Translation of a Mediterranean-Style Diet into the Australian Dietary Guidelines: A Nutritional, Ecological and Environmental Perspective
Abstract
1. Introduction
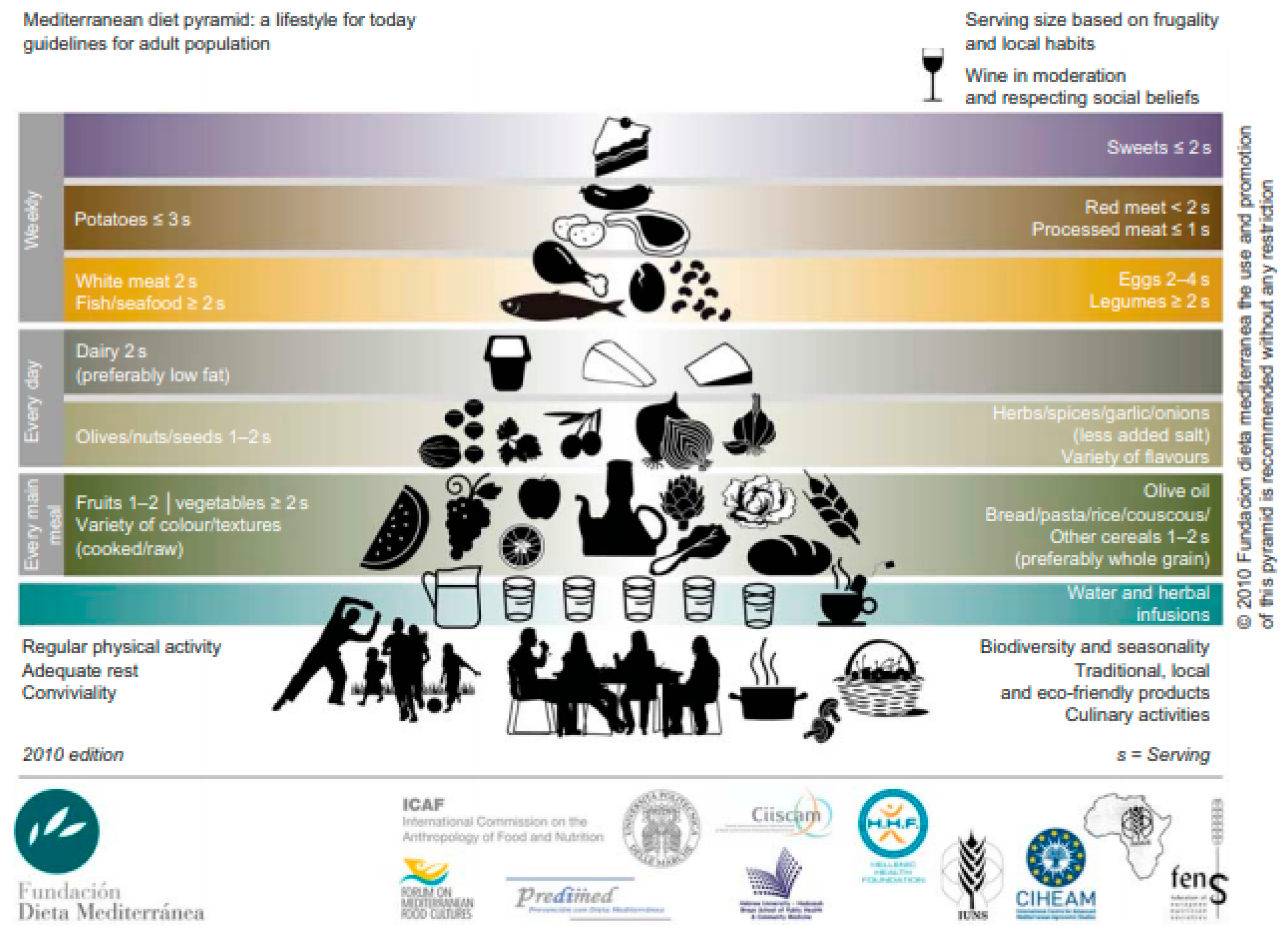
2. The Traditional Mediterranean Diet: A Win-Win Dietary Pattern?
3. Efficacy on Health-Related Primary Outcomes Using the Mediterranean Diet as a Dietary Intervention in Clinical Trials conducted in Australia: What Is the Evidence?
4. Assessing Adherence to a Mediterranean Diet in Clinical Trials Conducted in Australia: What is the Evidence?
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MedDiet | Mediterranean Diet |
| T2DM | Type 2 diabetes mellitus |
| CHD | coronary heart disease |
| SAT | subcutaneous adipose tissue |
| VAT | visceral adipose tissue |
| BP | blood pressure |
| CRP | C-reactive protein |
| CVD | cardiovascular disease |
| ADG | Australian Dietary Guidelines |
| SF-36 | self-administered short-form 36 health survey |
| NAFLD | non-alcoholic fatty liver disease |
| HbA1c | glycated haemoglobin |
| BMI | body mass index |
| n-3 | omega-3 |
| QoL | quality of life |
References
- Crosland, P.; Ananthapavan, J.; Davison, J.; Lambert, M.; Carter, R. The health burden of preventable disease in Australia: A systematic review. Aust. Nz J. Public Health 2019, 43, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Melaku, Y.A.; Renzaho, A.; Gill, T.K.; Taylor, A.W.; Dal Grande, E.; de Courten, B.; Baye, E.; Gonzalez-Chica, D.; Hyppönen, E.; Shi, Z.; et al. Burden and trend of diet-related non-communicable diseases in Australia and comparison with 34 OECD countries, 1990–2015: Findings from the Global Burden of Disease Study 2015. Eur. J. Nutr. 2019, 58, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, E.; Hendy, C.; Magnusson, R.; Colagiuri, S. Public support for government regulatory interventions for overweight and obesity in Australia. Bmc Public Health 2018, 18, 513. [Google Scholar] [CrossRef] [PubMed]
- Australian Bureau of Statistics: National Health Survey: First Results, 2017–2018. Available online: https://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/4364.0.55.001~2017-18~Main%20Features~Overweight%20and%20obesity~90 (accessed on 1 October 2019).
- Hayes, A.J.; Lung, T.W.; Bauman, A.; Howard, K. Modelling obesity trends in Australia: Unravelling the past and predicting the future. Int. J. Obes. 2005, 41, 178–185. [Google Scholar] [CrossRef]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Herforth, A.; Arimond, M.; Alvarez-Sanchez, C.; Coates, J.; Christianson, K.; Muehlhoff, E. A global review of food-based dietary guidelines. Adv. Nutr. 2019, 10, 590–605. [Google Scholar] [CrossRef]
- Nour, M.; Sui, Z.; Grech, A.; Rangan, A.; McGeechan, K.; Allman-Farinelli, M. The fruit and vegetable intake of young Australian adults: A population perspective. Public Health Nutr. 2017, 20, 2499–2512. [Google Scholar] [CrossRef]
- Galea, L.M.; Beck, E.J.; Probst, Y.C.; Cashman, C.J. Whole grain intake of Australians estimated from a cross-sectional analysis of dietary intake data from the 2011–13 Australian Health Survey. Public Health Nutr. 2017, 20, 2166–2172. [Google Scholar] [CrossRef]
- Garcia-Fernandez, E.; Rico-Cabanas, L.; Rosgaard, N.; Estruch, R.; Bach-Faig, A. Mediterranean Diet and cardiodiabesity: A review. Nutrients 2014, 6, 3474–3500. [Google Scholar] [CrossRef]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018, 72, 30–43. [Google Scholar] [CrossRef]
- George, E.; Kucianski, T.; Mayr, H.; Moschonis, G.; Tierney, A.; Itsiopoulos, C. A Mediterranean diet model in Australia: Strategies for translating the traditional Mediterranean diet into a multicultural setting. Nutrients 2018, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014, 17, 2769–2782. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.J.; Parletta, N. Implementing a Mediterranean-style diet outside the Mediterranean region. Curr. Atheroscler. Rep. 2018, 20, 28. [Google Scholar] [CrossRef] [PubMed]
- Middleton, G.; Keegan, R.; Smith, M.F.; Alkhatib, A.; Klonizakis, M. Implementing a Mediterranean diet intervention into a RCT: Lessons learned from a non-Mediterranean based country. J. Nutr. Health Ag. 2015, 19, 1019–1022. [Google Scholar] [CrossRef]
- Logan, K.J.; Woodside, J.V.; Young, I.S.; McKinley, M.C.; Perkins-Porras, L.; McKeown, P.P. Adoption and maintenance of a Mediterranean diet in patients with coronary heart disease from a Northern European population: A pilot randomised trial of different methods of delivering Mediterranean diet advice. J. Hum. Nutr. Diet. 2010, 23, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Donini, L.M.; Serra-Majem, L.; Bulló, M.; Gil, Á.; Salas-Salvadó, J. The Mediterranean diet: Culture, health and science. Br. J. Nutr. 2015, 113, 1–3. [Google Scholar] [CrossRef]
- Villani, A.; Sultana, J.; Doecke, J.; Mantzioris, E. Differences in the interpretation of a modernized Mediterranean diet prescribed in intervention studies for the management of type 2 diabetes: How closely does this align with a traditional Mediterranean diet? Eur. J. Nutr. 2018, 58, 1369–1380. [Google Scholar] [CrossRef]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean diet; a literature review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Orfanos, P.; Norat, T.; Bueno-de-Mesquita, B.; Ocké, M.C.; Peeters, P.H.; van der Schouw, Y.T.; Boeing, H.; Hoffmann, K.; Boffetta, P.; et al. Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. BMJ 2005, 330, 991. [Google Scholar] [CrossRef]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402–1406. [Google Scholar] [CrossRef] [PubMed]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Burlingame, B.; Dernini, S. Sustainable diets: The Mediterranean diet as an example. Public Health Nutr. 2011, 14, 2285–2287. [Google Scholar] [CrossRef] [PubMed]
- Dernini, S.; Berry, E.; Serra-Majem, L.; La Vecchia, C.; Capone, R.; Medina, F.; Aranceta-Bartrina, J.; Belahsen, R.; Burlingame, B.; Calabrese, G.; et al. Med Diet 4.0, the Mediterranean diet with four sustainable benefits. Public Health Nutr. 2017, 20, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Saez-Almendros, S.; Obrador, B.; Bach-Faig, A.; Serra-Majem, L. Environmental footprints of Mediterranean versus Western dietary patterns: Beyond the health benefits of the Mediterranean diet. BMC Environ. Health 2013, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A. Diversity v. globalization: Traditional foods at the epicenter. Public Health Nutr. 2012, 15, 951–954. [Google Scholar] [CrossRef]
- Germani, A.; Vitiello, V.; Giusti, A.M.; Pinto, A.; Donini, L.M.; del Blazo, V. Environmental and economic sustainability of the Mediterranean Diet. Int. J. Food Sci. Nutr. 2014, 65, 1008–1012. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Tilman, D.; Clark, M. Global diets link environmental sustainability and human health. Nature 2014, 515, 518–522. [Google Scholar] [CrossRef]
- Itsiopoulos, C.; Brazionis, L.; Kaimakamis, M.; Cameron, M.; Best, J.D.; O’Dea, K.; Rowley, K. Can the Mediterranean diet lower HbA1c in type 2 diabetes? Results from a randomized cross-over study. Nutr. Metab. Cardiovas. Dis. 2011, 21, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Itsiopoulos, C.; Kucianski, T.; Mayr, H.L.; van Gaal, W.J.; Martinez-Gonzalez, M.A.; Vally, H.; Kingsley, M.; Kouris-Blazos, A.; Radcliffe, J.; Segal, L.; et al. The AUStralian MEDiterranean Diet Heart Trial (AUSMED Heart Trial): A randomized clinical trial in secondary prevention of coronary heart disease in a multiethnic Australian population: Study protocol. Am. Heart J. 2018, 203, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Mayr, H.L.; Itsiopoulos, C.; Tierney, A.C.; Kucianski, T.; Radcliffe, J.; Garg, M.; Willcox, J.; Thomas, C.J. Ad libitum Mediterranean diet reduces subcutaneous but not visceral fat in patients with coronary heart disease: A randomised controlled pilot study. Clin. Nutr. ESPEN 2019, 32, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Mayr, H.L.; Tierney, A.C.; Kucianski, T.; Thomas, C.J.; Itsiopoulos, C. Australian patients with coronary heart disease achieve high adherence to 6-month Mediterranean diet intervention: Preliminary results of the AUSMED Heart Trial. Nutrition 2019, 61, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.R.; Bryan, J.; Hodgson, J.M.; Wilson, C.; Dhillon, V.; Murphy, K.J. A randomised controlled intervention trial evaluating the efficacy of an Australianised Mediterranean diet compared to the habitual Australian diet on cognitive function, psychological wellbeing and cardiovascular health in healthy older adults (MedLey study): Protocol paper. BMC Nutr. 2015, 1, 35. [Google Scholar] [CrossRef]
- Davis, C.R.; Hodgson, J.M.; Woodman, R.; Bryan, J.; Wilson, C.; Murphy, K.J. A Mediterranean diet lowers blood pressure and improves endothelial function: Results from the MedLey randomized intervention trial. Am. J. Clin. Nutr. 2017, 105, 1305–1313. [Google Scholar] [CrossRef]
- Davis, C.R.; Bryan, J.; Hodgson, J.M.; Woodman, R.; Murphy, K.J. A Mediterranean diet reduces F2-isoprostanes and triglycerides among older Australian men and women after 6 months. J. Nutr. 2017, 147, 1348–1355. [Google Scholar] [CrossRef]
- Wade, A.; Davis, C.; Dyer, K.; Hodgson, J.; Woodman, R.; Keage, H.; Murphy, K.J. A Mediterranean diet to improve cardiovascular and cognitive health: Protocol for a randomised controlled intervention study. Nutrients 2017, 9, 145. [Google Scholar] [CrossRef]
- Wade, A.T.; Davis, C.R.; Dyer, K.A.; Hodgson, J.M.; Woodman, R.J.; Murphy, K.J. A Mediterranean diet supplemented with dairy foods improves markers of cardiovascular risk: Results from the MedDairy randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 1166–1182. [Google Scholar] [CrossRef]
- Wade, A.T.; Davis, C.R.; Dyer, K.A.; Hodgson, J.M.; Woodman, R.J.; Keage, H.A.; Murphy, K.J. Including pork in the Mediterranean diet for an Australian population: Protocol for a randomised controlled trial assessing cardiovascular risk and cognitive function. Nutr. J. 2017, 16, 84. [Google Scholar] [CrossRef]
- Wade, A.T.; Davis, C.R.; Dyer, K.A.; Hodgson, J.M.; Woodman, R.J.; Murphy, K.J. Effects of Mediterranean diet supplemented with lean pork on blood pressure and markers of cardiovascular risk: Findings from the MedPork trial. Br. J. Nutr. 2019, 122, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.; Bryan, J.; Wilson, C.; Hodgson, J.; Davis, C.; Murphy, K. The Mediterranean diet and cognitive function among healthy older adults in a 6-month randomised controlled trial: The MedLey study. Nutrients 2016, 8, 579. [Google Scholar] [CrossRef] [PubMed]
- Wade, A.T.; Davis, C.R.; Dyer, K.A.; Hodgson, J.M.; Woodman, R.J.; Keage, H.A.; Murphy, K.J. A Mediterranean diet supplemented with dairy foods improves mood and processing speed in an Australian sample: Results from the MedDairy randomized controlled trial. Nutr. Neurosci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wade, A.T.; Davis, C.R.; Dyer, K.A.; Hodgson, J.M.; Woodman, R.J.; Keage, H.A.; Murphy, K.J. A Mediterranean Diet with fresh, lean pork improves processing speed and mood: Cognitive findings from the MedPork randomised controlled trial. Nutrients 2019, 11, 1521. [Google Scholar] [CrossRef] [PubMed]
- Properzi, C.; O’Sullivan, T.A.; Sherriff, J.L.; Ching, H.L.; Jeffrey, G.P.; Buckley, R.F.; Tibballs, J.; MacQuillan, G.C.; Garas, G.; Adams, L.A. Ad Libitum Mediterranean and Low-Fat Diets Both Significantly Reduce Hepatic Steatosis: A Randomized Controlled Trial. Hepatology 2018, 68, 1741–1754. [Google Scholar] [CrossRef]
- Jacka, F.N.; O’Neil, A.; Opie, R.; Itsiopoulos, C.; Cotton, S.; Mohebbi, M.; Castle, D.; Dash, S.; Mihalopoulos, C.; Chatterton, M.L.; et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’trial). BMC Med. 2017, 15, 23. [Google Scholar] [CrossRef]
- Parletta, N.; Zarnowiecki, D.; Cho, J.; Wilson, A.; Bogomolova, S.; Villani, A.; Itsiopoulos, C.; Niyonsenga, T.; Blunden, S.; Meyer, B.; et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A randomized controlled trial (HELFIMED). Nutr. Neurosci. 2019, 22, 474–487. [Google Scholar] [CrossRef]
- O’Neil, A.; Berk, M.; Itsiopoulos, C.; Castle, D.; Opie, R.; Pizzinga, J.; Brazionis, L.; Hodge, A.; Mihalopoulos, C.; Chatterton, M.L.; et al. A randomised, controlled trial of a dietary intervention for adults with major depression (the “SMILES” trial): Study protocol. BMC Psych. 2013, 13, 114. [Google Scholar] [CrossRef]
- Zarnowiecki, D.; Cho, J.; Wilson, A.; Bogomolova, S.; Villani, A.; Itsiopoulos, C.; Niyonsenga, T.; O’Dea, K.; Blunden, S.; Meyer, B.; et al. A 6-month randomised controlled trial investigating effects of Mediterranean-style diet and fish oil supplementation on dietary behaviour change, mental and cardiometabolic health and health-related quality of life in adults with depression (HELFIMED): Study protocol. BMC Nutr. 2016, 2, 52. [Google Scholar] [CrossRef]
- Crichton, G.E.; Bryan, J.; Hodgson, J.M.; Murphy, K.J. Mediterranean diet adherence and self-reported psychological functioning in an Australian sample. Appetite 2013, 70, 53–59. [Google Scholar] [CrossRef]
- Hodge, A.; English, D.; Itsiopoulos, C.; O’dea, K.; Giles, G. Does a Mediterranean diet reduce the mortality risk associated with diabetes: Evidence from the Melbourne Collaborative Cohort Study. Nutr. Metab. Cardiovas. Dis. 2011, 21, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Milte, C.M.; Thorpe, M.G.; Crawford, D.; Ball, K.; McNaughton, S.A. Associations of diet quality with health-related quality of life in older Australian men and women. Exp. Gerontol. 2015, 64, 8–16. [Google Scholar] [CrossRef] [PubMed]
- McClure, R.; Villani, A. Greater adherence to a Mediterranean Diet is associated with better gait speed in older adults with type 2 diabetes mellitus. Clin. Nutr. ESPEN 2019, 32, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Stanton, A.; Buckley, J.; Villani, A. Adherence to a Mediterranean Diet is not Associated with Risk of Sarcopenic Symptomology: A Cross-Sectional Analysis of Overweight and Obese Older Adults in Australia. J. Frailty Ag. 2019, 8, 146–149. [Google Scholar]
- Murphy, K.; Dyer, K.; Hyde, B.; Davis, C.; Hodgson, J.; Woodman, R. Australians Can Adopt Mediterranean Diet (MedDiet) Principles up to 1-Year Following Completion of the Medley Trial. Curr. Dev. Nutr. 2019. [Google Scholar] [CrossRef]
- National Health Medical Research Council. National Health and Medical Research Council: Australian Dietary Guidelines; National Health Medical Research Council: Canberra, Australia, 2013. [Google Scholar]
- Kromhout, D.; Keys, A.; Aravanis, C.; Buzina, R.; Fidanza, F.; Giampaoli, S.; Jansen, A.; Menotti, A.; Nedeljkovic, S.; Pekkarinen, M.; et al. Food consumption patterns in the 1960s in seven countries. Am. J. Clin. Nutr. 1989, 49, 889–894. [Google Scholar] [CrossRef]
- Toledo, E.; Hu, F.B.; Estruch, R.; Buil-Cosiales, P.; Corella, D.; Salas-Salvado, J.; Covas, M.I.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Effect of the Mediterranean diet on blood pressure in the PREDIMED trial: Results from a randomized controlled trial. BMC Med. 2013, 11, 207. [Google Scholar] [CrossRef]
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA 2004, 292, 1440–1446. [Google Scholar] [CrossRef]
- Casas, R.; Sacanella, E.; Urpí-Sardà, M.; Chiva-Blanch, G.; Ros, E.; Martínez-González, M.A.; Covas, M.I.; Rosa Ma, L.-R.; Salas-Salvadó, J.; Fiol, M.; et al. The effects of the Mediterranean diet on biomarkers of vascular wall inflammation and plaque vulnerability in subjects with high risk for cardiovascular disease. A randomized trial. PLoS ONE 2014, 9, e100084. [Google Scholar] [CrossRef]
- Kastorini, C.-M.; Milionis, H.J.; Esposito, K.; Giugliano, D.; Goudevenos, J.A.; Panagiotakos, D. The effect of Mediterranean diet on metabolic syndrome and its components: A meta-analysis of 50 studies and 534,906 individuals. J. Am. Coll. Cardiol. 2011, 57, 1299–1313. [Google Scholar] [CrossRef]
- Rees, K.; Hartley, L.; Flowers, N.; Clarke, A.; Hooper, L.; Thorogood, M.; Stranges, S. ‘Mediterranean’ dietary pattern for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2013, 12, CD009825. [Google Scholar] [CrossRef] [PubMed]
- De Lorgeril, M.; Renaud, S.; Salen, P.; Monjaud, I.; Mamelle, N.; Martin, J.L.; Monjaud, I.; Guidollet, J.; Touboul, P.; Delaye, J. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet 1994, 343, 1454–1459. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.Á.; Hershey, M.S.; Zazpe, I.; Trichopoulou, A. Transferability of the Mediterranean diet to non-Mediterranean countries. What is and what is not the Mediterranean diet. Nutrients 2017, 9, 1226. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. Nutrient Reference Values for Australia and New Zealand: Calcium 2006. Available online: https://www.nrv.gov.au/nutrients/calcium (accessed on 1 October 2019).
- Knight, A.; Bryan, J.; Murphy, K. Is the Mediterranean diet a feasible approach to preserving cognitive function and reducing risk of dementia for older adults in Western countries? New insights and future directions. Ageing Res. Rev. 2016, 25, 85–101. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. Australian Health Survey 2011-12, Consumption of food groups from the Australian Dietary Guidelines; Australian Buraeu of Statistic: Canberra, Australia, 2016. [Google Scholar]
- Australian Health Survey: Nutrition First Results-Foods and Nutrients, 2011–2012. Available online: https://www.abs.gov.au/ausstats/abs@.nsf/lookup/4364.0.55.007main+features12011-12 (accessed on 1 October 2019).
- Sui, Z.; Raubenheimer, D.; Cunningham, J.; Rangan, A. Changes in meat/poultry/fish consumption in Australia: From 1995 to 2011–2012. Nutrients 2016, 8, 753. [Google Scholar] [CrossRef]
- Sparks, E.; Farrand, C.; Santos, J.; McKenzie, B.; Trieu, K.; Reimers, J.; Davidson, C.; Johnson, C.; Webster, J. Sodium Levels of Processed Meat in Australia: Supermarket Survey Data from 2010 to 2017. Nutrients 2018, 10, 1686. [Google Scholar] [CrossRef]
- O’Connor, L.E.; Kim, J.E.; Campbell, W.W. Total red meat intake of ≥0.5 servings/d does not negatively influence cardiovascular disease risk factors: A systemically searched meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2016, 105, 57–69. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Satija, A.; Blondin, S.A.; Janiszewski, M.; Emlen, E.; O’Connor, L.E.; Campbell, W.W.; Hu, F.B.; Willett, W.C.; Stampfer, M.J. Meta-analysis of randomized controlled trials of red meat consumption in comparison with various comparison diets on cardiovascular risk factors. Circulation 2019, 139, 1828–1845. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.; Clifton, P. A review of potential metabolic etiologies of the observed association between red meat consumption and development of type 2 diabetes mellitus. Metabolism 2015, 64, 768–779. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.-M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Hyeon, J.; Lee, S.A.; Kwon, S.O.; Lee, H.; Keum, N.; Lee, J.K.; Park, S.M. Role of Total, Red, Processed, and White Meat Consumption in Stroke Incidence and Mortality: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. J. Am. Heart Assoc. 2017, 6, e005983. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Pan, L.; Sun, C.; Xi, Y.; Wang, L.; Li, D. Red meat consumption and the risk of stroke: A dose–response meta-analysis of prospective cohort studies. J. Stroke Cereb. Dis. 2016, 25, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Je, Y. Meat consumption and risk of metabolic syndrome: Results from the Korean population and a meta-analysis of observational studies. Nutrients 2018, 10, 390. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K.; International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Carr, P.R.; Walter, V.; Brenner, H.; Hoffmeister, M. Meat subtypes and their association with colorectal cancer: Systematic review and meta-analysis. Int. J. Cancer 2016, 138, 293–302. [Google Scholar] [CrossRef]
| Author | Primary Aims | Study Population | Study Duration | Sample Size | Control Diets | Primary Outcomes |
|---|---|---|---|---|---|---|
| Itsiopoulos et al. [32] | To examine the efficacy of a traditional Mediterranean-type cuisine on HbA1c and vascular risk. | Participants with well-controlled T2DM | 24-weeks (cross-over study; no wash out period used) | 27 | Habitual diet | Adherence to a traditional moderate-fat Mediterranean diet improves glycemic control and diet quality in patients with well-controlled T2DM. |
| Mayr et al. [34,35] | To determine the efficacy of an ad libitum MedDiet on cardiometabolic risk markers | Participants with pre-existing CHD | 6-months | 65 MedDiet n = 34; Low-fat n = 31 | Low-fat diet; standard low-fat diet recommendations consistent with ADG and National Heart Foundation recommendations | Adherence to the MedDiet intervention significantly reduced SAT but not VAT area. No significant between group differences were observed on markers of inflammation, oxidative stress, lipids, glucose and BP. |
| Davis et al. 1 [37,38] Knight et al. 2 [43] | 1. To assess the impact of a MedDiet pattern on BP and endothelial function in an older Australian population 2. To examine the examining effect of a MedDiet pattern on cognitive function | Otherwise healthy older adults aged ≥64 years | 6-months | 166 MedDiet n = 85; Habitual diet n = 81 | Habitual diet | 1. Adherence to a MedDiet intervention resulted in a small but significantly lower systolic blood pressure and improved endothelial function. However, no significant between group differences observed for lipoprotein profiles, glucose, insulin, CRP, BMI and waist-to-hip ratio. 2. No evidence of a beneficial effect for a MedDiet intervention on cognitive performance. |
| Wade et al. 1 [40] Wade et al. 2 [44] | 1. To determine the effect of a MedDiet intervention supplemented with dairy foods on cardiovascular risk factors 2. To determine the cognitive and psychological effects of a MedDiet supplemented with dairy foods | Males and females aged between 45–75 years with at least 2 CVD risk factors | 24-weeks (cross-over study; participants followed each intervention for 8-weeks with an 8-week washout period separating the interventions) | 41 | Low-fat diet; standard low-fat diet designed to replicate the control diet used in the PREDIMED trial [8] | 1. Adherence to a MedDiet intervention supplemented with additional dairy foods led to significant changes in markers of cardiovascular risk in participants at risk of CVD. 2. Adherence to a MedDiet supplemented with additional dairy foods led to improvements in mood and processing speed in participants at risk of CVD. |
| Wade et al. 1 [42] Wade et al. 2 [45] | 1. To assess the cardiovascular effects of a MedDiet intervention supplemented with fresh, lean pork 2. To assess a MedDiet supplemented with 2–3 weekly servings of fresh lean pork against measures of cognitive function and well-being | Males and females aged between 45–80 years with at least 2 CVD risk factors | 24-weeks (cross-over study; participants followed each intervention for 8-weeks with an 8-week washout period separating the interventions) | 33 | Low-fat diet; standard low-fat diet designed to replicate the control diet used in the PREDIMED trial [8] | The MedPork intervention resulted in no significant between group differences for blood pressure, lipids, glucose, insulin or CRP concentrations. Compared with the low-fat control diet, the MedPork intervention led to higher performance in the cognitive domain of processing speed and higher scores for the SF-36 subscale, emotional role functioning. |
| Properzi et al. [46] | To examine the efficacy of an ad libitum MedDiet on hepatic steatosis and cardiometabolic risk factors | Adult patients with a diagnosis of NAFLD | 12-weeks | 48 MedDiet n = 48; Low-fat n = 48 | Low-fat diet; standard low-fat diet recommendations consistent with ADG and American Heart Foundation recommendations | An ad libitum MedDiet intervention showed no significant between group differences in hepatic steatosis and measures of liver function. However, the MedDiet intervention lead to significant reductions in total cholesterol, triglycerides and HbA1c |
| Jacka et al. [47] | To investigate the efficacy of a MedDiet intervention for the treatment of major depressive episodes | Males and females with moderate to severe depression | 12-weeks | 67 MedDiet n = 33; Social support n = 34 | Social support group; nil dietary intervention | Adherence to a MedDiet intervention resulted in significant reductions in depressive symptomology, independent of any changes in BMI, self-efficacy, smoking rates and/or physical activity |
| Parletta et al. [48] | To investigate the efficacy of a MedDiet supplemented with n-3 fish oil on mental health and depressive symtomology | Males and females with self-reported depression | 6-months (3-month intervention, with 3-month follow-up) | 152 MedDiet n = 75; Social support n = 77 | Social support group; nil dietary intervention | Adherence to a MedDiet intervention supplemented with n-3 fish oil significantly reduced depressive episodes and improved mental health QoL scores |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantzioris, E.; Villani, A. Translation of a Mediterranean-Style Diet into the Australian Dietary Guidelines: A Nutritional, Ecological and Environmental Perspective. Nutrients 2019, 11, 2507. https://doi.org/10.3390/nu11102507
Mantzioris E, Villani A. Translation of a Mediterranean-Style Diet into the Australian Dietary Guidelines: A Nutritional, Ecological and Environmental Perspective. Nutrients. 2019; 11(10):2507. https://doi.org/10.3390/nu11102507
Chicago/Turabian StyleMantzioris, Evangeline, and Anthony Villani. 2019. "Translation of a Mediterranean-Style Diet into the Australian Dietary Guidelines: A Nutritional, Ecological and Environmental Perspective" Nutrients 11, no. 10: 2507. https://doi.org/10.3390/nu11102507
APA StyleMantzioris, E., & Villani, A. (2019). Translation of a Mediterranean-Style Diet into the Australian Dietary Guidelines: A Nutritional, Ecological and Environmental Perspective. Nutrients, 11(10), 2507. https://doi.org/10.3390/nu11102507





