoptosis and ROS production. In combination with paclitaxel, enhances cell proliferation inhibition effects. | [69] |
| Procyanidin B2 3,3″-di-O-gallate (B2G2) | Grape seed | Prostate | CSCs cell renewal ↓ Cleaved Notch1, HES-1, NF-κB, STAT3. | B2G2 targets both differentiated cells and CSCs in the tumor mass and impairs prostate cancer growth and relapse | [70] |
| Quercetin | Fruits Vegetables Red wine | Prostate | Cell proliferation inhibition ↓ PI3K, AKT, ERK1/2, p38, ABCG2, NF-κB Inhibition of migration in PC3 and CD44+/CD133+ ↓ PI3K/PTEN, MAPK, NF-κB | Downmodulation of growth factor midkine (MK) expression curbs migration, tumorigenesis and progression of CD44+/CD133+ and prostate cancer cells. Quercetin enhances MK inhibition, promoting apoptosis and effectively eliminating cancer cells. | [71] |
| Quercetin | Fruits Vegetables Red wine | Breast | Cell proliferation inhibition ↓ mTOR, PI3K, Akt, CyclinD, Bcl-2 Cell viability inhibition ↓ ERα | Quercetin inhibits PI3K/Akt/mTOR-signaling, decreasing proliferation in CD44+/CD24− CSCs, thereby decreasing breast CSC population. | [72] |
| Secoisolariciresinol diglucoside (SDG) | Flaxseed | Breast | Inhibition of tumor growth and macrophage infiltration Cell survival inhibition ↓ p65 and NF-κB | SDG treatment, and in particular its metabolite enterolactone, correlates with restrained breast tumor growth in ERα-negative breast cancer. Therefore, SDS could be effective as an adjuvant treatment to reduce recurrence. | [73] |
| β-Sitosterol-d-glucoside (β-SDG) | Sweet potato | Breast | Activation of tumor supressors ↑ miR-10a Cell signaling regulation ↓ PI3K/Akt, Bcl-2 Apoptosis ↑ caspase proteases | Inhibitory effects of β-SDG breast-cancer cell growth. Promising therapeutic agent for treating breast cancer. | [74] |
| Extract Source | Bioactive Fraction | Cancer Type | Molecular Mechanism | Anticancer Effect | Reference |
|---|---|---|---|---|---|
| Andrographis paniculata | Andrographolide | Prostate | Apoptosis Cell cycle and DNA repair modulation ATM, BLM, BRCA2, BRIP1, CLSPN, NBN, PALB | Andrographolide promotes DNA damage in tumor cells leading to cell death. | [75] |
| Aronia | 3-O-p-Coumaroyltormentic Acid | Breast | Cell proliferation inhibition Reduction of cancer cell subpopulations CD44high/CD24low, ALDH+ Self-renewal inhibition ↓ CD44, Sox2, Oct4 Cell survival inhibition ↓ c-Myc | Promotes CSCs cell death inhibiting survival and self-renewal potential. | [76] |
| Castor oil | ω-hydroxyundec-9-enoic (ω-HUA) | Breast | Increased apoptosis and ROS generation ↑ Caspase-3, PARP, p38, JNK | ω-HUA-induced cell death promotes tumor regression. | [77] |
| Ginger | Gingerols | Leukemia | Antiproliferative impact on methotrexate-resistant tumor cell lines not by modifying the expression levels of the ABCA2 and ABCA3 drug efflux genes. | Antitumor impact of ginger in combination with methotrexate on T-cell acute lymphoblastic leukemia (T-ALL). | [78] |
| Ginseng | Ginsenoside Rg3 | Colorectal | Cell survival inhibition ↓ NF-κB, Cyclin D1, Survivin, Cox-2, VEGF | Rg3 enhances radiotherapy by impairing cell survival, finally inhibiting tumor growth. | [79] |
| Grape seed extract | Monomeric, dimeric and trimeric proantho-cyanidins (OPCs) | Colorectal | Cell cycle and DNA replication inhibition ↓ CCNE2, E2F1 ↑ SFN, CDKN1A, MAD1L1 Cell migration inhibition ↓ MMP2, EZH2, WNT5A Upregulation tumor suppressor gene PTEN | OPCc block various oncogenic pathways and inhibit colorectal cancer growth through multiple cell pathways. | [80] |
| Isodon | Flexicaulin A | Colorectal | Cell proliferation inhibition ↑p21 | Flexicaulin A inhibits cancer cell proliferation, emerging as a promising support treatment in colorectal malignancies. | [81] |
| Orange peel | Nobiletin Sinensetin Sutellarein tetramethylether Tangeretin | Colorectal | Cell proliferation inhibition Cancer stemness and self-renewal inhibition ↓PROM1, LGR5 EMT transition modulation ↑CDH1 ↓ZEB1, SNAI1 | Orange peel extract reduces cell proliferation and modulating cancer stemness and self-renewal. Synergistical interaction with 5-fluorouracil. | [82] |
| Sorghum | Phenolic acids and flavonoids | Prostate | Apoptosis ↓ Bcl-2, Akt ↑ Bax Cell cycle arrest ↓ Cyclin D1, Cyclin E ↑ p21Waf/Cip1 | Donganme sorghum ethyl- acetate extract (DSEE) suppresses cell proliferation by activating apoptosis. | [83] |
| Rosemary and shark liver oil rich in alkylglycerols | Phenolic diterpenes | Colorectal | Modulation of expression of genes involved in immune-modulation, inflammation, oxidative stress, lipid metabolism, and tumorigenesis. | Activation of innate immune, cytotoxic and anti-inflammatory responses towards effector cells. Gene expression modulation supports its potential usefulness in cancer patients. | [84] |
| Thunder god vine | Triptolide | Breast | Cell proliferation inhibition Caspase-3-mediated apoptosis Autophagy induction | Triptolide could be an efficient anticancer agent specific for triple negative breast cancers. | [85] |
| Watercress and broccoli extracts | Phenethyl isothiocyanate (PEITC) and sulforaphane (SFN) | Colorectal | Impaired cell proliferation Decreased cell self-renewal Decreased cell adhesion ↓ E-cadherin Reversion of CSC ALDH1-mediated chemoresistance ↓ LGR5, PROM1, ALDH1 CSC proliferation Wnt/β-catenin/TCF7L2 | Chemotherapeutic potential of ITC-enriched extracts in CRC therapy by targeting critical aspects of tumor progression and tumor relapse. | [86] |
| Bioactive Foodstuff | Cancer Type | Nano-Formulation | Molecular Mechanisms | Anticancer Effect | Reference |
|---|---|---|---|---|---|
| Curcumin | Breast | H-ferritin (HFn) nanoparticle | HFn biopolymer specifically binds to the TfR1 receptor, found to be overexpressed in triple negative breast cancer cells. | HFn nanoparticles raises solubility, stability and bioavailability of curcumin, potentiating its effects as a doxorubicin sensitizer. | [87] |
| Curcumin | Breast | Fe3+-curcumin and Cu2+-curcumin complexes encapsulated into poly(styrene)-co-maleic acid (SMA) micelles. | Metal complexes prevent curcumin degradation. Its sequential encapsulation into SMA micelles improves their solubility and stability and their accumulation in tumors. | Improved chemical stability and tumor growth reduction. Higher stability in biological fluids. Increased ability to enter and accumulate in tumor cells. | [88] |
| Curcumin | Prostate | Dextran nanobubbles | Effective internalization into tumor cells and sustained release of curcumin, enhancing curcumin potential to inhibit cell migration and promote apoptosis. | Lower doses of curcumin are needed to get the same anti-cancer effects. Helping to prevent metastasis and relapse. | [89] |
| Curcumin in combination paclitaxel | Breast | Hyaluronic acid (HA) lipoid hybrid nanoparticles | HA interacts with the CD44 receptor, overexpressed in breast CSCs. | Enhanced anti-tumor impact by inhibiting cell growth and migration. | [90] |
| Curcumin in combination paclitaxel | Breast | Poly (ethylene glycol)-benzoic imine-poly(g-benzyl-L-aspartate)-b-poly(1-vinylimidazole) block copolymer | This pH polymer can switch its surface charge in order to facilitate their intake by tumor cells, solving issues regarding drug delivery into inner regions of solid tumors. | The formulation increases the extent of action of the curcumin-paclitaxel combination. | [91] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reglero, C.; Reglero, G. Precision Nutrition and Cancer Relapse Prevention: A Systematic Literature Review. Nutrients 2019, 11, 2799. https://doi.org/10.3390/nu11112799
Reglero C, Reglero G. Precision Nutrition and Cancer Relapse Prevention: A Systematic Literature Review. Nutrients. 2019; 11(11):2799. https://doi.org/10.3390/nu11112799
Chicago/Turabian StyleReglero, Clara, and Guillermo Reglero. 2019. "Precision Nutrition and Cancer Relapse Prevention: A Systematic Literature Review" Nutrients 11, no. 11: 2799. https://doi.org/10.3390/nu11112799
APA StyleReglero, C., & Reglero, G. (2019). Precision Nutrition and Cancer Relapse Prevention: A Systematic Literature Review. Nutrients, 11(11), 2799. https://doi.org/10.3390/nu11112799





