Food Consumption during Pregnancy and Post-Partum. ECLIPSES Study
Abstract
1. Introduction
2. Materials and Methods
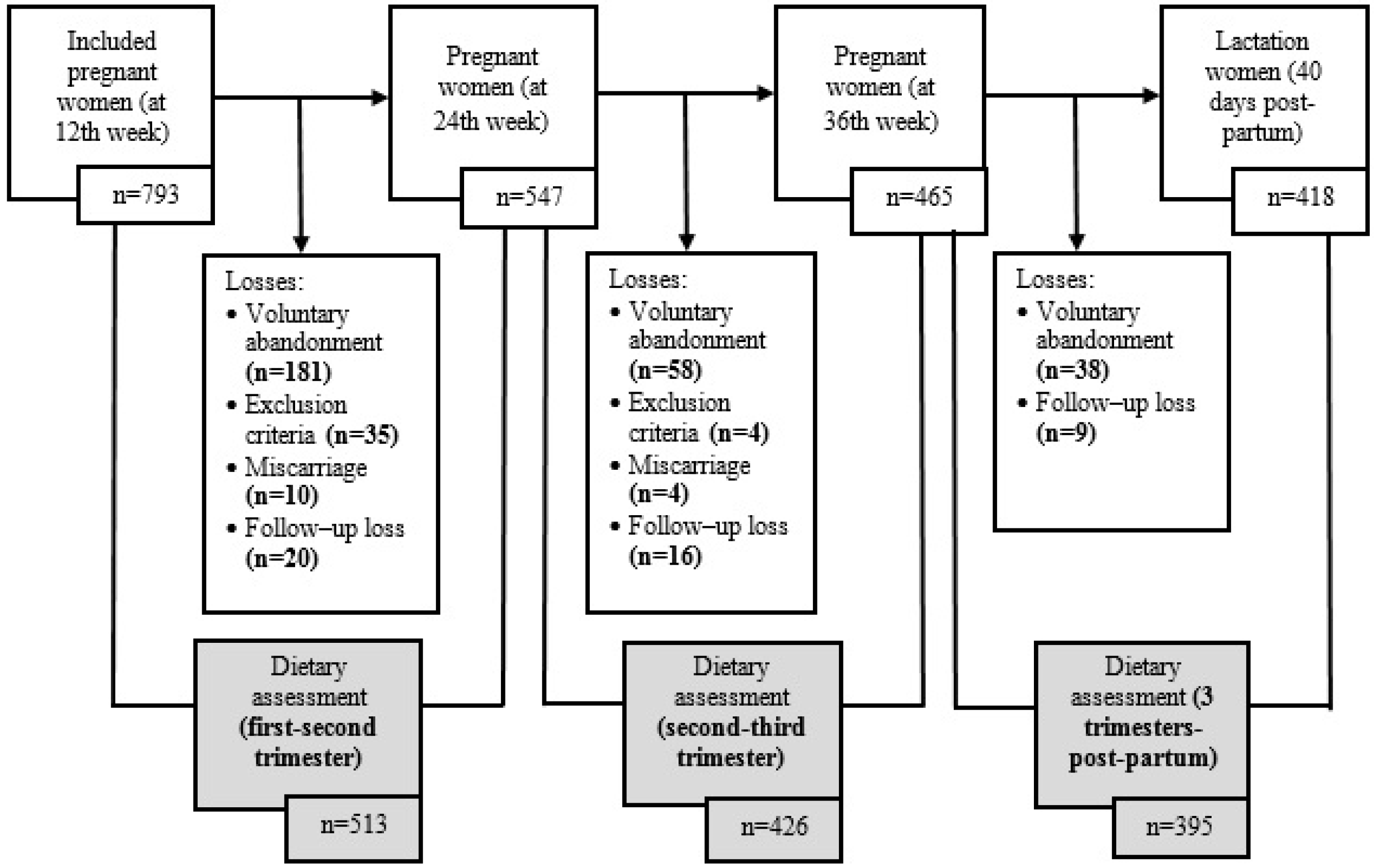
2.1. Population and Study Design
Selection Criteria
2.2. Data Collection and Processing
2.2.1. Medical History
2.2.2. Anthropometric Measures
2.2.3. Lifestyle Habits
2.2.4. Food Consumption and Energy and Nutrient Intake
2.2.5. Adherence to a Relative Mediterranean Diet (rMED Score)
2.3. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
3.2. Food Consumption
3.3. Food Consumption throughout Pregnancy and Post-Partum
3.4. Influence of Maternal Factors on Food Consumption
4. Discussion
4.1. Adherence to the Spanish Dietary Guidelines and to the Mediterranean Diet Pattern
4.2. Diet Changes during Pregnancy and Post-Partum
4.3. Association with Socio-Economic and Cultural Characteristics and Lifestyles
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tieu, J.; Shepherd, E.; Middleton, P.; Crowther, C.A. Dietary advice interventions in pregnancy for preventing gestational diabetes mellitus. Cochrane Databqase Syst. Rev. 2017, 1, CD006674. [Google Scholar] [CrossRef] [PubMed]
- Trandafir, L.M.; Temneanu, O.R. Pre and post-natal risk and determination of factors for child obesity. J. Med. Life 2016, 9, 386–391. [Google Scholar] [PubMed]
- Puszko, B.; Sánchez, S.; Vilas, N.; Pérez, M.; Barretto, L.; López, L. El impacto de la educación alimentaria nutricional en el embarazo: Una revisión de las experiencias de intervención. Rev. Chil. Nutr. 2017, 44, 11. [Google Scholar] [CrossRef]
- Lowensohn, R.I.; Stadler, D.D.; Naze, C. Current Concepts of Maternal Nutrition. Obstet. Gynecol. Surv. 2016, 71, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Arija, V.; Cucó, G.; Vila, J.; Iranzo, R.; Fernández, J. Consumo, hábitos alimentarios y estado nutricional de la población de Reus en la etapa preconcepcional, el embarazo y el posparto. Med. Clin. 2004, 123, 5–11. [Google Scholar] [CrossRef]
- Ferrer, C.; García-Esteban, R.; Mendez, M.; Romieu, I.; Torrent, M.; Sunyer, J. Determinantes sociales de los patrones dietéticos durante el embarazo. Gac. Sanit. 2009, 23, 38–43. [Google Scholar] [CrossRef]
- Flores-Quijano, E.; Heller-Rouassant, S. Embarazo y lactancia. Gac. Med. Mex. 2016, 152, 6–12. [Google Scholar]
- Institute of Medicine (US). Nutrition During Pregnancy; National Academies Pres: Washington, DC, USA, 1990. [Google Scholar]
- Sociedad Española de Nutrición Comunitaria (SENC). Alimentación, embarazo y lactancia. In Guía de la Alimentación Saludable Para Atención Primaria y Colectivos Ciudadanos, 1st ed.; Editorial Planeta, S.A.: Barcelona, Spain, 2019; pp. 106–117. [Google Scholar]
- Agnoli, C.; Sieri, S.; Ricceri, F.; Giraudo, M.T.; Masala, G.; Assedi, M.; Panico, S.; Mattiello, A.; Tumino, R.; Giurdanella, M.C.; et al. Adherence to a Mediterranean diet and long-term changes in weight and waist circumference in the EPIC-Italy cohort. Nutr. Diabetes. 2018, 8, 22. [Google Scholar] [CrossRef]
- Carlos, S.; De La Fuente-Arrillaga, C.; Bes-Rastrollo, M.; Razquin, C.; Rico-Campa, A.; Martinez-Gonzalez, M.A.; Ruiz-Canela, M. Mediterranean diet and health outcomes in the SUN cohort. Nutrients 2018, 10, 439. [Google Scholar] [CrossRef]
- Englund-Ögge, L.; Brantsæter, A.L.; Sengpiel, V.; Haugen, M.; Birgisdottir, B.E.; Myhre, R.; Meltzer, H.M.; Jacobsson, B.; Brantsaeter, A.L. Maternal dietary patterns and preterm delivery: Results from large prospective cohort study. BMJ 2014, 348, g1446. [Google Scholar] [CrossRef]
- Karamanos, B.; Thanopoulou, A.; Anastasiou, E.; Assaad-Khalil, S.; Albache, N.; Bachaoui, M.; Slama, C.B.; El Ghomari, H.; Jotic´, A.; The MGSD-GDM Study Group; et al. Relation of the Mediterranean diet with the incidence of gestational diabetes. Eur. J. Clin. Nutr. 2013, 68, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Brantsaeter, A.L.; Haugen, M.; Myhre, R.; Sengpiel, V.; Englund-Ögge, L.; Nilsen, R.M.; Borgen, I.; Duarte-Salles, T.; Papadopoulou, E.; Vejrup, K.; et al. Diet matters, particularly in pregnancy—Results from MoBa studies of maternal diet and pregnancy outcomes. Norsk Epidemiologi 2014, 24, 63–77. [Google Scholar] [CrossRef]
- Bacopoulou, F.; Landis, G.; Rentoumis, A.; Tsitsika, A.; Efthymiou, V. Mediterranean diet decreases adolescent waist circumference. Eur. J. Clin. Investig. 2017, 47, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Mistretta, A.; Marventano, S.; Antoci, M.; Cagnetti, A.; Giogianni, G.; Nolfo, F.; Rametta, S.; Pecora, G.; Marranzano, M. Mediterranean diet adherence and body composition among Southern Italian adolescents. Obes. Res. Clin. Pract. 2017, 11, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Bernal, C.; Ramón, R.; Quiles, J.; Murcia, M.; Navarrete-Muñoz, E.M.; Vioque, J.; Ballester, F.; Regablito, M. Dietary intake in pregnant women in a Spanish Mediterranean area: As good as it is supposed to be? Public Health Nutr. 2012, 16, 1379–1389. [Google Scholar] [CrossRef]
- Wen, L.M.; Flood, V.M.; Simpson, J.M.; Rissel, C.; Baur, L.A. Dietary behaviours during pregnancy: Findings from first-time mothers in southwest Sydney, Australia. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 13. [Google Scholar] [CrossRef]
- Stråvik, M.; Jonsson, K.; Hartvigsson, O.; Sandin, A.; Wold, A.E.; Sandberg, A.-S.; Barman, M. Food and Nutrient Intake during Pregnancy in Relation to Maternal Characteristics: Results from the NICE Birth Cohort in Northern Sweden. Nutrients 2019, 11, 1680. [Google Scholar] [CrossRef]
- Cuervo, M.; Sayon-Orea, C.; Santiago, S.; Martínez, J.A. Dietary and health profiles of spanish women in preconception, pregnancy and lactation. Nutrients 2014, 6, 4434–4451. [Google Scholar] [CrossRef]
- Arija, V.; Fargas, F.; March, G.; Abajo, S.; Basora, J.; Canals, J.; Ribot, B.; Aparicio, E.; Serrat, N.; Hernández-Martínez, C.; et al. Adapting iron dose supplementation in pregnancy for greater effectiveness on mother and child health: Protocol of the ECLIPSES randomized clinical trial. BMC Pregnancy Child. 2014, 14, 33. [Google Scholar] [CrossRef]
- De Catalunya, G. Classificació Catalana D’ocupacions 2011 (CCO-2011), Adaptació de la CNO-2011; Institut d’Estadística de Catalunya: Barcelona, Spain, 2013. [Google Scholar]
- World Health Organization (WHO). Global Dabatase on Body Mass Index. 2006. Available online: http://apps.who.int/bmi/index.jsp.?introPage=intro_3.html (accessed on 18 January 2019).
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-Country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Zapata, C.D.; Granada, E.P.; Giraldo, J.C. Caracterización de la población Risaraldense con relación a la práctica de la actividad física: 2006. Rev. Med. de Risaralda 2007, 13, 13–21. [Google Scholar]
- Trinidad, I.; Fernández, J.; Cucó, G.; Biarnés, E.; Arija, V. Validación de un cuestionario de frecuencia de consumo alimentario corto: Reproducibilidad y validez. Nutr. Hosp. 2008, 23, 242–252. [Google Scholar]
- Trichopoulou, A.; Kouris-Blazos, A.; Wahlqvist, M.L.; Gnardellis, C.; Lagiou, P.; Polychronopoulos, E.; Vassilakou, T.; Lipworth, L.; Trichopoulos, D. Diet and overall survival in elderly people. BMJ 1995, 311, 1457–1460. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Barrés, S.; Romaguera, D.; Valvi, D.; Martínez, D.; Vioque, J.; Navarrete-Muñoz, E.M.; Amiano, P.; Gonzalez-Palacios, S.; Guxens, M.; Pereda, E.; et al. Mediterranean dietary pattern in pregnant women and offspring risk of overweight and abdominal obesity in early childhood: The INMA birth cohort study. Pediatr. Obes. 2016, 11, 491–499. [Google Scholar] [CrossRef]
- Mousa, A.; Naqash, A.; Lim, S. Macronutrient and Micronutrient Intake during Pregnancy: An Overview of Recent Evidence. Nutrients 2019, 11, 443. [Google Scholar] [CrossRef]
- Irles Rocamora, J.A.; Iglesias Bravo, E.M.; Avilés Mejías, S.; Bernal López, E.; Benito de Valle Galindo, P.; Moriones López, L.; Maetzu Aznar Mingo Canal, A.D. valor Nutricional de la dieta en embarazadas sanes. Resultados de una encuesta dietética en gestantes. Nutr. Hosp. 2003, 18, 248–252. [Google Scholar]
- Cucó, G.; Fernández-Ballart, J.; Sala, J.; Viladrich, C.; Iranzo, R.; Vila, J.; Arija, V. Dietary patterns and associated lifestyles in preconception, pregnancy and postpartum. Eur. J. Clin. Nutr. 2006, 60, 364–371. [Google Scholar] [CrossRef]
- Savard, C.; Lemieux, S.; Carbonneau, É.; Provencher, V.; Gagnon, C.; Robitaille, J.; Morisset, A.S. Trimester-Specific Assessment of Diet Quality in a Sample of Canadian Pregnant Women. Int. J. Environ. Res. Public Health 2019, 16, 311. [Google Scholar] [CrossRef]
- Lee, A.; Muggli, E.; Halliday, J.; Lewis, S.; Gasparini, E.; Forster, D. What do pregnant women eat, and are they meeting the recommended dietary requirements for pregnancy? Midwifery 2018, 67, 70–76. [Google Scholar] [CrossRef]
- Biagi, C.; Di Nunzio, M.; Bordoni, A.; Gori, D.; Lanari, M. Effect of adherence to mediterranean diet during pregnancy on children’s health: A systematic review. Nutrients 2019, 11, 997. [Google Scholar] [CrossRef] [PubMed]
- Chatzi, L.; Mendez, M.; Garcia, R.; Roumeliotaki, T.; Ibarluzea, J.; Tardón, A.; Amiano, P.; Lertxundi, A.; Iñiguez, C.; Vioque, J.; et al. Mediterranean diet adherence during pregnancy and fetal growth: INMA (Spain) and RHEA (Greece) mother-child cohort studies. Br. J. Nutr. 2012, 107, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Silva-del Valle, M.A.; Sánchez-Villegas, A.; Serra-Majem, L. Asociación entre el seguimiento de la dieta Mediterránea con el sobrepeso y la obesidad en gestantes de Gran Canaria. Nutr. Hosp. 2013, 28, 654–659. [Google Scholar] [PubMed]
- Wen, L.M.; Simpson, J.M.; Rissel, C.; Baur, L.A. Maternal “Junk Food” Diet During Pregnancy as a Predictor of High Birthweight: Findings from the Healthy Beginnings Tria. Birth 2013, 40, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Gamba, R.J.; Leung, C.W.; Petito, L.; Abrams, B.; Laraia, B.A. Sugar sweetened beverage consumption during pregnancy is associated with lower diet quality and greater total energy intake. PLoS ONE 2019, 14, e0215686. [Google Scholar] [CrossRef]
- Shin, D.; Lee, K.W.; Song, W.O. Dietary patterns during pregnancy are associated with risk of gestational diabetes mellitus. Nutrients 2015, 7, 9369–9382. [Google Scholar] [CrossRef]
- Moran, L.J.; Sui, Z.; Cramp, C.S.; Dodd, J.M. A decrease in diet quality occurs during pregnancy in overweight and obese women which is maintained post-partum. Int. J. Obes. 2013, 37, 704–711. [Google Scholar] [CrossRef]
- Looman, M.; Geelen, A.; Samlal, R.A.K.; Heijligenberg, R.; Klein Gunnewiek, J.M.T.; Balvers, M.G.J.; Wijnberger, L.D.E.; Brouwer-Brolsma, E.M.; Feskens, E.J.M. Changes in micronutrient intake and status, diet quality and glucose tolerance from preconception to the second trimester of pregnancy. Nutrients 2019, 11, 460–476. [Google Scholar] [CrossRef]
- Rifas-Shiman, S.L.; Rich-Edwards, J.W.; Willett, W.C.; Kleinman, K.P.; Oken, E.; Gillman, M.W. Changes in dietary intake from the first to the second trimester of pregnancy. Paediatr. Perinat. Epidemiol. 2006, 20, 35–42. [Google Scholar] [CrossRef]
- Rad, N.T.; Ritterath, C.; Siegmund, T.; Wascher, C.; Siebert, G.; Henrich, W.; Buhling, K.J. Longitudinal analysis of changes in energy intake and macronutrient composition during pregnancy and 6 weeks post-partum. Arch. Gynecol. Obstet. 2011, 283, 185–190. [Google Scholar]
- Sánchez, C.L.; Rodríguez, A.B.; Sánchez, J.; González, R.; Rivero, M.; Barriga, C.; Cubero, J. Calcium intake nutritional status in breastfeeding women. Arch. Latinoam. Nutr. 2008, 58, 371–376. [Google Scholar] [PubMed]
- de Jersey, S.J.; Nicholson, J.M.; Callaway, L.K.; Daniels, L.A. An observational study of nutrition and physical activity behaviours, knowledge, and advice in pregnancy. BMC Pregnancy Childbirth 2013, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Blondin, J.H.; LoGiudice, J.A. Pregnant women’s knowledge and awareness of nutrition. Appl. Nurs. Res. 2018, 39, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Bookari, K.; Yeatman, H.; Williamson, M. Informing Nutrition Care in the Antenatal Period: Pregnant Women’s Experiences and Need for Support. Biomed. Res. Int. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Generalitat de Cataluña. Guia per a Embarassades, Edició revisada 2018; Generalitat de Catalunya, Departament de Salut: Barcelona, Spain, 2018. [Google Scholar]
- Doyle, I.M.; Borrmann, B.; Grosser, A.; Razum, O.; Spallek, J. Determinants of dietary patterns and diet quality during pregnancy: A systematic review with narrative synthesis. Public Health Nutr. 2017, 20, 1009–1028. [Google Scholar] [CrossRef]
- Wesołowska, E.; Jankowska, A.; Trafalska, E.; Kałużny, P.; Grzesiak, M.; Dominowska, J.; Hanke, W.; Calamandrei, G.; Polańska, K. Sociodemographic, lifestyle, environmental and pregnancy-related determinants of dietary patterns during pregnancy. Int. J. Environ. Res. Public. Health 2019, 16, 754–769. [Google Scholar] [CrossRef]
- Rifas-Shiman, S.L.; Rich-Edwards, J.W.; Kleinman, K.P.; Oken, E.; Gillman, M.W. Dietary Quality during Pregnancy Varies by Maternal Characteristics in Project Viva: A US Cohort. J. Am. Diet. Assoc. 2009, 109, 1004–1011. [Google Scholar] [CrossRef]
- Coathup, V.; Northstone, K.; Gray, R.; Wheeler, S.; Smith, L. Dietary Patterns and Alcohol Consumption During Pregnancy: Secondary Analysis of Avon Longitudinal Study of Parents and Children. Alcohol. Clin. Exp. Res. 2017, 41, 1120–1128. [Google Scholar] [CrossRef]
| General Characteristics | n = 793 | |
|---|---|---|
| Maternal age (years) | 30.5 (4.9) | |
| Country of origin, Spain (%) | 84.1 (667) | |
| Planned pregnancy (%) | 80.3 (611) | |
| Primipara (%) | 37.5 (297) | |
| Gestational age (weeks) | 39.7 (0.9) | |
| Type of delivery (%) | Normal vaginal | 80 (634) |
| Caesarean | 20 (159) | |
| Maternal educational level (%) | Primary studies | 31.4 (249) |
| Secondary studies | 40.9 (324) | |
| University studies | 27.7 (220) | |
| Social class (%) | Low | 16.0 (127) |
| Middle | 68.3 (542) | |
| High | 15.6 (124) | |
| Maternal smoking (%) | 15.3 (121) | |
| Maternal alcohol consumption (%) | 15.4 (122) | |
| Weight (kg) | 65.1 (11.9) | |
| Height (m) | 1.61 (0.1) | |
| BMI (kg/m2) | 24.8 (4.5) | |
| BMI categories (%) | Normal weight | 63.4 (503) |
| Excess weight | 36.6 (290) | |
| Physical activity categories (%) | Active | 7.1 (56) |
| Sedentary | 92.9 (737) | |
| Type of feeding at 40 days (%) | Breastfeeding | 72.5 (575) |
| Mixed feeding | 9.5 (75) | |
| Infant formula | 18 (143) |
| n = 793 | |||||
|---|---|---|---|---|---|
| Food Group | Recommended * (Servings) | Servings/d | g/d | ||
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ||
| Red and processed meat | Occasionally | 0.95 (0.5) | 1 (0.7) | 57.1 (31.8) | 51.7 (43.1) |
| Poultry, fish, and eggs | 2 per day | 1.1 (0.5) | 1.1 (0.6) | 97.4 (44.6) | 98.6 (57.1) |
| Fruits | 3–4 per day | 1.7 (1.0) | 1.7 (1.3) | 229.8 (141.9) | 228.6 (195.6) |
| Vegetables | 2–3 per day | 1.2 (0.7) | 1.1 (0.9) | 74.8 (44.8) | 71.4 (48.21) |
| Dairy products | 3–4 per day | 2.2 (1.2) | 2.2 (1.3) | 309.3 (175.7) | 317.1 (184.2) |
| Salted cereals ‡ | 4–5 per day | 1.8 (0.8) | 1.8 (0.9) | 124.5 (54.4) | 122.1 (60.83) |
| Sweet cereals † | Occasionally | 1.0 (0.8) | 0.9 (0.9) | 33.9 (28.3) | 30.4 (30.7) |
| Legumes | 2–4 per week | 0.2 (0.2) | 0.2 (0.1) | 14.6 (11.4) | 15.2 (8.6) |
| Nuts | 3–7 per week | 0.2 (0.2) | 0.1 (0.3) | 2.8 (3.5) | 2.0 (4.3) |
| Sweets | Occasionally | 0.3 (0.4) | 0.2 (0.4) | 5.5 (6.6) | 3.6 (6.0) |
| Sweetened beverages | Occasionally | 0.2 (0.3) | 0.1 (0.3) | 49.3 (64.3) | 28.6 (57.1) |
| Alcoholic drinks | None | 0.02 (0.1) | 0.0 (0.0) | 2.5 (12.1) | 0.0 (0.0) |
| rMED Score • | - | 9.5 (2.6) | 9.8 (3.0) | - | - |
| Comparison First Trimester Versus Second Trimester | Comparison Second Trimester Versus Third Trimester | Comparison Third Trimester Versus Post-Partum | Comparison Mean Three Trimesters Versus Post-Partum | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First Trimester a n = 513 | Second Trimester b | p-Value between (a-b) | Third TRIMESTER c | p-Value between (b-c) | Post-Partum d | p-Value between (c-d) | Mean Three Trimesters e | Post-Partum d | p-Value between (d-e) | |
| n = 513 | n = 513 | n = 426 | n = 395 | n = 395 | n = 395 | |||||
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |||||
| Red and processed meat | 58.6 (37.1) | 60.0 (38.4) | 0.306 | 58.6 (37.2) | 0.315 | 58.6 (35.0) | 0.566 | 59.2 (34.0) | 58.6 (35.0) | 0.444 |
| Poultry, fish, and eggs | 96.8 (60.4) | 100.7 (53.0) | 0.577 | 92.1 (55.9) | 0.311 | 96.9 (50.7) | 0.752 | 99.5 (51.0) | 96.9 (50.7) | 0.989 |
| Fruits | 228.6 (208.9) | 214.3 (185.7) | 0.045 | 209.8 (178) | 0.088 | 189.0 (189.3) | 0.003 | 221.4 (159.5) | 189.0 (189.3) | <0.001 |
| Vegetables Dairy products | 71.4 (50.1) | 72.9 (51.4) | 0.881 | 72.9 (48.9) | 0.252 | 71.4 (45.4) | 0.873 | 75.7 (46.9) | 71.4 (45.4) | 0.003 |
| Dairy products | 319.3 (163.9) | 337.1 (141.4) | <0.001 | 337.1 (141.2) | 0.789 | 319.3 (137.1) | 0.022 | 332.1 (135.6) | 319.3 (137.1) | 0.242 |
| Salted cereals ‡ Sweet cereals † | 121.1 (63.6) | 110.7 (59.8) | 0.110 | 116.4 (11 | 116.4 (11) | 0.040. | ||||
| 116.4 (59.8) | <0.001 | 110.7 (59.8) | 0.011 | 116.4 (58.5) | 0.049 | 117.4 (27.0) | 116.4 (58.5) | 0.047 | ||
| Sweet cereals † | 30.0 (34.4) | |||||||||
| 30.9 (30.6) | 0.764 | 30.0 (30.8) | 0.449 | 29.3 (28.6) | 0.710 | 31.2 (26.9) | 29.3 (28.6) | 0.033 | ||
| Legumes | 17.1 (8.6) | 17.1 (8.6) | 0.398 | 12.9 (8.6) | 0.201 | 17.1 (8.6) | 0.042 | 14.3 (11.4) | 17.1 (8.6) | 0.653 |
| Nuts | 2.0 (4.3) | 2.1 (4.3) | 0.947 | 1.8 (4.3) | 0.280 | 2.1 (4.3) | 0.123 | 2.1 (3.6) | 2.1 (4.3) | 0.739 |
| Sweets | 3.6 (7.6) | 4.3 (7.6) | 0.006 | 4.3 (7.1) | 0.418 | 3.9 (7.4) | 0.409 | 4.6 (6.6) | 3.9 (7.4) | 0.996 |
| Sweetened beverages | 28.6 (85.7) | 32.3 (74.7) | 0.004 | 32.6 (73.8) | 0.868 | 28.6 (85.7) | 0.629 | 37.5 (71.4) | 28.6 (85.7) | 0.579 |
| Alcoholic drinks | 0.0 (0.0) | 0.0 (0.0) | 0.682 | 0.0 (0) | 0.515 | 0.0 (0.0) | <0.001 | 0.0 (0.0) | 0.0 (0.0) | <0.001 |
| rMED Score • | 10.0 (4.0) | 10.0 (4.0) | 0.833 | 10.0 (4) | 0.819 | 10.0 (4.0) | 0.288 | 10.0 (3.0) | 10.0 (4.0) | 0.110 |
| Food Group (g/d) | Socio-economic Level | Maternal Educational Level | Country of Origin | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low n = 107 | Medium n = 440 | High n = 110 | p-Value | Primary n = 206 | Secondary n = 252 | University n = 199 | p-Value | Spain n = 544 | Latin America n = 61 | Arab n = 52 | p-Value | ||
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |||||
| Red and processed meat | 55.0 (50.9) | 52.7 (43.1) | 52.1 (41.6) | 0.734 | 55.0 (43.0) | 55.0 (47.2) | 51.4 (38.8) | 0.308 | 55.0 (42.9) | 50.0 (54.7) | 40.0 (42.1) | 0.011 | |
| Poultry, fish, and eggs | 102.3 (61.9) | 98.5(54.5) | 102.3 (52.3) | 0.423 | 101.1 (53.9) | 100.7 (56.0) | 102.3 (48.6) | 0.661 | 101.2 (53.6) | 101.4 (60.2) | 102.3 (56.0) | 0.564 | |
| Fruits | 234.4 (185.7) | 228.6 (185.1) | 234.4 (180.4) | 0.904 | 227.6 (228.6) | 228.6 (176.8) | 234.4 (174.3) | 0.518 | 220.7 (177.4) | 285.7 (133.8) | 248.2 (253.6) | 0.003 | |
| Vegetables | 64.3 (44.5) | 70.7 (41.4) | 79.3 (51.8) | <0.001 | 63.6 (41.9) | 70.0 (42.5) | 78.6 (45.0) | <0.001 | 75.7 (48.6) | 65.7 (37.5) | 60.7 (52.0) | 0.008 | |
| Dairy products | 333.6 (157.9) | 319.6 (204.5) | 322.5 (152.5) | 0.745 | 308.2 (227.1) | 330.0 (156.6) | 333.6 (167.9) | 0.417 | 322.1 (180.3) | 333.3 (160.3) | 321.5 (227.5) | 0.748 | |
| Salted cereals ‡ | 121.3 (81.1) | 119.5 (58.7) | 124.3 (39.5) | 0.016 | 124.3 (73.9) | 124.3 (55.4) | 119.6 (52.1) | 0.174 | 120.7 (54.1) | 140.7 (106.4) | 123.4 (81.6) | 0.009 | |
| Sweet cereals † | 35.0 (37.9) | 32.1 (29.4) | 29.5 (24.1) | 0.166 | 32.4 (38.1) | 35 (29.1) | 26.4 (25.5) | 0.063 | 31.3 (27.5) | 35.9 (43.0) | 35.0 (37.4) | 0.947 | |
| Legumes | 15.2 (17.1) | 15.1 (8.6) | 15.2 (8.6) | 0.059 | 15.2 (9.6) | 15.2 (8.6) | 15.2 (8.6) | 0.688 | 15.2 (8.6) | 15.2 (21.6) | 12.9 (18.2) | 0.959 | |
| Nuts | 0.5 (2.9) | 2.1 (4.3) | 2.9 (5.9) | <0.001 | 1.0 (2.9) | 2.1 (4.3) | 2.1 (3.8) | <0.001 | 2.1 (4.3) | 0.5 (2.9) | 2.3 (6.4) | 0.043 | |
| Sweets | 2.9 (7.6) | 3.6 (6.0) | 3.9 (5.7) | 0.933 | 3.1 (5.7) | 3.8 (7.1) | 3.6 (5.7) | 0.642 | 3.6 (6.0) | 2.1 (6.0) | 2.9 (5.9) | 0.013 | |
| Sweetened beverages | 28.6 (57.1) | 28.6 (85.7) | 28.6 (57.1) | 0.432 | 28.6 (85.7) | 28.6 (57.1) | 28.6 (57.1) | 0.324 | 28.6 (57.1) | 14.3 (56.0) | 20.5 (57.1) | 0.048 | |
| Alcoholic drinks | 0.0 | 0.0 | 0.0 | 0.825 | 0.0 | 0.0 | 0.0 | 0.643 | 0.0 | 0.0 | 0.0 | 0.278 | |
| rMED Score • | 9.8 (3.0) | 9.8 (3.0) | 10.0 (3.0) | 0.065 | 9.8 (4.0) | 9.8 (3.8) | 10.0 (4.0) | 0.012 | 9.8 (3.0) | 9.8 (4.0) | 10.0 (3.0) | 0.070 | |
| Food group (g/d) | Age (years) | Maternal alcohol consumption | Maternal smoking | Planned pregnancy | |||||||||
| <25 n = 85 | 25–29 n = 168 | ≥30 n = 404 | p-value | No n = 671 | Yes n = 122 | p-value | No n = 546 | Yes n = 111 | p-value | No n = 129 | Yes n = 520 | p-value | |
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |||||
| Red and processed meat | 46.2 (42.6) | 56.8 (37.5) | 50.7 (41.6) | 0.014 | 55.7 (40.0) | 27.0 (32.0) | <0.001 | 51.6 (43.3) | 55.7 (42.9) | 0.292 | 50.7 (45.8) | 54.6 (41.6) | 0.535 |
| Poultry, fish, and eggs | 92.9 (59.5) | 99.3 (57.8) | 102.3 (52.6) | 0.505 | 93.6 (58.8) | 102.3 (9.8) | 0.053 | 101,43 (51.3) | 101.4 (69.6) | 0.361 | 101.9 (65.4) | 101.4 (52) | 0.818 |
| Fruits | 214.3 (221.4) | 228.6 (197.0) | 234.4 (173.9) | 0.510 | 214.3 (200.0) | 234.4 (37.1) | 0.002 | 234.4 (179.3) | 185.7 (214.3) | 0.005 | 233.3 (189.8) | 228.6 (185.7) | 0.433 |
| Vegetables | 51.4 (50.0) | 67.1 (49.0) | 78.6 (44.3) | <0.001 | 65.7 (51.4) | 78.6 (3.5) | <0.001 | 74.3 (45.5) | 64.3 (51.4) | 0.070 | 67.1 (48.4) | 74.3 (47.1) | 0.018 |
| Dairy products | 297.9 (230.4) | 308.6 (207.9) | 335.1 (151.9) | 0.140 | 308.6 (217.1) | 335.1 (16.6) | 0.045 | 323 (173.3) | 320 (222.1) | 0.643 | 315.7 (183) | 322.1 (178.7) | 0.408 |
| Salted cereals ‡ | 124.3 (88.0) | 124.3 (62.2) | 120.9 (54.3) | 0.141 | 117.9 (70.6) | 124.3 (4.0) | 0.183 | 123.4 (60.1) | 117.9 (68.6) | 0.164 | 124.3 (71.5) | 120.7 (57.4) | 0.179 |
| Sweet cereals † | 35.0 (47.1) | 32.9 (33.2) | 30.1 (28.8) | 0.068 | 27.1 (35.0) | 35.9 (8.0) | 0.003 | 32.1 (28.2) | 30 (33.8) | 0.600 | 30.4 (33.8) | 31.6 (29.2) | 0.700 |
| Legumes | 15.2 (20.4) | 15.2 (8.6) | 15.2 (8.6) | 0.957 | 8.6 (8.6) | 15.2 (6.6) | 0.368 | 15.2 (8.6) | 17.1 (8.6) | 0.618 | 8.6 (12.9) | 15.2 (8.6) | 0.031 |
| Nuts | 1.0 (2.9) | 1.1 (4.3) | 2.1 (4.3) | 0.003 | 1.1 (4.3) | 2.9 (0.7) | <0.001 | 2.1 (4.3) | 1.1 (2.9) | 0.102 | 1.1 (2.9) | 2.1 (4.3) | 0.058 |
| Sweets | 3.6 (6.4) | 3.6 (7.8) | 3.6 (5.7) | 0.678 | 3.0 (6.6) | 6.1 (2.6) | <0.001 | 3.6 (5.9) | 3.6 (8.5) | 0.884 | 2.9 (5.6) | 3.6 (6.4) | 0.135 |
| Sweetened beverages | 28.6 (85.7) | 28.6 (85.7) | 28.6 (57.1) | 0.918 | 26.7 (57.1) | 56.0 (27.4) | <0.001 | 28.6 (57.1) | 56 (114.3) | 0.044 | 28.6 (78.6) | 28.6 (57.1) | 0.867 |
| Alcoholic drinks | 0.0 | 0.0 | 0.0 | 0.531 | 0.0 | 6.3 (9.8) | <0.001 | 0 (0) | 0 (0) | 0.500 | 0 (0) | 0 (0) | 0.650 |
| rMED Score • | 9.0 (3.5) | 9.9 (3.0) | 9.8 (3.0) | 0.026 | 10.0 (4.0) | 9.8 (1.8) | 0.004 | 9.8 (3) | 9 (4.7) | 0.079 | 9.8 (3) | 9.8 (3) | 0.172 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jardí, C.; Aparicio, E.; Bedmar, C.; Aranda, N.; Abajo, S.; March, G.; Basora, J.; Arija, V.; Study Group, t.E. Food Consumption during Pregnancy and Post-Partum. ECLIPSES Study. Nutrients 2019, 11, 2447. https://doi.org/10.3390/nu11102447
Jardí C, Aparicio E, Bedmar C, Aranda N, Abajo S, March G, Basora J, Arija V, Study Group tE. Food Consumption during Pregnancy and Post-Partum. ECLIPSES Study. Nutrients. 2019; 11(10):2447. https://doi.org/10.3390/nu11102447
Chicago/Turabian StyleJardí, Cristina, Estefania Aparicio, Cristina Bedmar, Núria Aranda, Susana Abajo, Gemma March, Josep Basora, Victoria Arija, and the ECLIPSES Study Group. 2019. "Food Consumption during Pregnancy and Post-Partum. ECLIPSES Study" Nutrients 11, no. 10: 2447. https://doi.org/10.3390/nu11102447
APA StyleJardí, C., Aparicio, E., Bedmar, C., Aranda, N., Abajo, S., March, G., Basora, J., Arija, V., & Study Group, t. E. (2019). Food Consumption during Pregnancy and Post-Partum. ECLIPSES Study. Nutrients, 11(10), 2447. https://doi.org/10.3390/nu11102447






