1. Introduction
Anemia is a major public health problem in the low- and middle-income countries [
1]. Anemia in children, defined as a hemoglobin level < 11.0 g/dL, is associated with impaired cognitive performance; increased mortality and morbidity; and poorer educational attainment in children [
2]. Iron deficiency (ID) is considered as the most common cause of anemia, with the widely held assumption that half of all anemia cases are caused by ID [
3]. The World Health Organization recommends Micronutrient Powder (MNP), a powdered formulation consisting of key micronutrients, including iron, as an intervention to prevent childhood anemia [
4]. Accordingly, the Bangladesh Government has also adopted this intervention to prevent childhood anemia. However, an increasing number of studies have shown that the supplementation of iron/MNP is associated with side effects, such as diarrhea, nausea, vomiting, bloody stool, malaria, and respiratory tract infections [
5,
6,
7]. Of note, iron is a pro-oxidant and can have deleterious effects if an excessive amount of free iron is present in the body system [
8]. Iron in the body is maintained by a tightly controlled regulatory system, and uptake of iron in the body depends on the iron status of the body, and/or the presence of inflammation and infection. In the presence of a sufficient reserve of body iron or systemic inflammation, the intestinal uptake of iron may be limited due to hepcidin-mediated regulation [
9,
10]. The unabsorbed iron in the gut might affect the composition of the gut microbiome, leading to the side effects [
11,
12]. In this context, trials have been conducted assessing the efficacy of low-iron MNPs in African settings with a high infection burden. Findings have shown that despite there being efficacy with the low-iron formulations in improving hemoglobin levels, increased side-effects were documented compared to placebo [
13,
14]. Groundwater iron has been an evolving area of research in anemia science [
15,
16]. Iron is one of the most abundant metals on Earth, and is ubiquitous in groundwater sources depending on the environment over which the water flows [
17,
18]. Recent studies have shown a significant association between iron status and daily iron intake from drinking groundwater in different population groups [
15,
19]. Further, iron status was observed to be good in Bangladeshi populations who are drinking from groundwater with a high level of iron [
15,
18,
19,
20]. In the country, the MNP program for the prevention of childhood anemia suffers poor coverage (~2%–3%, personal communication), and the side-effects are documented [
21]. To date, no study has been conducted to examine the usage of MNPs/iron supplements in iron-replete children, whose potable supplies are iron-rich groundwater. Hence, the present study examined the effect of a low-iron MNP compared with the standard MNP on the hemoglobin concentration, and the associated morbidities, in Bangladeshi children exposed to high-iron groundwater.
4. Discussion
This randomized controlled trial examined the effect of a low-dose iron MNP against the standard MNP on hemoglobin and iron status in rural Bangladeshi children (2–5 years old), who drink from the “high-iron” groundwater. Using the intention-to-treat analysis, we observed, the lower bound of the 95% CI for the difference of the treatment effect of the low-iron MNP with the standard MNP was −0.3 g/dL. This was above the priori non-inferior margin of the acceptable difference of −0.5 g/dL, thus establishing the non-inferiority of the low-iron MNP against the standard treatment. The per-protocol analysis also yielded similar findings (
Table S1). The finding of the low baseline (5.4%–5.8%) and end-point (1%–2.5%) prevalence of anemia warrants discussion. Our study site resides in the areas with a very high concentration of iron in groundwater [
17]. Of note, in the present study samples, the median value of iron concentration in groundwater was 4.54 mg/L (mean: ~8 mg/L), which was much higher than the cut-off for defining the “high” level of iron in groundwater [
18]. There were hardly any children who were iron deficient (baseline < 2%, end-point 0%). Taking these into considerations, the low prevalence of anemia was not surprising. Further, we used a venous blood sample to measure hemoglobin concentration. Studies have shown that the capillary blood sampling, which is commonly employed for measuring hemoglobin concentration in surveys and studies, tend to overestimate anemia estimates [
44,
45,
46]. The reasons for the difference between the methods are the measurement errors (mostly happens with capillary sampling) or the biological variability, which is difficult to minimize [
44,
46].
We observed the usage of low-iron MNP (5 mg iron) resulted in significantly fewer incidences of side-effects, such as diarrhea, nausea and fever, compared with the usage of the standard MNP (12.5 mg iron). The lower incidence of side-effects from a low-iron MNP is expected since these morbidities commonly occur with iron supplementation [
5,
6]. The findings of the low incidence of side-effects with the low-iron formulation are promising for the MNP programs in Bangladesh that suffers from suboptimum coverage, and side-effects were identified as an important underlying cause of the poor coverage [
21].
Studies examining the efficacy and morbidities of the low-iron MNP are scarce. Samuel et al. [
13], in Ethiopian children, have shown that a low-iron MNP containing 6 mg of iron in combination with an infant and young child feeding (IYCF) intervention effected in a marginal improvement of hemoglobin compared with the non-intervention group (no-iron), but caused a higher incidence of diarrhea [
13]. This was relatively consistent with our finding, as we observed fewer incidences of diarrhea with the low-iron formulation compared with the standard MNP, which contain a higher amount of iron. Paganini et al. observed in young Kenyan infants that the MNP with 5 mg of highly bioavailable iron resulted in a 50% reduction in anemia over a 4-month intervention when compared with the control (no iron) [
14]. These trials, e.g., Samuel et al. and Paganini et al., demonstrated the superior efficacy of the low-iron MNP (5–6 mg of iron) against the control (0.0 mg of iron) on hemoglobin concentration, while the present study showed a non-inferior efficacy of the low-iron MNP (5 mg of iron) against the standard MNP (12.5 mg of iron), which is a logical outcome.
Paganini et al. employed a highly bioavailable iron in their low-iron formulation (containing 2.5 mg ferrous fumarate + 2.5 mg NaFeEDTA + 190 FTU phytase), and they observed an 18.8% absorption of iron [
14]. In the present study, the low-iron MNP contained 5 mg of iron as ferrous fumarate. Tondeur et al. showed that ferrous fumarate in MNP, mixed in a cereal-based diet, had an absorption rate of 4.65% in the iron-replete children [
47]. Using the ferrous fumarate and presumably with a much lower rate of absorption of iron than in Paganini et al.’s trial, the present study demonstrated the efficacy of low-iron MNP in preventing low hemoglobin levels, which could be explained by the consumption of iron from groundwater. Iron in groundwater remains mostly in a reducing and bioavailable (ferrous) state [
15,
48], and is reported to have a high absorption rate [
49]. We considered the intake of iron from all sources—diet, groundwater and MNP—and calculated the amount of potentially bioavailable iron, considering the differential absorption potentials for different sources. Based on a study of the absorption of iron from iron-rich natural water [
49], we assumed an estimated absorption potential for iron from groundwater. Accordingly, the estimated lowest amount of potentially bioavailable iron from all sources combined in children taking the low-iron MNP was 0.85 mg/day (
Supplementary Text 2), which is sufficient to meet the daily requirement in this group of children [
50].
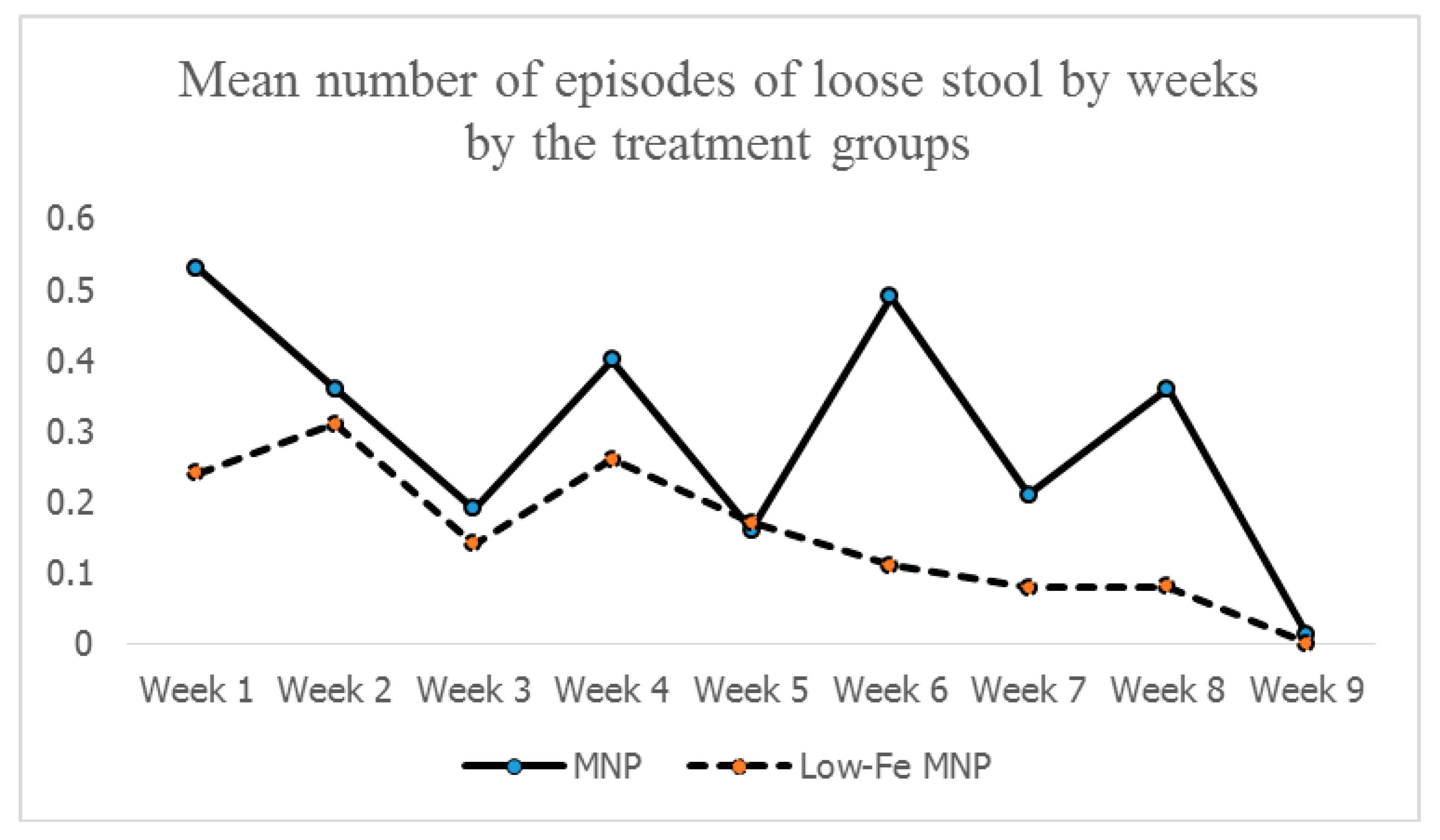
The body iron reserve was sufficient, with >550 mg of baseline values in all groups. There was a similar magnitude of the increment of the iron reserve from baseline to end-point in both treatment groups, though the intake of supplemental iron in the standard MNP group was ~2.5 times (633.6 vs. 261.1 mg) higher than that in the low-iron MNP group. This suggests that, relative to the dose of iron, the amount of absorption of iron was smaller in the standard MNP group compared to its counterpart. This might have led to a higher amount of unabsorbed iron in the intestinal tract for the standard MNP group, which might have contributed to a significantly higher number of diarrheal and loose stool episodes observed in that group than in the low-iron MNP group. Further research is needed in this setting to examine the iron-induced adversities on the composition of gut microbiota, which is linked with iron supplementation and the occurrence of diarrhea and loose stool, to support the present findings.
The combined intake of iron from all sources (diet + groundwater + MNP) were 18.25 and 12.37 mg in the standard MNP and the low-iron MNP groups, respectively (
Table S2). There was no group difference for intakes from dietary and groundwater sources; the difference was attributed to the intake of iron from the different MNPs. An intake of 18.25 mg iron from all sources did not show any association with the body-iron reserve in the standard MNP group (
r = 0.02;
p = 0.87). However, the intake of 12.37 mg iron in the low-iron MNP group showed a significant association (
r = 0.28,
p = 0.03). One possible explanation for the differential outcome between the groups is that the higher amount of iron in the standard MNP group might have initiated the stimulation of hepcidin at some point, through the iron-transferrin transportation complex [
51]. This might have led to the subsequent inhibition of the absorption of further iron from the intestine [
51], thus limiting the buildup of an iron reserve in the standard MNP group. This was reflected in the similarities of the levels of body-iron reserves between the groups at the end-point. However, for the low-iron MNP, a moderate degree of association indicates that the amount of iron (i.e., from low-iron MNP and other sources) present in the duodenum maintained a positive gradient of absorption of iron with minimal/no inhibition of absorption. This suggests that the dose of iron (5 mg) in the low-iron formulation was optimum in Bangladeshi children exposed to a high level of iron from groundwater. As the absorption was efficient, there might be less iron remaining unabsorbed, leading to lower incidences of side-effects (e.g., diarrhea, loose stool, nausea and fever) compared with the standard MNP group. This was further complemented by the findings of the mean number of loose stools by weeks during the 2-months intervention, which after initial occurrences in both the groups, declined and stabilized in the low-iron MNP group from the 4th week onwards. However, it continued to occur in higher numbers in the standard MNP group.
A baseline prevalence of ~5.5% anemia in a high iron groundwater area may question the relevance of the iron supplementation program for the prevention of anemia in children. However, the iron level in groundwater is considerably variable in the tube-wells [
15,
17]. In a predominantly high iron groundwater area, there are the wells that contain either no iron or a negligible level of iron (<0.3 mg/L, the WHO aesthetic limit) [
52]. Hence, in the context of a less diversified traditional diet with suboptimum dietary iron [
27], the absence of the supplementation program might be counterproductive to some children even in the high iron groundwater areas. In this setting, the low-iron MNP with a reduced risk of side-effects can be an optimum measure.
A limitation of the study was that one of the main investigators, who did the preliminary analyses, could not be blinded to the treatment group coding. This might have introduced some risk of bias. Unfortunately, this could not be avoided as the MNP preparations were imported from India and the customs clearance required the declaration of the composition of the different MNP preparations. However, all field personnel engaged in the distribution and recording of the compliance of MNP consumption and morbidity data, and parents of the children remained blind to the treatment group coding. Morbidity data were collected on the weekly recalls. The method, though widely practiced, is subject to recall bias. However, we provided extensive training to the monitoring staffs to collect data objectively. Among the strengths of the study, the uptake of the interventions was satisfactory (~86% MNPs were consumed) (
Table S3). Dropouts were fewer (<5% in the groups), which improved the precision of the findings.