Metabolic Effects of the Sweet Protein MNEI as a Sweetener in Drinking Water. A Pilot Study of a High Fat Dietary Regimen in a Rodent Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Study Design
2.2. Determination of Body Composition and Energy Balance
2.3. Glucose Tolerance Test and Plasma Parameters
2.4. Composition, Inflammation and Insulin Signaling in Skeletal Muscle
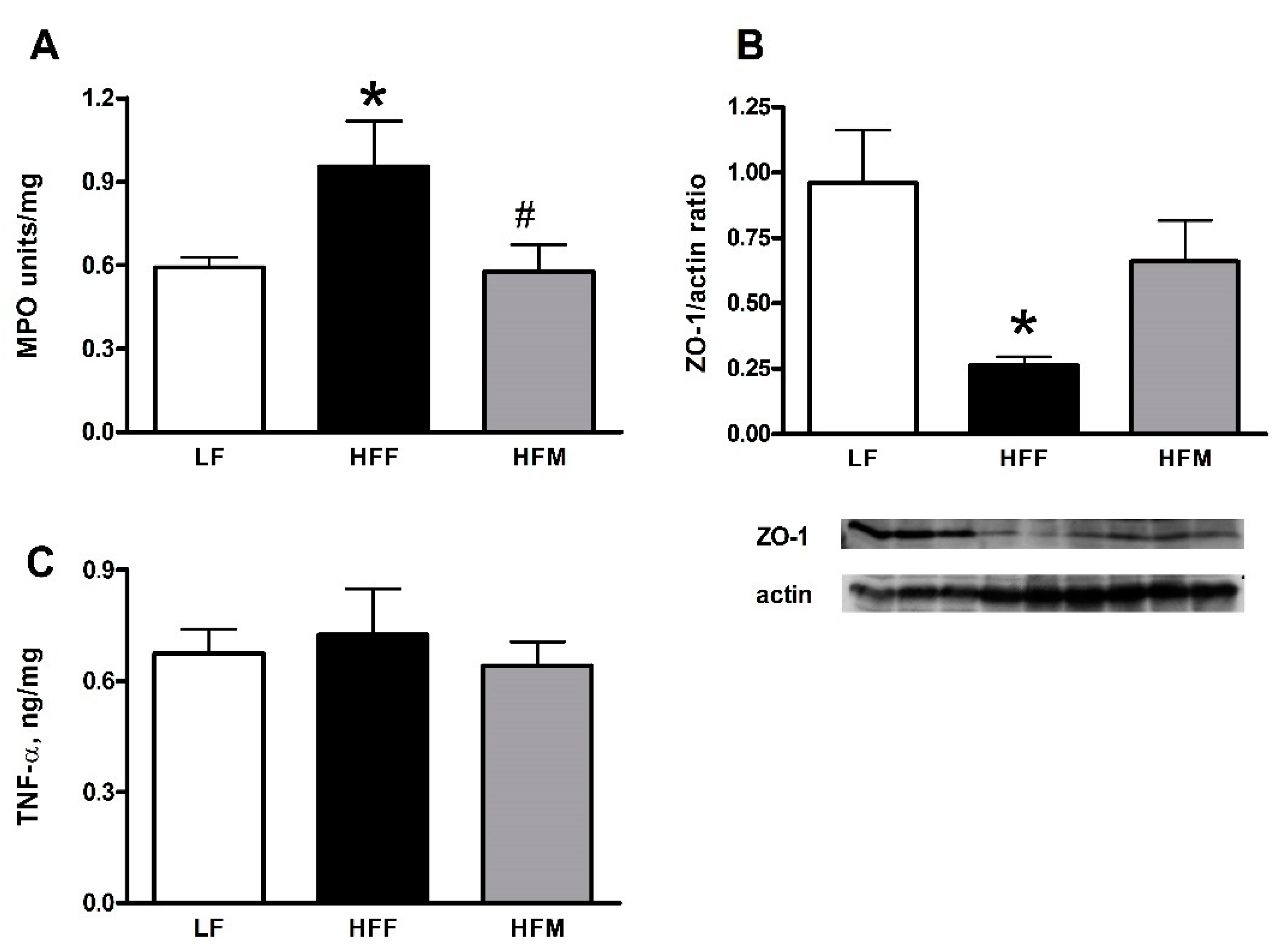
2.5. Markers of Inflammation and Leakiness in the Colon
2.6. Data Analysis and Statistics
2.7. Chemicals
3. Results
3.1. Whole Body Compartments
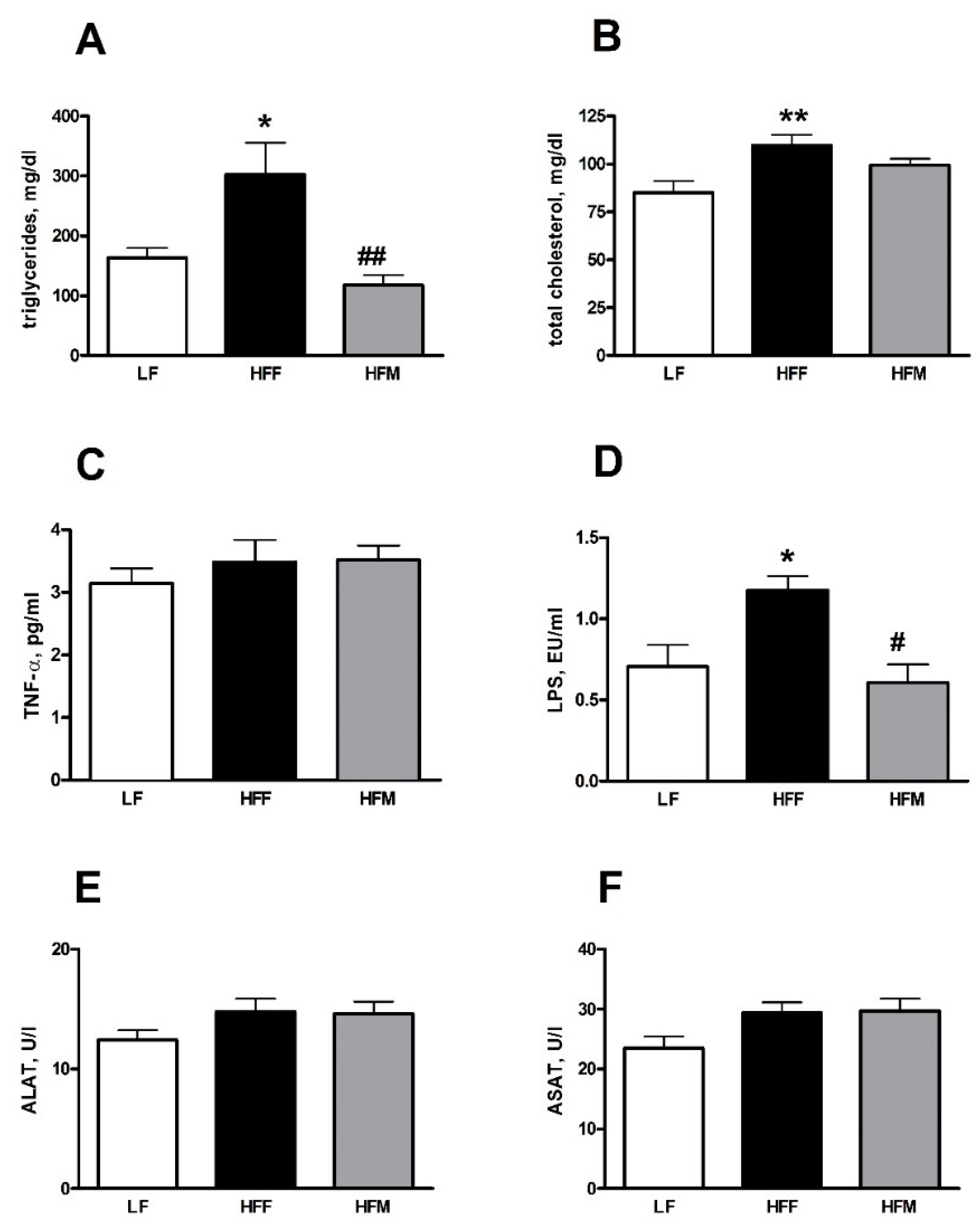
3.2. Plasma Markers of Inflammation and Lipid Profile
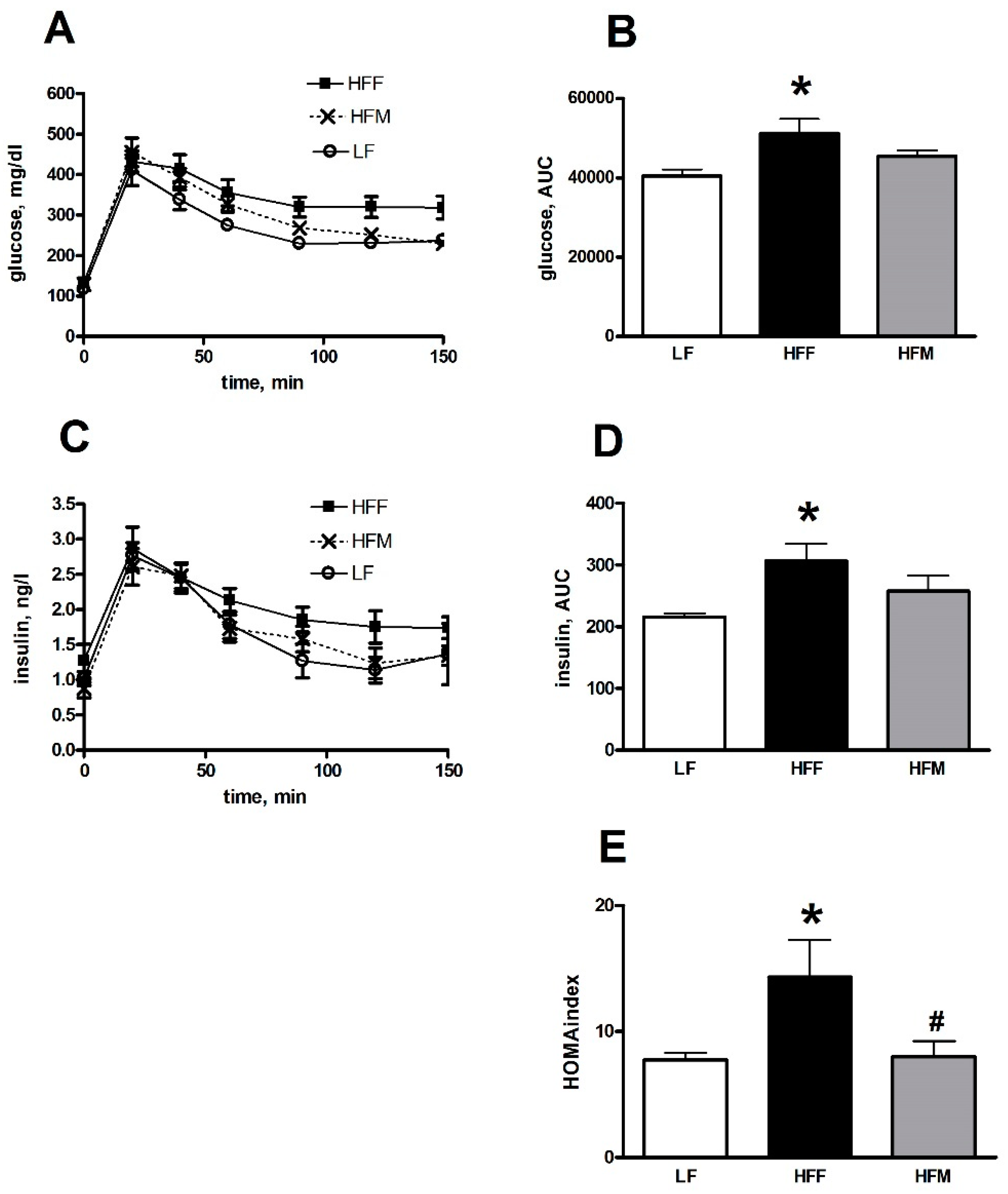
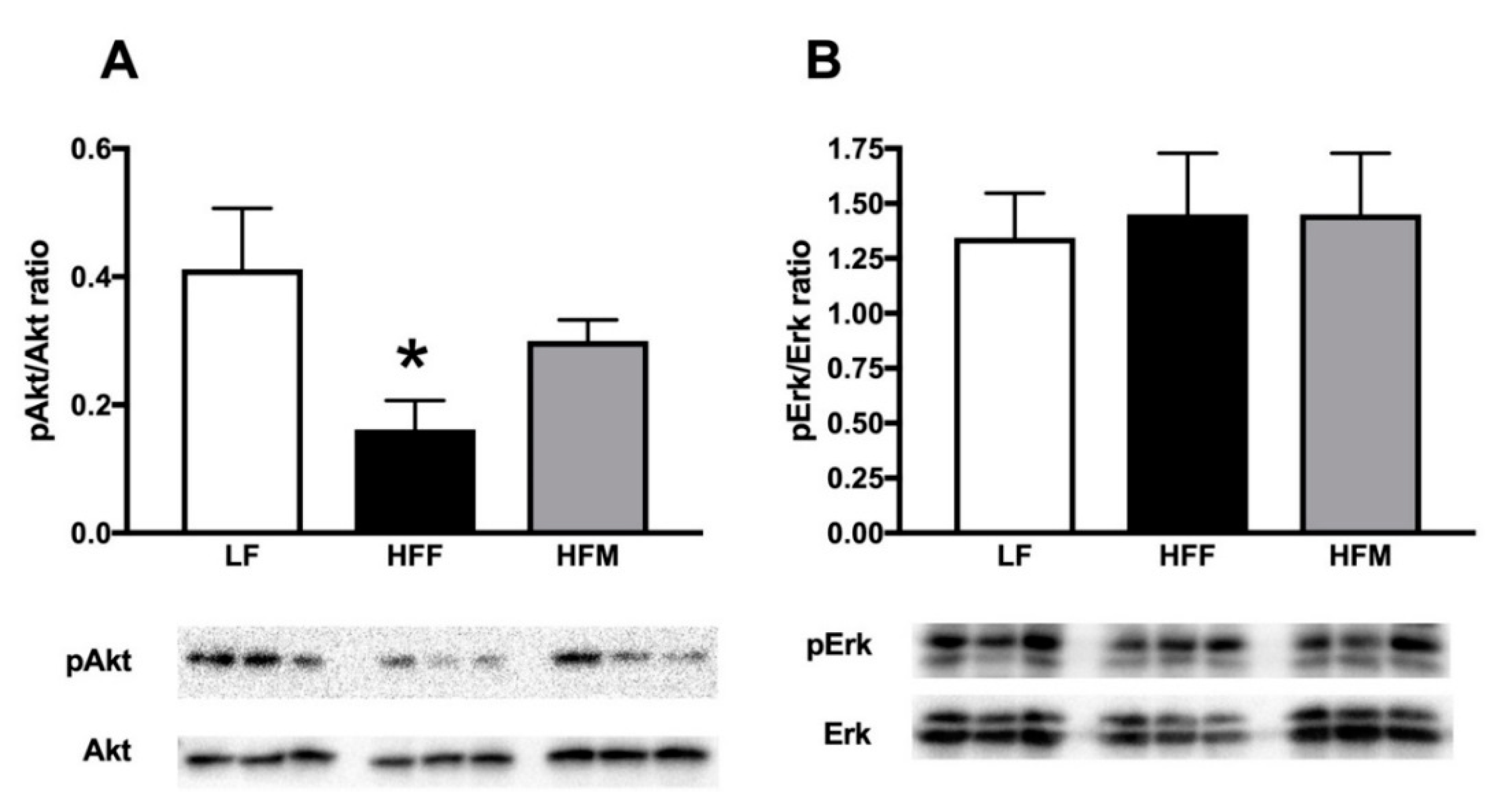
3.3. Glucose Tolerance and Skeletal Muscle Lipid Profile
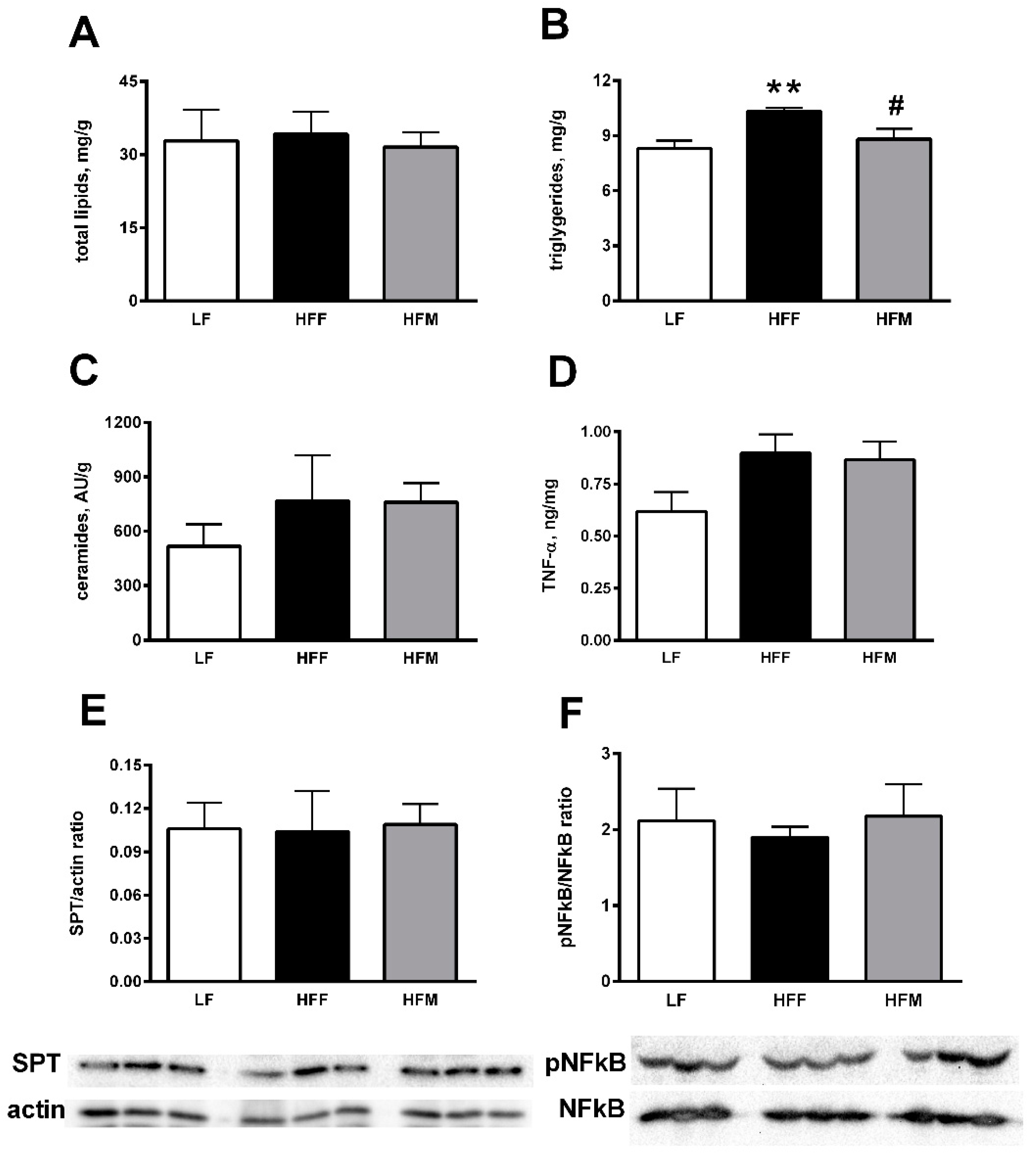
3.4. Colon Markers of Inflammation and Leakiness
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schiffman, S.S. Rationale for Further Medical and Health Research on High-Potency Sweeteners. Chem. Senses 2012, 37, 671–679. [Google Scholar] [CrossRef] [Green Version]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Kuperman, Y. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Di Luccia, B.; Crescenzo, R.; Mazzoli, A.; Cigliano, L.; Venditti, P.; Walser, J.-C.; Iossa, S. Rescue of fructose-induced metabolic syndrome by antibiotics or faecal transplantation in a rat model of obesity. PLoS ONE 2015, 10, e0134893. [Google Scholar] [CrossRef]
- Crescenzo, R.; Mazzoli, A.; Di Luccia, B.; Bianco, F.; Cancelliere, R.; Cigliano, L.; Liverini, G.; Baccigalupi, L.; Iossa, S. Dietary fructose causes defective insulin signaling and ceramide accumulation in the liver that can be reversed by gut microbiota modulation. Food Nutr. Res. 2017, 61, 1331657. [Google Scholar] [CrossRef]
- Kant, R. Sweet proteins—Potential replacement for artificial low calorie sweeteners. Nutr. J. 2005, 4, 5. [Google Scholar] [CrossRef]
- Picone, D.; Temussi, P.A. Dissimilar sweet proteins from plants: Oddities or normal components? Plant Sci. 2012, 195, 135–142. [Google Scholar] [CrossRef]
- Di Monaco, R.; Miele, N.A.; Volpe, S.; Picone, D.; Cavella, S. Temporal sweetness profile of MNEI and comparison with commercial sweeteners. J. Sens. Stud. 2014, 29, 385–394. [Google Scholar] [CrossRef]
- Leone, S.; Pica, A.; Merlino, A.; Sannino, F.; Temussi, P.A.; Picone, D. Sweeter and stronger: Enhancing sweetness and stability of the single chain monellin MNEI through molecular design. Sci. Rep. 2016, 6, 34045. [Google Scholar] [CrossRef]
- Leone, S.; Picone, D. Molecular dynamics driven design of Ph-stabilized mutants of MNEI, a sweet protein. PLoS ONE 2016, 11, e0158372. [Google Scholar] [CrossRef]
- Masuda, T.; Ohta, K.; Ojiro, N.; Murata, K.; Mikami, B.; Tani, F.; Kitabatake, N. A hypersweet protein: Removal of the specific negative charge at Asp21 enhances thaumatin sweetness. Sci. Rep. 2016, 6, 20255. [Google Scholar] [CrossRef]
- Cai, C.; Li, L.; Lu, N.; Zheng, W.; Yang, L.; Liu, B. Expression of a high sweetness and heat-resistant mutant of sweet-tasting protein, monellin, in Pichia pastoris with a constitutive GAPDH promoter and modified N-terminus. Biotechnol. Lett. 2016, 38, 1–6. [Google Scholar] [CrossRef]
- Higginbotham, J.D.; Snodin, D.J.; Eaton, K.K.; Daniel, J.W. Safety evaluation of thaumatin (Talin protein). Food Chem. Toxicol. 1983, 21, 815–823. [Google Scholar] [CrossRef]
- Rega, M.F.; Siciliano, A.; Gesuele, R.; Lofrano, G.; Carpentieri, A.; Picone, D.; Guida, M. Ecotoxicological survey of MNEI and Y65R-MNEI proteins as new potential high-intensity sweeteners. Environ. Sci. Pollut. Res. Int. 2017, 24, 9734–9740. [Google Scholar] [CrossRef]
- Morris, J.A.; Cagan, R.H. Purification of monellin, the sweet principle of Dioscoreophyllum cumminsii. Biochim. Biophys. Acta BBA Gen. Subj. 1972, 261, 114–122. [Google Scholar] [CrossRef]
- Crescenzo, R.; Bianco, F.; Coppola, P.; Mazzoli, A.; Cigliano, L.; Liverini, G.; Iossa, S. The effect of high-fat—High-fructose diet on skeletal muscle mitochondrial energetics in adult rats. Eur. J. Nutr. 2015, 54, 183–192. [Google Scholar] [CrossRef]
- Leone, S.; Sannino, F.; Tutino, M.L.; Parrilli, E.; Picone, D. Acetate: Friend or Foe? Efficient Production of a Sweet Protein in Escherichia coli BL21 Using Acetate as a Carbon Source. Microb. Cell Fact. 2015, 14, 106. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Iossa, S.; Mollica, M.P.; Lionetti, L.; Crescenzo, R.; Botta, M.; Liverini, G. Skeletal muscle oxidative capacity in rats fed high-fat diet. Int. J. Obes. 2002, 26, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Cacho, J.; Sevillano, J.; de Castro, J.; Herrera, E.; Ramos, M.P. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am. J. Physiol. 2008, 295, E1269–E1276. [Google Scholar] [CrossRef]
- Crescenzo, R.; Bianco, F.; Coppola, P.; Mazzoli, A.; Cigliano, L.; Liverini, G.; Iossa, S. Increased skeletal muscle mitochondrial efficiency in rats with fructose-induced alteration in glucose tolerance. Br. J. Nutr. 2013, 110, 1996–2003. [Google Scholar] [CrossRef] [Green Version]
- Krawisz, J.E.; Sharon, P.; Stenson, W.F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 1984, 87, 1344–1350. [Google Scholar] [CrossRef]
- Kim, J.J.; Shajib, M.; Manocha, M.M.; Khan, W.I. Investigating intestinal inflammation in DSS-induced model of IBD. J. Vis. Exp. 2012, 60, 3678. [Google Scholar] [CrossRef]
- Bourque, C.W. Central mechanisms of osmosensation and systemic osmoregulation. Nat. Rev. Neurosci. 2008, 9, 519–531. [Google Scholar] [CrossRef]
- Ackroff, K.; Sclafani, A. Rats integrate meal cost and post oral changes in caloric density. Physiol. Behav. 1996, 60, 927–932. [Google Scholar] [CrossRef]
- Defronzo, R.A. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef]
- Schinner, S.; Scherbaum, W.A.; Bornstein, S.R.; Barthel, A. Molecular mechanisms of insulin resistance. Diabet. Med. 2005, 22, 674–682. [Google Scholar] [CrossRef]
- Molina, S.A.; Moriarty, H.K.; Infield, D.T.; Imhoff, B.R.; Vance, R.J.; Kim, A.H.; Hansen, J.M.; Hunt, W.R.; Koval, M.; McCarty, N.A. Insulin signaling via the PI3-kinase/Akt pathway regulates airway glucose uptake and barrier function in a CFTR-dependent manner. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 312, L688–L702. [Google Scholar] [CrossRef] [Green Version]
- Matthew, S.H.; Sankar, G. Regulation of NF-κB by TNF family cytokines. Semin. Immunol. 2014, 26, 253–266. [Google Scholar] [CrossRef]
- Amati, F.; Dubé, J.J.; Alvarez-Carnero, E.; Edreira, M.M.; Chomentowski, P.; Coen, P.M.; Goodpaster, B.H. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: Another paradox in endurance-trained athletes? Diabetes 2011, 60, 2588–2597. [Google Scholar] [CrossRef]
- Holland, W.L.; Bikman, B.T.; Wang, L.P.; Yuguang, G.; Sargent, K.M.; Bulchand, S.; Pagliassotti, M.J. Lipid-induced insulin resistance mediated by the proinflammator y receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Investig. 2011, 121, 1858–1870. [Google Scholar] [CrossRef]
- De La Serre, C.B.; Ellis, C.L.; Lee, J.; Hartman, A.L.; Rutledge, J.C.; Raybould, H.E. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G440–G448. [Google Scholar] [CrossRef]
| LF | HFF | HFM | |
|---|---|---|---|
| DIET | |||
| Standard chow * | 50 | 50 | 50 |
| Sunflower oil | 2 | 2 | 2 |
| Casein | 9 | 14.8 | 14.8 |
| AIN-76 mineral mix ** | 1.6 | 1.6 | 1.6 |
| AIN-76 vitamin mix *** | 0.4 | 0.4 | 0.4 |
| Choline | 0.1 | 0.1 | 0.1 |
| Methionine | 0.1 | 0.1 | 0.1 |
| Cornstarch | 36.8 | 10 | 10 |
| Lard | --- | 21 | 21 |
| Total weight, g | 100 | 100 | 100 |
| Water content, % | 4 | 6 | 6 |
| Metabolizable energy content, kJ **** | 1398 | 1838 | 1838 |
| Protein (J/100 J) | 22.0 | 22.0 | 22.0 |
| Lipids (J/100 J) | 9.4 | 50.2 | 50.2 |
| Carbohydrates (J/100 J) | 68.6 | 27.8 | 27.8 |
| BEVERAGES | |||
| Water, ml | 100 | 100 | 100 |
| Fructose, g | --- | 10 | --- |
| MNEI, g | --- | --- | 0.004 |
| LF | HFF | HFM | |
|---|---|---|---|
| Initial body weight, g | 460 ± 23 | 461 ± 10 | 461 ± 12 |
| Final body weight, g | 499 ± 28 | 516 ± 10 | 518 ± 18 |
| Body weight gain, g | 39 ± 6 | 55 ± 4 | 57 ± 8 |
| Epididymal fat weight, g (100 gbw)−1 | 1.5 ± 0.1 | 2.2 ± 0.2 * | 2.1 ± 0.2 * |
| Body lipids, g (100 gbw)−1 | 14.7 ± 0.9 | 17.8 ± 0.9 * | 17.7 ± 0.9 * |
| Water intake, mL | 1324 ± 39 | 2018 ± 215 ** | 1371 ± 108 # |
| Drink energy intake, kJ | 0 | 2990 ± 363 * | 0 *# |
| Diet energy intake, kJ | 11,010 ± 293 | 8324 ± 371 ** | 10,750 ± 386 ## |
| Metabolizable energy intake, kJ | 11,010 ± 293 | 11,314 ± 415 | 10,750 ± 386 |
| Energy expenditure, kJ | 9564 ± 251 | 10,072 ± 388 | 8960 ± 187 |
| Energy gain, kJ | 830 ± 100 | 1439 ± 140 * | 1458 ± 143 * |
| Lipid gain, kJ | 894 ± 88 | 1599 ± 120 * | 1598 ± 150 * |
| Metabolic efficiency, % | 7.3 ± 0.7 | 12.5 ± 2.0 * | 13.8 ± 2.0 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cancelliere, R.; Leone, S.; Gatto, C.; Mazzoli, A.; Ercole, C.; Iossa, S.; Liverini, G.; Picone, D.; Crescenzo, R. Metabolic Effects of the Sweet Protein MNEI as a Sweetener in Drinking Water. A Pilot Study of a High Fat Dietary Regimen in a Rodent Model. Nutrients 2019, 11, 2643. https://doi.org/10.3390/nu11112643
Cancelliere R, Leone S, Gatto C, Mazzoli A, Ercole C, Iossa S, Liverini G, Picone D, Crescenzo R. Metabolic Effects of the Sweet Protein MNEI as a Sweetener in Drinking Water. A Pilot Study of a High Fat Dietary Regimen in a Rodent Model. Nutrients. 2019; 11(11):2643. https://doi.org/10.3390/nu11112643
Chicago/Turabian StyleCancelliere, Rosa, Serena Leone, Cristina Gatto, Arianna Mazzoli, Carmine Ercole, Susanna Iossa, Giovanna Liverini, Delia Picone, and Raffaella Crescenzo. 2019. "Metabolic Effects of the Sweet Protein MNEI as a Sweetener in Drinking Water. A Pilot Study of a High Fat Dietary Regimen in a Rodent Model" Nutrients 11, no. 11: 2643. https://doi.org/10.3390/nu11112643





