Impaired Glucose Tolerance is Associated with Enhanced Platelet-Monocyte Aggregation in Short-Term High-Fat Diet-Fed Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Blood Collection for Haematology Characteristics and Flow Cytometry Analysis
2.3. Instrument Set-Up and Optimization
2.4. Measurement of Baseline PMA Levels
2.5. Measurement of PMA Post-Stimulation with ADP
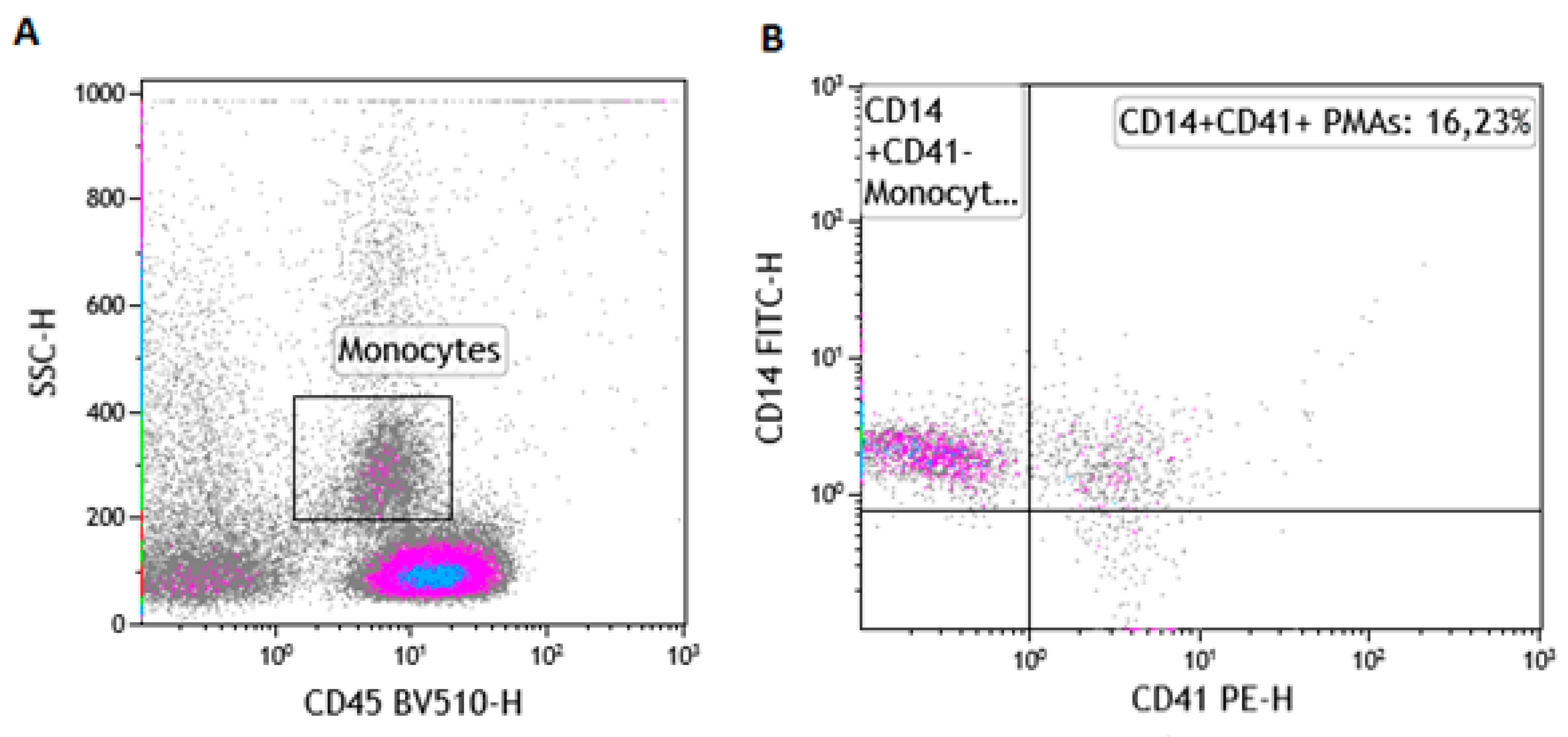
2.6. The Gating Strategy for the Enumeration of PMAs
2.7. Statistical Analysis
3. Results
3.1. The Impact of HFD on Baseline Metabolic Parameters and Glucose Tolerance
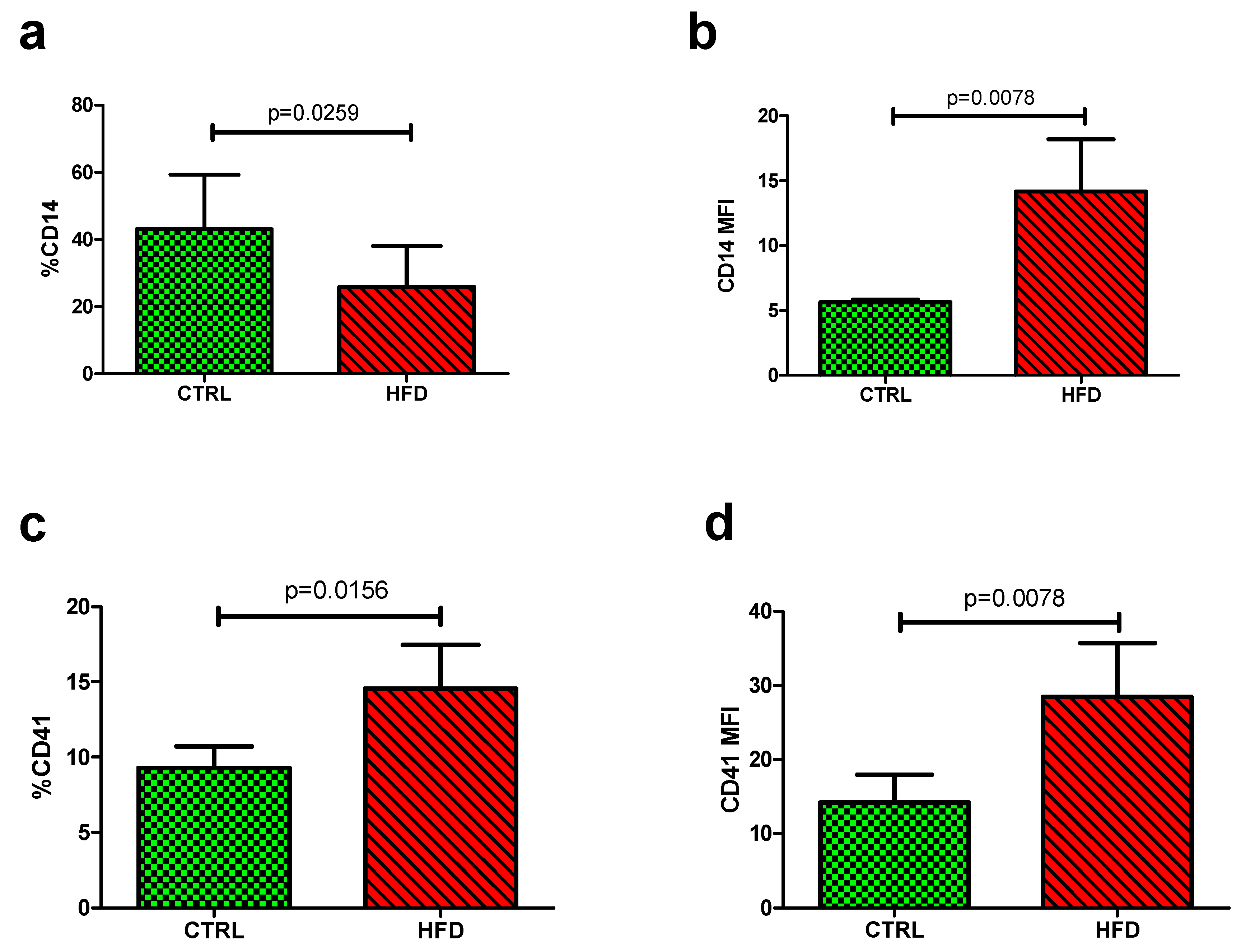
3.2. The Impact of HFD on Platelet-Monocyte Aggregates
3.3. The Impact of HFD on the Modulation of PMAs Post-Stimulation with ADP
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosqvist, F.; Iggman, D.; Kullberg, J.; Cedernaes, J.; Johansson, H.E.; Larsson, A.; Johansson, L.; Ahlström, H.; Arner, P.; Dahlman, I.; et al. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes 2014, 63, 2356–2368. [Google Scholar] [CrossRef] [PubMed]
- Yngen, M.; Östenson, C.G.; Hu, H.; Li, N.; Hjemdahl, P.; Wallén, N.H. Enhanced P-selectin expression and increased soluble CD40 ligand in patients with type 1 diabetes mellitus and microangiopathy: Evidence for platelet hyperactivity and chronic inflammation. Diabetologia 2004, 47, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Bournazos, S.; Rennie, J.; Hart, S.P.; Fox, K.A.A.; Dransfield, I. Monocyte functional responsiveness after PSGL-1-mediated platelet adhesion is dependent on platelet activation status. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Duan, Z.; Li, D.; Li, D.; Wang, Z.; Ren, L.; Shen, T.; Shao, Y. Higher levels of circulating monocyte-platelet aggregates are correlated with viremia and increased sCD163 levels in HIV-1 infection. Cell. Mol. Immunol. 2015, 12, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Hui, H.; Fuller, K.A.; Erber, W.N.; Linden, M.D. Imaging flow cytometry in the assessment of leukocyte-platelet aggregates. Methods 2017, 112, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Dorsam, R.T.; Kunapuli, S.P. Central role of the P2Y 12 receptor in platelet activation. J. Clin. Investig. 2004, 113, 10–15. [Google Scholar] [CrossRef]
- Yau, J.W.; Teoh, H.; Verma, S. Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 2015, 15, 130. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and oxidative stress in an obese state and the protective effects of gallic acid. Nutrients 2019, 11, 23. [Google Scholar] [CrossRef]
- Michelson, A.D.; Barnard, M.R.; Krueger, L.A.; Valeri, C.R.; Furman, M.I. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: Studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation 2001, 104, 1533–1537. [Google Scholar] [CrossRef]
- Van Gils, J.M.; Zwaginga, J.J.; Hordijk, P.L. Molecular and functional interactions among monocytes, platelets, and endothelial cells and their relevance for cardiovascular diseases. J. Leukoc. Biol. 2009, 85, 195–204. [Google Scholar] [CrossRef]
- Li, Z.; Yang, F.; Dunn, S.; Gross, A.K.; Smyth, S.S. Platelets as immune mediators: Their role in host defense responses and sepsis. Thromb. Res. 2011, 127, 184–188. [Google Scholar] [CrossRef] [Green Version]
- Davison, G.M.; Nkambule, B.B.; Mkandla, Z.; Hon, G.M.; Kengne, A.P.; Erasmus, R.T.; Matsha, T.E. Platelet, monocyte and neutrophil activation and glucose tolerance in South African Mixed Ancestry individuals. Sci. Rep. 2017, 7, 40329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbieri, J.; Fontela, P.C.; Winkelmann, E.R.; Zimmermann, C.E.; Sandri, Y.P.; Mallet, E.K.; Frizzo, M.N. Anemia in patients with type 2 diabetes mellitus. Anemia 2015, 2015, 354737. [Google Scholar] [CrossRef]
- Andrikopoulos, S.; Blair, A.R.; Deluca, N.; Fam, B.C.; Proietto, J. Evaluating the glucose tolerance test in mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1323–E1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patkó, Z.; Császár, A.; Acsády, G.; Ôry, I.; Takács, É.V.A.; Fûrész, J. Elevation of monocyte-platelet aggregates is an early marker of type 2 diabetes. Interv. Med. Appl. Sci. 2012, 4, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Furman, M.I.; Benoit, S.E.; Barnard, M.R.; Valeri, C.R.; Borbone, M.L.; Becker, R.C.; Hechtman, H.B.; Michelson, A.D. Increased platelet reactivity and circulating monocyte-platelet aggregates in patients with stable coronary artery disease. J. Am. Coll. Cardiol. 1998, 31, 352–358. [Google Scholar] [CrossRef]
- Dang, Y.; Xia, Y.; Li, Y.; Yu, D.C.W. Anemia and type 2 diabetes mellitus associated with peripheral arterial disease progression in Chinese male patients. Clin. Biochem. 2013, 46, 1673–1677. [Google Scholar] [CrossRef]
- Bonakdaran, S.; Gharebaghi, M.; Vahedian, M. Prevalence of Functional Dyspepsia in a Rural Medical College Hospital. J. Evol. Med Dent. Sci. 2014, 3, 1934–1939. [Google Scholar]
- Chiou, T.T.-Y.; Lee, J.-J.; Wang, M.-C.; Chung, M.-S.; Pan, L.-L.; Hsieh, C.-J.; Huang, S.-T.; Chang, H.-W.; Yang, K.D.; Lee, C.-T.; et al. Genetic disposition and modifiable factors independently associated with anemia in patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2015, 108, 164–169. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Lee, M.-Y.; Lin, K.-D.; Hsu, W.-H.; Lee, Y.-J.; Hsiao, P.-J.; Shin, S.-J. Diabetes mellitus increases severity of thrombocytopenia in dengue-infected patients. Int. J. Mol. Sci. 2015, 16, 3820–3830. [Google Scholar] [CrossRef]
- Pretorius, E.; Bester, J.; Vermeulen, N.; Alummoottil, S.; Soma, P.; Buys, A.V.; Kell, D.B. Poorly controlled type 2 diabetes is accompanied by significant morphological and ultrastructural changes in both erythrocytes and in thrombingenerated fibrin: Implications for diagnostics. Cardiovasc. Diabetol. 2015, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Unruh, D.; Srinivasan, R.; Benson, T.; Haigh, S.; Coyle, D.; Batra, N.; Keil, R.; Sturm, R.; Blanco, V.; Palascak, M.; et al. Red Blood Cell Dysfunction Induced by High-Fat Diet. Circulation 2015, 132, 1898–1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadok, V.A.; Voelker, D.R.; Campbell, P.A.; Cohen, J.J.; Bratton, D.L.; Henson, P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992, 148, 2207–2216. [Google Scholar] [PubMed]
- Nagata, S.; Suzuki, J.; Segawa, K.; Fujii, T. Exposure of phosphatidylserine on the cell surface. Cell Death Differ. 2016, 23, 952–961. [Google Scholar] [CrossRef] [Green Version]
- Projahn, D.; Koenen, R.R. Platelets: Key players in vascular inflammation. J. Leukoc. Biol. 2012, 92, 1167–1175. [Google Scholar] [CrossRef]
- Furman, M.I.; Barnard, M.R.; Krueger, L.A.; Fox, M.L.; Shilale, E.A.; Lessard, D.M.; Marchese, P.; Frelinger, A.L., 3rd; Goldberg, R.J.; Michelson, A.D. Circulating monocyte-platelet aggregates are an early marker of acute myocardial infarction. J. Am. Coll. Cardiol. 2001, 38, 1002–1006. [Google Scholar] [CrossRef] [Green Version]
- Sudic, D.; Razmara, M.; Forslund, M.; Ji, Q.; Hjemdahl, P.; Li, N. High glucose levels enhance platelet activation: Involvement of multiple mechanisms. Br. J. Haematol. 2006, 133, 315–322. [Google Scholar] [CrossRef]
- Von Hentig, N.; Förster, A.K.; Kuczka, K.; Klinkhardt, U.; Klauke, S.; Gute, P.; Staszewski, S.; Harder, S.; Graff, J. Platelet-leucocyte adhesion markers before and after the initiation of antiretroviral therapy with HIV protease inhibitors. J. Antimicrob. Chemother. 2008, 62, 1118–11121. [Google Scholar] [CrossRef]
- Barnard, M.R.; Linden, M.D.; Frelinger, A.L.; Li, Y.; Fox, M.L.; Furman, M.I.; Michelson, A.D. Effects of platelet binding on whole blood flow cytometry assays of monocyte and neutrophil procoagulant activity. J. Thromb. Haemost. 2005, 3, 2563–2570. [Google Scholar] [CrossRef]
- Wrigley, B.J.; Shantsila, E.; Tapp, L.D.; Lip, G.Y.H. Increased Formation of monocyte-platelet aggregates in ischemic heart failure. Circ. Heart Fail. 2013, 6, 127–135. [Google Scholar] [CrossRef]
- Rutten, B.; Tersteeg, C.; Vrijenhoek, J.E.P.; Van Holten, T.C.; Elsenberg, E.H.A.M.; Mak-Nienhuis, E.M.; De Borst, G.J.; Jukema, J.W.; Pijls, N.H.J.; Waltenberger, J.; et al. Increased platelet reactivity is associated with circulating platelet-monocyte complexes and macrophages in human atherosclerotic plaques. PLoS ONE 2014, 9, e105019. [Google Scholar] [CrossRef] [PubMed]
| Ingredients | Low-fat Diet (Control) a | High-fat Diet b |
|---|---|---|
| Casein, 30 mesh | 200.00 | 200.00 |
| L-Cystine | 3.00 | 3.00 |
| Corn starch | 506.20 | - |
| Lodex 10 | 125.00 | 125.00 |
| Sucrose | 72.80 | 72.80 |
| Solka Floc, FCC200 (Fiber) | 50.00 | 50.00 |
| Soybean Oil | 25.00 | 25.00 |
| Lard | 20.00 | 245.00 |
| Mineral mix S10026B | 50.00 | 50.00 |
| Choline Bitartrate | 2.00 | 2.00 |
| Vitamin mix V10001C | 1.00 | 1.00 |
| Dye, Yellow FD&C #5, Alum. Lake 35–42% | 0.04 | - |
| Dye, Blue FD&C #1, Alum. Lake 35–42% | 0.01 | 0.05 |
| Parameter | Control (n = 8) | High-fat Diet (n = 8) | p-Value |
|---|---|---|---|
| Weight gain (g) | 25.0 ± 2.5 | 26.0 ± 1.9 | 0.43 |
| Glucose levels (mmol/L) | 6.1 (5.4–6.9) | 8.7 (8.5–9.2) | 0.008 |
| AUC mmol/L × 120 min | 636.0 (559.9–702.0) | 765.0 (715.5–784.5) | 0.032 |
| Insulin levels (µIU/mL) | 4.5 (4.4–4.6) | 4.8 (4.6–8.1) | 0.026 |
| White cell count (103/µL) | 5.35 (3.68–9.00) | 7.50 (4.80–8.40) | 0.4699 |
| Red blood cell count (106/µL) | 7.17 (7.04–7.69) | 6.910 (5.53–7.17) | 0.0178 |
| Haemoglobin (g/dL) | 25.85 (20.00–29.23) | 22.40 (16.75–25.00) | 0.6683 |
| Haematocrit (%) | 31.10 (30.05–33.30) | 29.00 (23.00–31.40) | 0.0433 |
| Mean cell volume (fL) | 43.00 (43.00–43.75) | 42.00 (41.00–44.00) | 0.0025 |
| Platelet count (103/µL) | 782.9 ± 206.4 | 697.2 ± 151.1 | 0.5789 |
| Mean platelet volume (fL) | 5.30 (5.03–5.50) | 5.20 (5.10–5.40) | 0.6957 |
| Neutrophil count (%) | 7.75 (7.00–9.48) | 8.000 (6.90–9.30) | 0.9640 |
| Lymphocyte count (%) | 89.85 (88.15–90.73) | 89.20 (87.80–90.50) | 0.5271 |
| Monocyte count (%) | 1.96 ± 0.24 | 2.05 ± 0.56 | 0.6840 |
| Basophil (%) | 0.25 (0.13–0.50) | 0.2000 (0.10–0.80) | 0.7997 |
| %CD14 | 31.05 (30.74–31.61) | 31.13 (29.65–35.78) | 0.4695 |
| CD14 MFI | 5.325 (5.14–5.45) | 5.59 (5.32–5.79) | 0.0350 |
| %CD41 | 7.06 (5.81–8.06) | 5.82 (4.43–6.83) | 0.2757 |
| CD41 MFI | 6.36 (6.04–7.52) | 6.12 (5.81–6.74) | 0.7892 |
| Control diet | Unstimulated (n = 3) | Post-ADP (n = 3) | p-value |
| %CD14 | 63.16 (61.10–63.80) | 17.99 (8.46–20.31) | 0.0074 |
| CD14 MFI | 6.13 (5.98–6.55) | 18.00 (12.53–64.61) | 0.2596 |
| %CD41 | 12.55 (12.49–16.34) | 25.97 (20.02–33.22) | 0.0438 |
| CD41 MFI | 25.84 (22.45–31.69) | 34.22 (24.06–41.80) | 0.2854 |
| High-fat diet | Unstimulated (n = 8) | Post-ADP (n = 7) | p-value |
| %CD14 | 13.52 (4.590–16.08) | 14.37 (8.430–18.98) | 0.0405 |
| CD14 MFI | 16.53 (11.28–45.47) | 20.32 (14.66–73.78) | 0.3125 |
| %CD41 | 29.96 ± 11.40 | 28.94 ± 10.79 | 0.4375 |
| CD41 MFI | 67.22 ± 28.19 | 40.08 ± 14.95 | 0.0938 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mkandla, Z.; Mutize, T.; Dludla, P.V.; Nkambule, B.B. Impaired Glucose Tolerance is Associated with Enhanced Platelet-Monocyte Aggregation in Short-Term High-Fat Diet-Fed Mice. Nutrients 2019, 11, 2695. https://doi.org/10.3390/nu11112695
Mkandla Z, Mutize T, Dludla PV, Nkambule BB. Impaired Glucose Tolerance is Associated with Enhanced Platelet-Monocyte Aggregation in Short-Term High-Fat Diet-Fed Mice. Nutrients. 2019; 11(11):2695. https://doi.org/10.3390/nu11112695
Chicago/Turabian StyleMkandla, Zibusiso, Tinashe Mutize, Phiwayinkosi V. Dludla, and Bongani B. Nkambule. 2019. "Impaired Glucose Tolerance is Associated with Enhanced Platelet-Monocyte Aggregation in Short-Term High-Fat Diet-Fed Mice" Nutrients 11, no. 11: 2695. https://doi.org/10.3390/nu11112695





