Combined Predictive Value of Extracellular Fluid/Intracellular Fluid Ratio and the Geriatric Nutritional Risk Index for Mortality in Patients Undergoing Hemodialysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Data Collection
2.3. Follow-Up Study
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. The GNRI and The ECF/ICF Ratio and Their Relationship
3.3. Univariate Association of The GNRI and The ECF/ICF Ratio with Mortality
3.4. Multivariate Association of The GNRI and The ECF/ICF Ratio with Mortality
3.5. Model Discrimination
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Rambod, M.; Bross, R.; Zitterkoph, J.; Benner, D.; Pithia, J.; Colman, S.; Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Association of Malnutrition-Inflammation Score with quality of life and mortality in hemodialysis patients: A 5-year prospective cohort study. Am. J. Kidney Dis. 2009, 53, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Furuya, R.; Takita, T.; Maruyama, Y.; Yamaguchi, Y.; Ohkawa, S.; Kumagai, H. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am. J. Clin. Nutr. 2008, 87, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, H.; Inoue, K.; Shimizu, K.; Hiraga, K.; Takahashi, E.; Otaki, K.; Yoshikawa, T.; Furuta, K.; Tokunaga, C.; Sakakibara, T.; et al. Tokai Renal Nutrition Study Group. Comparison of nutritional risk scores for predicting mortality in Japanese chronic hemodialysis patients. J. Ren. Nutr. 2017, 27, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, I.; Ishimura, E.; Kato, Y.; Okuno, S.; Yamamoto, T.; Yamakawa, T.; Mori, K.; Inaba, M.; Nishizawa, Y. Geriatric Nutritional Risk Index, a simplified nutritional screening index, is a significant predictor of mortality in chronic dialysis patients. Nephrol. Dial. Transplant. 2010, 25, 3361–3365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, H.; Ito, Y.; Ishii, H.; Aoyama, T.; Kamoi, D.; Kasuga, H.; Yasuda, K.; Maruyama, S.; Matsuo, S.; Murohara, T.; et al. Geriatric nutritional risk index accurately predicts cardiovascular mortality in incident hemodialysis patients. J. Cardiol. 2014, 64, 32–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, J.; Wang, M.; Zhang, Y.; Nie, L.; He, T.; Wang, Y.; Huang, Y.; Feng, B.; Zhang, J.; Zhao, J. Association of Geriatric Nutritional Risk index with mortality in hemodialysis patients: A meta-analysis of cohort studies. Kidney Blood Press. Res. 2018, 43, 1878–1889. [Google Scholar] [CrossRef]
- Johansen, K.L.; Dalrymple, L.S.; Delgado, C.; Kaysen, G.A.; Kornak, J.; Grimes, B.; Chertow, G.M. Association between body composition and frailty among prevalent hemodialysis patients: A US Renal Data System special study. J. Am. Soc. Nephrol. 2014, 25, 381–389. [Google Scholar] [CrossRef]
- Earthman, C.; Traughber, D.; Dobratz, J.; Howell, W. Bioimpedance spectroscopy for clinical assessment of fluid distribution and body cell mass. Nutr. Clin. Pract. 2007, 22, 389–405. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Heimbürger, O.; Lindholm, B.; Kaysen, G.A.; Bergström, J. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome). Nephrol. Dial. Transpl. 2000, 15, 953–960. [Google Scholar] [CrossRef] [Green Version]
- Pupim, L.B.; Caglar, K.; Hakim, R.M.; Shyr, Y.; Ikizler, T.A. Uremic malnutrition is a predictor of death independent of inflammatory status. Kidney Int. 2004, 66, 2054–2060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wizemann, V.; Wabel, P.; Chamney, P.; Zaluska, W.; Moissl, U.; Rode, C.; Malecka-Masalska, T.; Marcelli, D. The mortality risk of overhydration in haemodialysis patients. Nephrol. Dial. Transpl. 2009, 24, 1574–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoccali, C.; Moissl, U.; Chazot, C.; Mallamaci, F.; Tripepi, G.; Arkossy, O.; Wabel, P.; Stuard, S. Chronic Fluid Overload and Mortality in ESRD. J. Am. Soc. Nephrol. 2017, 28, 2491–2497. [Google Scholar] [CrossRef] [Green Version]
- Niebauer, J.; Volk, H.D.; Kemp, M.; Dominguez, M.; Schumann, R.R.; Rauchhaus, M.; Poole-Wilson, P.A.; Coats, A.J.; Anker, S.D. Endotoxin and immune activation in chronic heart failure: A prospective cohort study. Lancet 1999, 353, 1838–1842. [Google Scholar] [CrossRef]
- Pecoits-Filho, R.; Lindholm, B.; Stenvinkel, P. The malnutrition, inflammation, and atherosclerosis (MIA) syndrome—The heart of the matter. Nephrol. Dial. Transpl. 2002, 17, S28–S31. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Seo, J.W.; Yoon, J.W.; Lee, S.K.; Kim, S.J.; Lee, Y.K.; Noh, J.W.; Koo, J.R. The malnutrition-inflammation-depression-arteriosclerosis complex is associated with an increased risk of cardiovascular disease and all-cause death in chronic hemodialysis patients. Nephron. Clin. Pract. 2012, 122, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Tycho Vuurmans, J.L.; Boer, W.H.; Bos, W.J.; Blankestijn, P.J.; Koomans, H.A. Contribution of volume overload and angiotensin II to the increased pulse wave velocity of hemodialysis patients. J. Am. Soc. Nephrol. 2002, 13, 177–183. [Google Scholar] [PubMed]
- Kim, E.J.; Choi, M.J.; Lee, J.H.; Oh, J.E.; Seo, J.W.; Lee, Y.K.; Yoon, J.W.; Kim, H.J.; Noh, J.W.; Koo, J.R. Extracellular fluid/intracellular fluid volume ratio as a novel risk indicator for all-cause mortality and cardiovascular disease in hemodialysis patients. PLoS ONE 2017, 12, e0170272. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Pencina, M.J.; D’Agostino, R.B., Sr.; D’Agostino, R.B., Jr.; Vasan, R.S. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat. Med. 2008, 27, 157–172. [Google Scholar] [CrossRef]
- Zimmermann, J.; Herrlinger, S.; Pruy, A.; Metzger, T.; Wanner, C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999, 55, 648–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qureshi, A.R.; Alvestrand, A.; Divino-Filho, J.C.; Gutierrez, A.; Heimbürger, O.; Lindholm, B.; Bergström, J. Inflammation, malnutrition, and cardiac disease as predictors of mortality in hemodialysis patients. J. Am. Soc. Nephrol. 2002, 13, S28–S36. [Google Scholar] [PubMed]
- Kalantar-Zadeh, K.; Kopple, J.D.; Humphreys, M.H.; Block, G. Comparing outcome predictability of markers of malnutrition-inflammation complex syndrome in haemodialysis patients. Nephrol. Dial. Transplant. 2004, 19, 1507–1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yajima, T.; Yajima, K.; Takahashi, H.; Yasuda, K. The impact of abdominal fat levels on all-cause mortality risk in patients undergoing hemodialysis. Nutrients 2018, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Yajima, T.; Arao, M.; Yajima, K.; Takahashi, H.; Yasuda, K. The associations of fat tissue and muscle mass indices with all-cause mortality in patients undergoing hemodialysis. PLoS ONE 2019, 14, e0211988. [Google Scholar] [CrossRef] [PubMed]
| All Patients (N = 234) | G1 (N = 76) | G2 (N = 41) | G3 (N = 41) | G4 (N = 76) | p-Value | |
|---|---|---|---|---|---|---|
| Age (years) | 65.1 ± 12.6 | 56.5 ± 14.3 | 62.1 ± 14.3 | 66.8 ± 10.9 | 70.7 ± 10.1 | <0.0001 |
| Male (%) | 69.2 | 65.8 | 48.8 | 75.6 | 80.3 | 0.0039 |
| Underlying kidney disease | 0.0027 | |||||
| Diabetic kidney disease (%) | 42.7 | 47.3 | 22.0 | 53.7 | 43.4 | |
| Chronic glomerulonephritis (%) | 29.9 | 36.8 | 43.9 | 9.8 | 26.3 | |
| Nephrosclerosis (%) | 19.7 | 11.8 | 22.0 | 29.3 | 21.1 | |
| Others (%) | 7.7 | 4.1 | 12.1 | 7.2 | 9.2 | |
| HD duration (years) | 0.8 (0.6–5.1) | 1.1 (0.6–5.1) | 1.2 (0.5–5.7) | 0.8 (0.6–3.9) | 0.7 (0.6–5.7) | 0.65 |
| Alcohol (%) | 20.9 | 17.1 | 19.5 | 29.3 | 21.1 | 0.50 |
| Smoking (%) | 25.6 | 27.6 | 23.9 | 24.4 | 25.0 | 0.97 |
| Hypertension (%) | 92.3 | 94.7 | 85.4 | 100 | 89.5 | 0.016 |
| Diabetes (%) | 45.3 | 47.3 | 26.8 | 58.5 | 46.1 | 0.030 |
| History of CVD (%) | 63.2 | 53.9 | 51.2 | 80.5 | 69.7 | 0.0061 |
| BMI (kg/m2) | 22.0 ± 3.8 | 23.3 ± 3.5 | 20.1 ± 4.3 | 23.4 ± 2.8 | 21.0 ± 3.7 | <0.0001 |
| BUN (mg/dL) | 59.0 ± 16.8 | 65.9 ± 17.3 | 60.7 ± 13.9 | 57.6 ± 14.6 | 52.0 ± 16.0 | <0.0001 |
| Creatinine (mg/dL) | 8.8 ± 3.0 | 10.0 ± 3.4 | 9.4 ± 2.8 | 8.0 ± 2.6 | 7.7 ± 2.5 | <0.0001 |
| Albumin (g/dL) | 3.7 ± 0.4 | 3.9 ± 0.2 | 3.5 ± 0.3 | 3.8 ± 0.2 | 3.4 ± 0.3 | <0.0001 |
| Hemoglobin (g/dL) | 10.8 ± 1.4 | 11.0 ± 1.3 | 11.0 ± 1.1 | 10.8 ± 1.0 | 10.3 ± 1.6 | 0.0048 |
| T-Cho (mg/dL) | 154 ± 36 | 158 ± 36 | 161 ± 32 | 165 ± 36 | 143 ± 35 | 0.0028 |
| HDL-C (mg/dL) | 44.5 ± 15.2 | 47.1 ± 16.9 | 46.8 ± 16.5 | 42.2 ± 12.4 | 42.0 ± 13.5 | 0.10 |
| TG (mg/dL) | 121 ± 77 | 138 ± 90 | 121 ± 84 | 136 ± 86 | 96 ± 40 | 0.0039 |
| Uric acid (mg/dL) | 7.0 ± 1.8 | 7.5 ± 1.7 | 7.5 ± 1.6 | 7.0 ± 1.9 | 6.3 ± 1.6 | 0.0001 |
| Ca (mg/dL) | 8.8 ± 0.8 | 8.9 ± 0.8 | 8.5 ± 0.7 | 9.0 ± 0.8 | 8.8 ± 0.9 | 0.047 |
| P (mg/dL) | 5.1 ± 1.3 | 5.5 ± 1.3 | 5.3 ± 1.0 | 4.7 ± 1.1 | 4.8 ± 1.5 | 0.0008 |
| Glucose (mg/dL) | 137 ± 58 | 138 ± 60 | 124 ± 59 | 158 ± 66 | 132 ± 48 | 0.050 |
| CRP (mg/dL) | 0.17 (0.07–0.48) | 0.12 (0.06–0.24) | 0.12 (0.03–0.67) | 0.12 (0.07–0.44) | 0.35 (0.13–0.92) | <0.0001 |
| GNRI | 93.5 ± 6.5 | 98.9 ± 3.4 | 89.0 ± 4.6 | 97.9 ± 2.2 | 88.2 ± 5.2 | <0.0001 |
| CTR (%) | 49.3 ± 5.1 | 48.2 ± 4.8 | 48.2 ± 5.5 | 50.7 ± 5.7 | 50.2 ± 4.7 | 0.013 |
| Dry weight (kg) | 57.2 ± 12.1 | 61.5 ± 12.6 | 50.7 ± 12.0 | 60.6 ± 9.3 | 54.5 ± 10.7 | <0.0001 |
| Δ body weight (kg) | 2.0 ± 0.9 | 2.4 ± 0.9 | 1.7 ± 0.6 | 1.9 ± 0.8 | 1.9 ± 1.0 | 0.0002 |
| TBF (kg) | 27.6 ± 5.5 | 28.4 ± 5.8 | 24.3 ± 5.1 | 28.8 ± 3.8 | 27.9 ± 5.5 | 0.0002 |
| ICF (kg) | 17.3 ± 3.8 | 19.7 ± 3.9 | 16.7 ± 3.3 | 16.8 ± 2.5 | 15.4 ± 3.2 | <0.0001 |
| ECF (kg) | 10.3 ± 3.3 | 8.7 ± 2.4 | 7.5 ± 2.1 | 12.0 ± 2.0 | 12.5 ± 3.3 | <0.0001 |
| ECF/ICF ratio | 0.61 ± 0.22 | 0.44 ± 0.08 | 0.45 ± 0.08 | 0.72 ± 0.12 | 0.82 ± 0.21 | <0.0001 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variables | β | p-Value | β | p-Value |
| Age | 0.430 | <0.0001 | 0.107 | 0.12 |
| Male gender | 0.180 | 0.0059 | 0.212 | 0.0001 |
| Diabetes | 0.216 | 0.0009 | 0.171 | 0.0021 |
| History of CVD | 0.152 | 0.020 | 0.002 | 0.966 |
| CTR | 0.314 | <0.0001 | 0.200 | 0.0010 |
| Creatinine | −0.399 | <0.0001 | −0.133 | 0.051 |
| Phosphorus | −0.310 | <0.0001 | −0.118 | 0.053 |
| Log CRP | 0.329 | <0.0001 | 0.136 | 0.017 |
| GNRI | −0.387 | <0.0001 | −0.247 | <0.0001 |
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| All-cause mortality | ||||
| GNRI (continuous) | 0.88 (0.85–0.92) | <0.0001 | 0.90 (0.87–0.94) | <0.0001 |
| ECF/ICF (/0.01) (continuous) | 1.05 (1.04–1.06) | <0.0001 | 1.04 (1.03–1.05) | <0.0001 |
| Lower GNRI | 4.23 (2.54–7.37) | <0.0001 | 3.48 (2.01–6.25) | <0.0001 |
| Higher ECF/ICF | 15.17 (7.56–34.95) | <0.0001 | 11.38 (5.29–27.89) | <0.0001 |
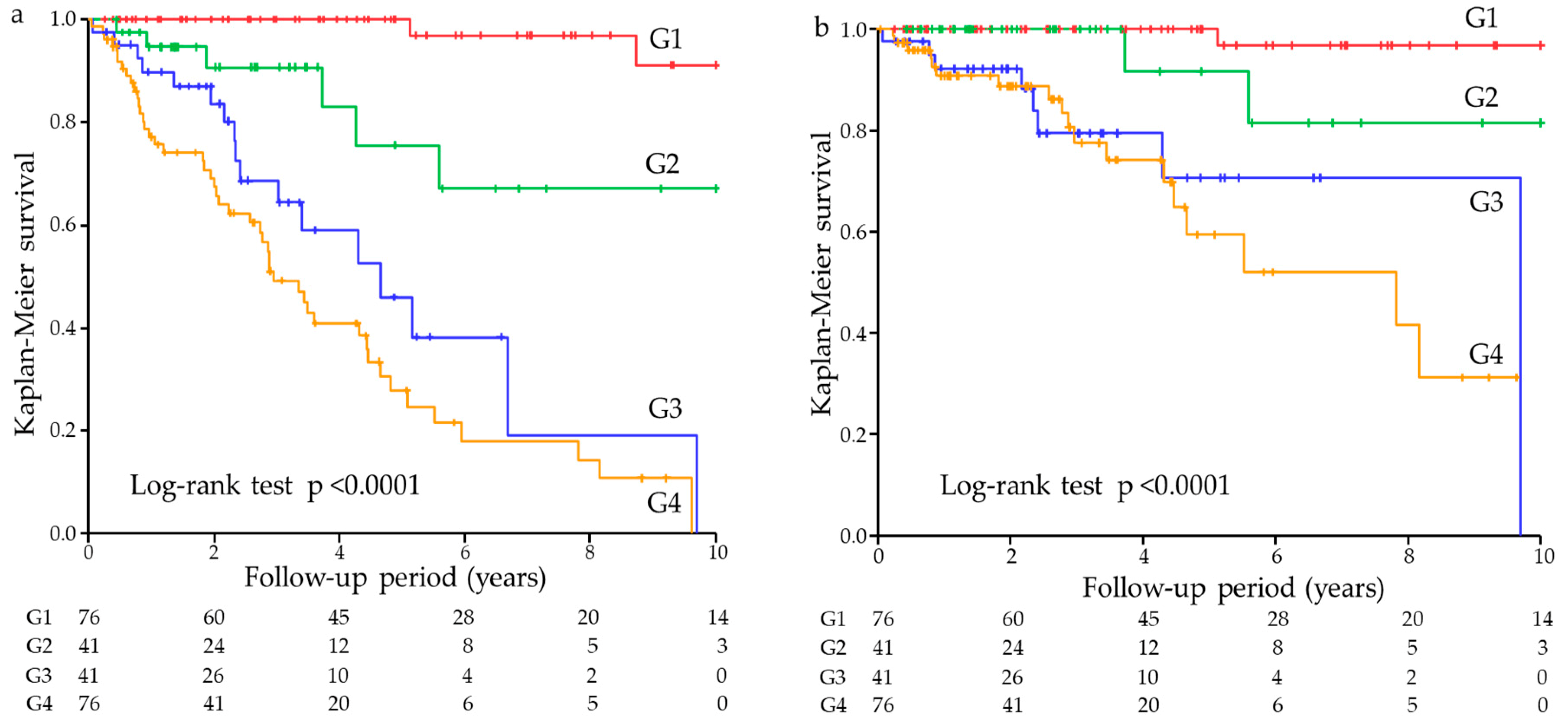
| Cross-classified (vs. G1) | <0.0001 | <0.0001 | ||
| G2 | 9.31 (2.14–63.66) | 0.0027 | 9.85 (2.20–68.67) | 0.0025 |
| G3 | 33.99 (9.57–216.32) | <0.0001 | 27.39 (7.27–180.60) | <0.0001 |
| G4 | 54.70 (16.53–339.15) | <0.0001 | 43.42 (12.22–279.83) | <0.0001 |
| Cardiovascular disease mortality | ||||
| GNRI (continuous) | 0.89 (0.84–0.94) | 0.0001 | 0.89 (0.84–0.96) | 0.0018 |
| ECF/ICF (/0.01) (continuous) | 1.05 (1.03–1.06) | <0.0001 | 1.03 (1.02–1.05) | 0.0003 |
| Lower GNRI | 3.46 (1.60–8.08) | 0.0014 | 3.19 (1.39–7.88) | 0.0056 |
| Higher ECF/ICF | 18.46 (6.20–80.24) | <0.0001 | 11.87 (3.65–55.91) | <0.0001 |
| Cross-classified (vs. G1) | <0.0001 | <0.0001 | ||
| G2 | 6.52 (0.62–140.54) | 0.11 | 9.07 (0.82–203.31) | 0.071 |
| G3 | 36.99 (6.53–696.71) | <0.0001 | 25.44 (4.11–504.39) | 0.0001 |
| G4 | 47.26 (9.20–872.61) | <0.0001 | 36.56 (6.31–719.66) | <0.0001 |
| Variables | C-Index | p-Value | NRI | p-Value | IDI | p-Value |
|---|---|---|---|---|---|---|
| All-cause mortality | ||||||
| Established risk factors+ | 0.722 (0.650–0.794) | Ref. | Ref. | Ref. | ||
| GNRI | 0.755 (0.687–0.824) | 0.13 | 0.444 | 0.0009 | 0.064 | 0.0001 |
| ECF/ICF | 0.819 (0.761–0.876) | 0.0021 | 0.793 | <0.0001 | 0.142 | <0.0001 |
| GNRI and ECF/ICF | 0.834 (0.778–0.890) | 0.0004 | 0.920 | <0.0001 | 0.170 | <0.0001 |
| Cardiovascular disease mortality | ||||||
| Established risk factors+ | 0.773 (0.676–0.871) | Ref. | Ref. | Ref. | ||
| +GNRI | 0.786 (0.691–0.880) | 0.52 | 0.403 | 0.021 | 0.024 | 0.045 |
| +ECF/ICF | 0.834 (0.761–0.908) | 0.061 | 0.787 | <0.0001 | 0.046 | 0.010 |
| +GNRI and ECF/ICF | 0.841 (0.773–0.909) | 0.048 | 0.826 | <0.0001 | 0.061 | 0.0024 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yajima, T.; Yajima, K.; Takahashi, H.; Yasuda, K. Combined Predictive Value of Extracellular Fluid/Intracellular Fluid Ratio and the Geriatric Nutritional Risk Index for Mortality in Patients Undergoing Hemodialysis. Nutrients 2019, 11, 2659. https://doi.org/10.3390/nu11112659
Yajima T, Yajima K, Takahashi H, Yasuda K. Combined Predictive Value of Extracellular Fluid/Intracellular Fluid Ratio and the Geriatric Nutritional Risk Index for Mortality in Patients Undergoing Hemodialysis. Nutrients. 2019; 11(11):2659. https://doi.org/10.3390/nu11112659
Chicago/Turabian StyleYajima, Takahiro, Kumiko Yajima, Hiroshi Takahashi, and Keigo Yasuda. 2019. "Combined Predictive Value of Extracellular Fluid/Intracellular Fluid Ratio and the Geriatric Nutritional Risk Index for Mortality in Patients Undergoing Hemodialysis" Nutrients 11, no. 11: 2659. https://doi.org/10.3390/nu11112659
APA StyleYajima, T., Yajima, K., Takahashi, H., & Yasuda, K. (2019). Combined Predictive Value of Extracellular Fluid/Intracellular Fluid Ratio and the Geriatric Nutritional Risk Index for Mortality in Patients Undergoing Hemodialysis. Nutrients, 11(11), 2659. https://doi.org/10.3390/nu11112659





