Sex-Specific Glucose Homeostasis and Anthropometric Responses to Sleeve Gastrectomy in Obese Patients
Abstract
1. Introduction
2. Materials and Methods
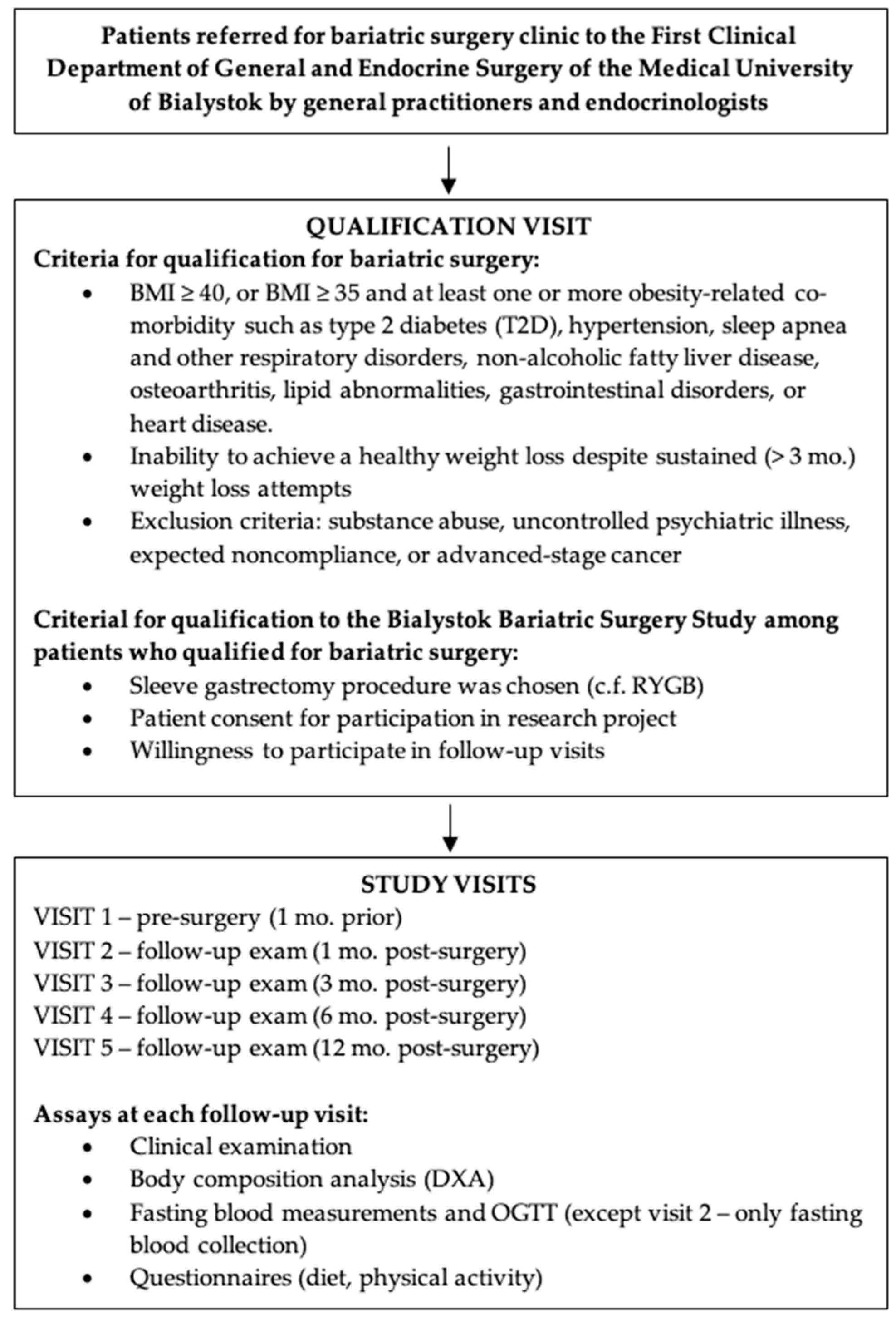
2.1. Study Design
2.2. Study Population
2.3. Assay Protocols and Measurements
2.4. Statistical Analysis
3. Results
3.1. Cohort-Wide Characteristics
3.2. Baseline Sex-Specific Differences
3.3. Model Comparison
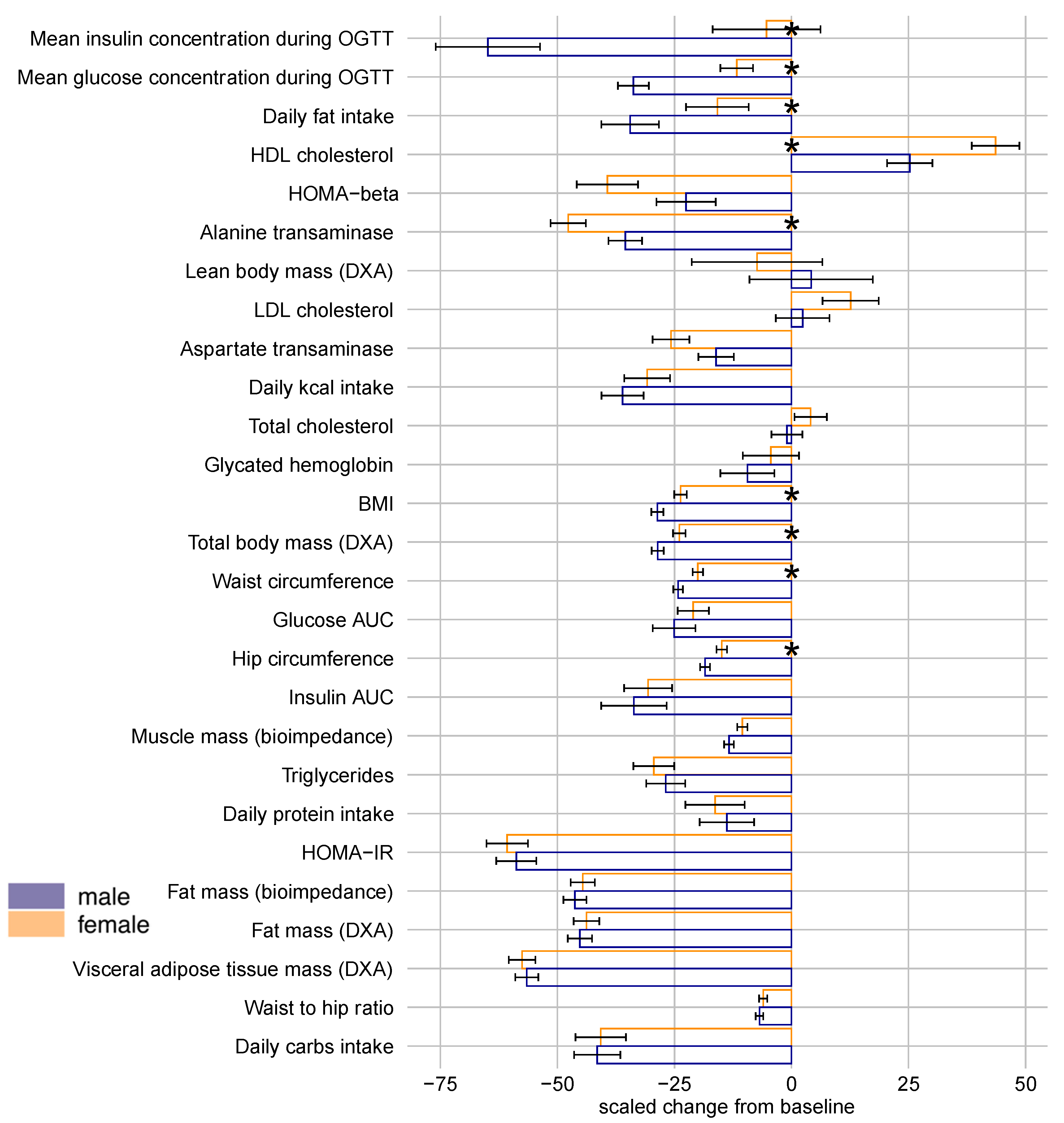
3.4. Cohort-Wide (Across-Sex) Responses to Bariatric Surgery
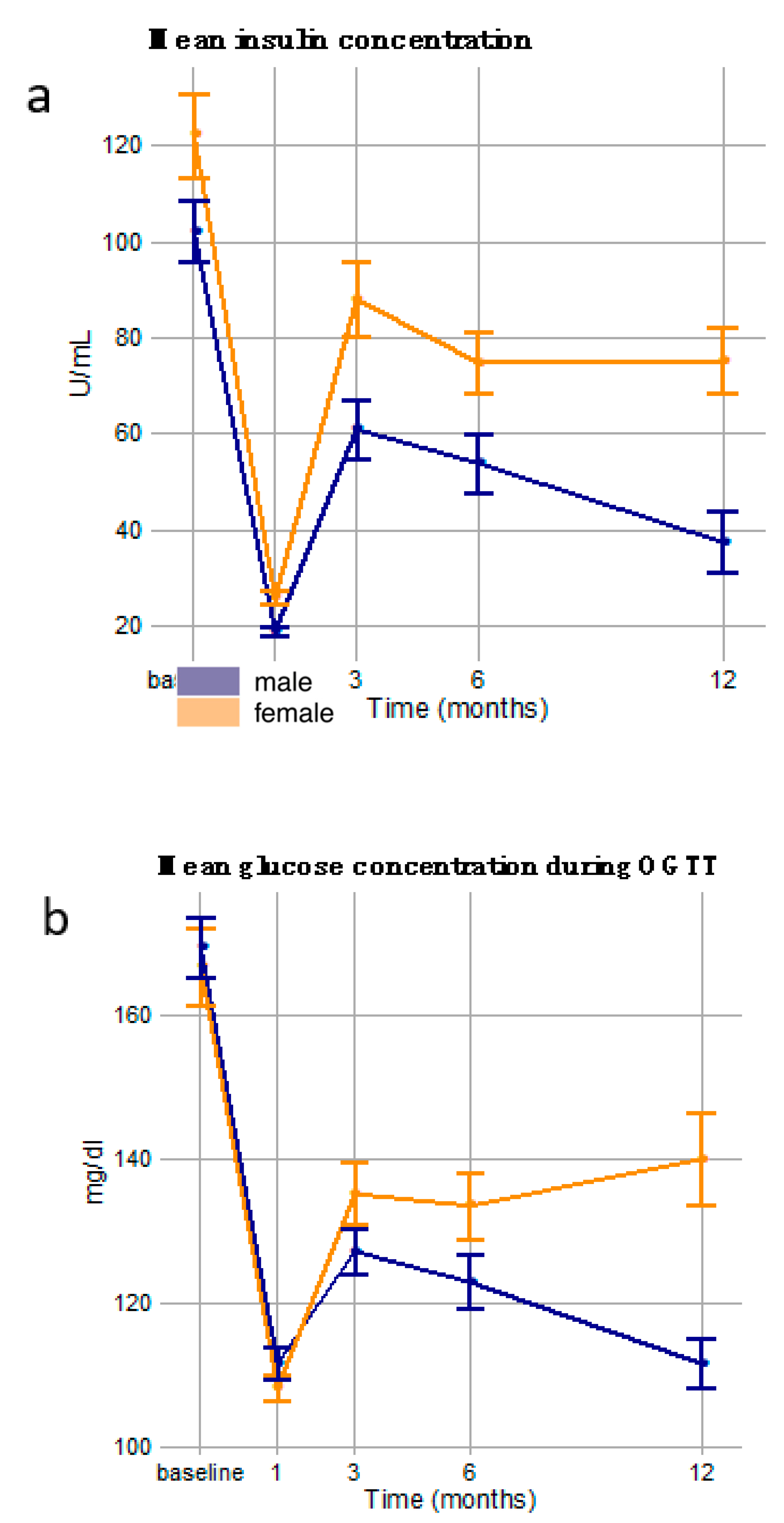
3.5. Cross-Sectional (Sex-Specific) Responses to Bariatric Surgery
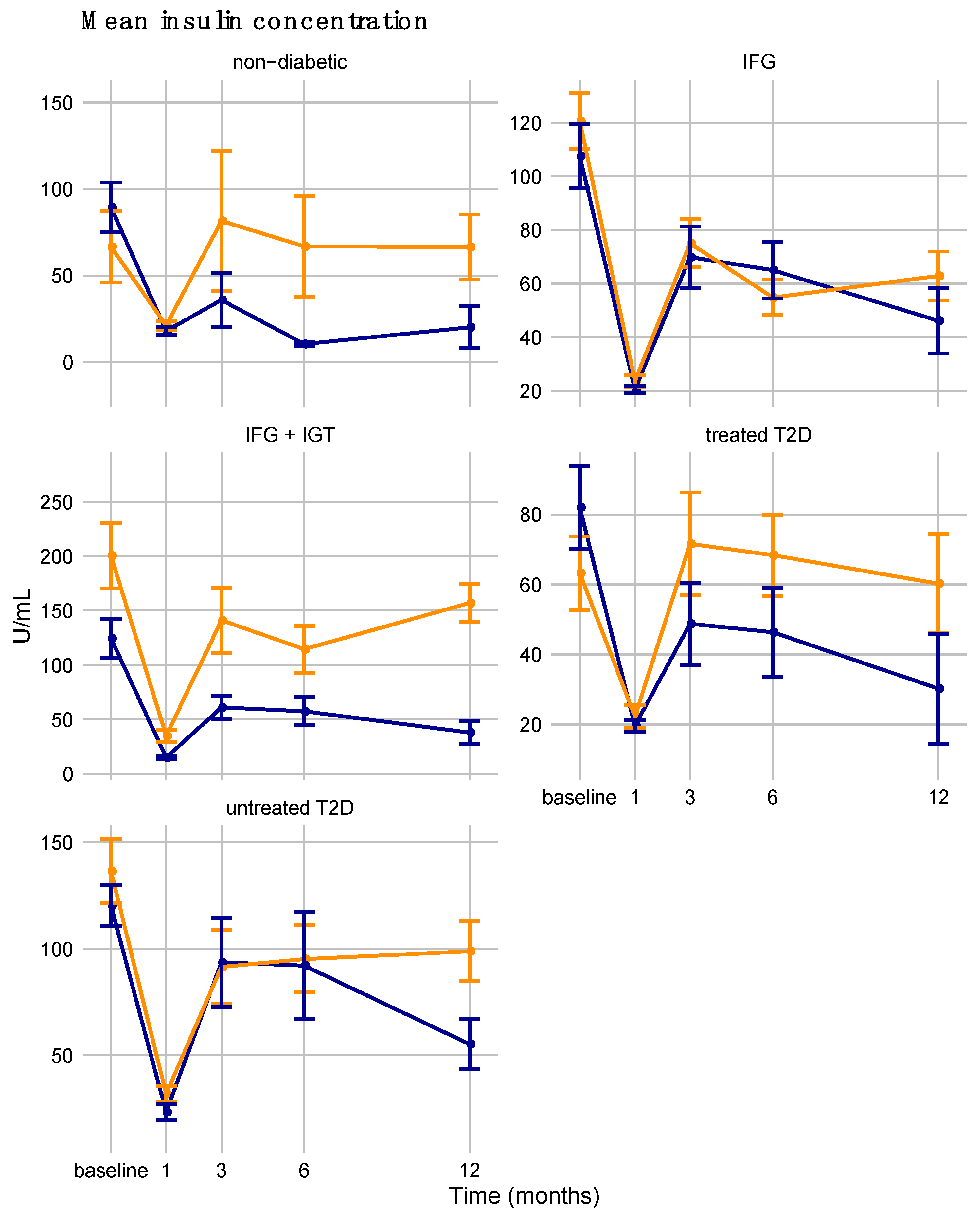
3.6. Dysglycemia Diagnosis Differences in Sex-Specific Responses to Bariatric Surgery
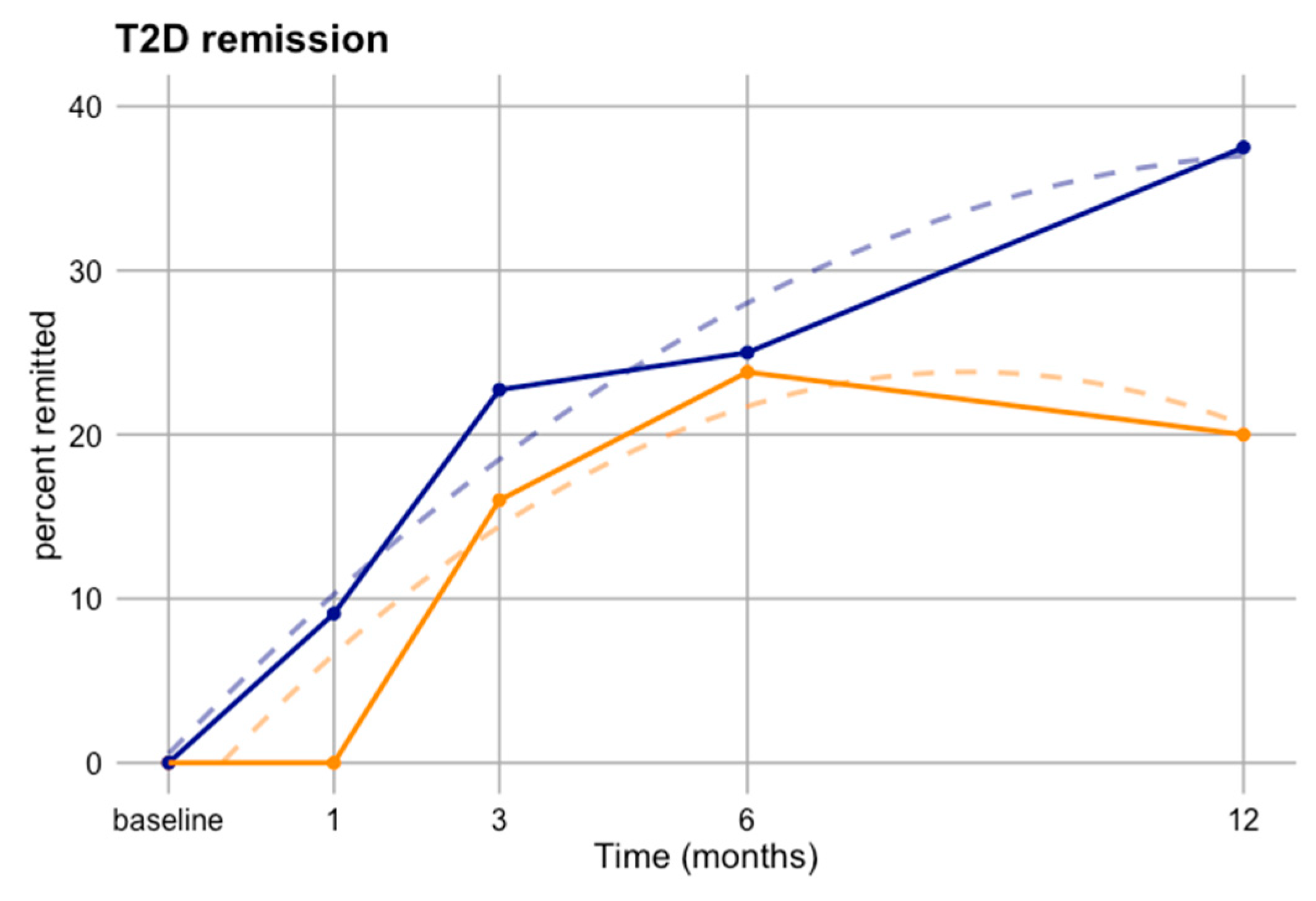
3.7. Sex-Spcific Trends in T2D Remission
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Obesity and Overweight: Fact Sheet; WHO: Geneva, Switzerland, 2018; Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 3 August 2019).
- Buchwald, H.; Avidor, Y.; Braunwald, E.; Jensen, M.D.; Pories, W.; Fahrbach, K.; Schoelles, K. Bariatric surgery: A systematic review and meta-analysis. J. Am. Med. Assoc. 2004, 292, 1724–1737. [Google Scholar] [CrossRef] [PubMed]
- Maggard, M.A.; Shugarman, L.R.; Suttorp, M.; Maglione, M.; Sugarman, H.J.; Livingston, E.H.; Nguyen, N.T.; Li, Z.; Mojica, W.A.; Hilton, L.; et al. Meta-analysis: Surgical treatment of obesity. Ann. Intern. Med. 2005, 142, 547. [Google Scholar] [CrossRef]
- English, W.J.; DeMaria, E.J.; Brethauer, S.A.; Mattar, S.G.; Rosenthal, R.J.; Morton, J.M. American Society for Metabolic and Bariatric Surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surg. Obes. Relat. Dis. 2018, 14, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Angrisani, L.; Santonicola, A.; Iovino, P.; Formisano, G.; Buchwald, H.; Scopinaro, N. Bariatric Surgery Worldwide 2013. Obes. Surg. 2015, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, H.; Estok, R.; Fahrbach, K.; Banel, D.; Jensen, M.D.; Pories, W.J.; Bantle, J.P.; Sledge, I. Weight and Type 2 Diabetes after Bariatric Surgery: Systematic Review and Meta-analysis. Am. J. Med. 2009, 122, 248–256. [Google Scholar] [CrossRef]
- Cunneen, S.A.; Phillips, E.; Fielding, G.; Banel, D.; Estok, R.; Fahrbach, K.; Sledge, I. Studies of Swedish adjustable gastric band and Lap-Band: Systematic review and meta-analysis. Surg. Obes. Relat. Dis. 2008, 4, 174–185. [Google Scholar] [CrossRef]
- Young, M.T.; Phelan, M.J.; Nguyen, N.T. A decade analysis of trends and outcomes of male vs. female patients who underwent bariatric surgery. J. Am. Coll. Surg. 2016, 222, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, H.F.; Broderick, R.C.; Harnsberger, C.R.; Chang, D.C.; Sandler, B.J.; Jacobsen, G.R.; Horgan, S. Benefits of Bariatric Surgery Do Not Reach Obese Men. J. Laparoendosc. Adv. Surg. Tech. 2015, 25, 196–201. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Chang, S.H.; Stoll, C.R.T.; Song, J.; Varela, J.E.; Eagon, C.J.; Colditz, G.A. The effectiveness and risks of bariatric surgery an updated systematic review and meta-analysis, 2003–2012. JAMA Surg. 2014, 149, 275–287. [Google Scholar] [CrossRef]
- Padwal, R.; Klarenbach, S.; Wiebe, N.; Birch, D.; Karmali, S.; Manns, B.; Hazel, M.; Sharma, A.M.; Tonelli, M. Bariatric surgery: A systematic review and network meta-analysis of randomized trials. Obes. Rev. 2011, 12, 602–621. [Google Scholar] [CrossRef] [PubMed]
- Lyon, M.; Bashian, C.; Sheck, C.; Kushnir, L.; Slotman, G.J. Outcomes following laparoscopic Roux-en-Y gastric bypass (LRYGB)vary by sex: Analysis of 83,059 women and men with morbid obesity. Am. J. Surg. 2019, 217, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Kochkodan, J.; Telem, D.A.; Ghaferi, A.A. Physiologic and psychological gender differences in bariatric surgery. Surg. Endosc. 2018, 32, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- MacHado, M.B.; Velasco, I.T.; Scalabrini-Neto, A. Gastric bypass and cardiac autonomic activity: Influence of gender and age. Obes. Surg. 2009, 19, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Infanger, D.; Baldinger, R.; Branson, R.; Barbier, T.; Steffen, R.; Horber, F.F. Effect of Significant Intermediate-term Weight Loss on Serum Leptin Levels and Body Composition in Severely Obese Subjects. Obes. Surg. 2003, 13, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Andreu, A.; Moizé, V.; Rodríguez, L.; Flores, L.; Vidal, J. Protein intake, body composition, and protein status following bariatric surgery. Obes. Surg. 2010, 20, 1509–1515. [Google Scholar] [CrossRef]
- Tymitz, K.; Kerlakian, G.; Engel, A.; Bollmer, C. Gender differences in early outcomes following hand-assisted laparoscopic Roux-en-Y gastric bypass surgery: Gender differences in bariatric surgery. Obes. Surg. 2007, 17, 1588–1591. [Google Scholar] [CrossRef] [PubMed]
- Frezza, E.E.; Barton, A.; Herbert, H.; Wachtel, M.S. Laparoscopic sleeve gastrectomy with endoscopic guidance in morbid obesity. Surg. Obes. Relat. Dis. 2008, 4, 575–579. [Google Scholar] [CrossRef]
- Powers, P.S.; Rosemurgy, A.; Boyd, F.; Perez, A. Outcome of gastric restriction procedures: Weight, psychiatric diagnoses, and satisfaction. Obes. Surg. 1997, 7, 471–477. [Google Scholar] [CrossRef]
- Ranasinghe, W.K.B.; Wright, T.; Attia, J.; McElduff, P.; Doyle, T.; Bartholomew, M.; Hurley, K.; Persad, R.A. Effects of bariatric surgery on urinary and sexual function. BJU Int. 2011, 107, 88–94. [Google Scholar] [CrossRef]
- Dubnov-Raz, G.; Inge, T.H.; Ben-Ami, M.; Pienik, R.; Vusiker, I.; Yardeni, D. Body composition changes in adolescents after laparoscopic sleeve gastrectomy. Surg. Obes. Relat. Dis. 2016, 12, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Colquitt, J.L.; Pickett, K.; Loveman, E.; Frampton, G.K. Surgery for weight loss in adults. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Bashian, C.; Kushnir, L.; Nituica, C.; Slotman, G.J. Variation in clinical characteristics of women versus men preoperative for laparoscopic Roux-en-Y gastric bypass: Analysis of 83,059 patients. Am. Surg. 2017, 83, 947–951. [Google Scholar] [PubMed]
- Livingston, E.H.; Huerta, S.; Arthur, D.; Lee, S.; De Shields, S.; Heber, D. Male gender is a predictor of morbidity and age a predictor of mortality for patients undergoing gastric bypass surgery. Ann. Surg. 2002, 236, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Muennig, P.; Lubetkin, E.; Jia, H.; Franks, P. Gender and the burden of disease attributable to obesity. Am. J. Public Health 2006, 96, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Perrone, F.; Bianciardi, E.; Benavoli, D.; Tognoni, V.; Niolu, C.; Siracusano, A.; Gaspari, A.L.; Gentileschi, P. Gender Influence on Long-Term Weight Loss and Comorbidities After Laparoscopic Sleeve Gastrectomy and Roux-en-Y Gastric Bypass: A Prospective Study With a 5-Year Follow-up. Obes. Surg. 2016, 26, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, H.; Cao, Z.; Sun, X.; Zhang, C.; Cai, W.; Liu, R.; Hu, S.; Qin, M. A Randomized Clinical Trial of Laparoscopic Roux-en-Y Gastric Bypass and Sleeve Gastrectomy for the Treatment of Morbid Obesity in China: A 5-Year Outcome. Obes. Surg. 2014, 24, 1617–1624. [Google Scholar] [CrossRef]
- Sabinsky, M.S.; Toft, U.; Raben, A.; Holm, L. Overweight men’s motivations and perceived barriers towards weight loss. Eur. J. Clin. Nutr. 2007, 61, 526. [Google Scholar] [CrossRef][Green Version]
- Lipowska, M.; Lipowski, M.; Olszewski, H.; Dykalska-Bieck, D. Gender differences in body-esteem among seniors: Beauty and health considerations. Arch. Gerontol. Geriatr. 2016, 67, 160–170. [Google Scholar] [CrossRef]
- Davy, S.R.; Benes, B.A.; Driskell, J.A. Sex Differences in Dieting Trends, Eating Habits, and Nutrition Beliefs of a Group of Midwestern College Students. J. Am. Diet. Assoc. 2006, 106, 1673–1677. [Google Scholar] [CrossRef]
- Fagerli, R.A.; Wandel, M. Gender differences in opinions and practices with regard to a “Healthy Diet”. Appetite 1999, 32, 171–190. [Google Scholar] [CrossRef] [PubMed]
- Freedman, M.R. Gender, residence and ethnicity affect freshman BMI and dietary habits. Am. J. Health Behav. 2010, 34, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Grayson, B.E.; Schneider, K.M.; Woods, S.C.; Seeley, R.J. Improved rodent maternal metabolism but reduced intrauterine growth after vertical sleeve gastrectomy. Sci. Transl. Med. 2013, 5, 199ra112. [Google Scholar] [CrossRef] [PubMed]
- Grayson, B.E.; Gutierrez-Aguilar, R.; Sorrell, J.E.; Matter, E.K.; Adams, M.R.; Howles, P.; Karns, R.; Seeley, R.J.; Sandoval, D.A. Bariatric surgery emphasizes biological sex differences in rodent hepatic lipid handling. Biol. Sex Differ. 2017, 8, 401. [Google Scholar] [CrossRef] [PubMed]
- Griffin, C.; Hutch, C.R.; Abrishami, S.; Stelmak, D.; Eter, L.; Li, Z.; Chang, E.; Agarwal, D.; Zamarron, B.; Varghese, M.; et al. Inflammatory responses to dietary and surgical weight loss in male and female mice. Biol. Sex Differ. 2019, 10, 16. [Google Scholar] [CrossRef]
- Taylor, R.; Al-Mrabeh, A.; Sattar, N. Understanding the mechanisms of reversal of type 2 diabetes. Lancet Diabetes Endocrinol. 2019, 7, 726–736. [Google Scholar] [CrossRef]
- Szczerbinski, L.; Taylor, M.A.; Citko, A.; Gorska, M.; Larsen, S.; Hady, H.R.; Kretowski, A. Clusters of Glycemic Response to Oral Glucose Tolerance Test Explain Multivariate Metabolic and Anthropometric Outcomes of Obese Patients. J. Clin. Med. 2019, 8, 1091. [Google Scholar] [CrossRef]
- Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am. J. Clin. Nutr. 1992, 55, 615S–619S. [CrossRef]
- Araszkiewicz, A.; Bandurska-Stankiewicz, E.; Budzyński, A.; Cypryk, K.; Czech, A.; Czupryniak, L.; Drzewoski, J.; Dzida, G.; Dziedzic, T.; Franek, E.; et al. 2019 Guidelines on the management of diabetic patients. A position of Diabetes Poland. Clin. Diabetol. 2019, 8, 1–95. [Google Scholar] [CrossRef]
- Brethauer, S.A.; Kim, J.; el Chaar, M.; Papasavas, P.; Eisenberg, D.; Rogers, A.; Ballem, N.; Kligman, M.; Kothari, S. Standardized Outcomes Reporting in Metabolic and Bariatric Surgery. Obes. Surg. 2015, 11, 489–506. [Google Scholar] [CrossRef]
- 2. Classification and diagnosis of diabetes: Standards of medical care in diabetesd 2019. Diabetes Care 2019, 42, S13–S28. [CrossRef] [PubMed]
- IPAQ Research Committee. IPAQ Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ)—Short and Long Forms. Available online: https://www.academia.edu/5346814/Guidelines_for_Data_Processing_and_Analysis_of_the_International_Physical_Activity_Questionnaire_IPAQ_Short_and_Long_Forms_Contents (accessed on 7 March 2017).
- Team, R.C. R: A Language and Environment for Statistical Computing. R Found. Stat. Comput. 2016. [Google Scholar] [CrossRef]
- Conover, W.J. Practical Nonparametric Statistics, 3rd ed.; Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Doble, B.; Wordsworth, S.; Rogers, C.A.; Welbourn, R.; Byrne, J.; Blazeby, J.M.; Blazeby, J.; Welbourn, R.; Byrne, J.; Reeves, B.C.; et al. What Are the Real Procedural Costs of Bariatric Surgery? A Systematic Literature Review of Published Cost Analyses. Obes. Surg. 2017, 27, 2179–2192. [Google Scholar] [CrossRef] [PubMed]
- Arman, G.A.; Himpens, J.; Dhaenens, J.; Ballet, T.; Vilallonga, R.; Leman, G. Long-term (11+ years) outcomes in weight, patient satisfaction, comorbidities, and gastroesophageal reflux treatment after laparoscopic sleeve gastrectomy. Surg. Obes. Relat. Dis. 2016, 12, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Khorgami, Z.; Shoar, S.; Andalib, A.; Aminian, A.; Brethauer, S.A.; Schauer, P.R. Trends in utilization of bariatric surgery, 2010–2014: Sleeve gastrectomy dominates. Surg. Obes. Relat. Dis. 2017, 13, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; King, W.C.; Gourash, W.; Belle, S.H.; Hinerman, A.; Pomp, A.; Dakin, G.; Courcoulas, A.P. Long-term weight change and health outcomes for sleeve gastrectomy (SG) and matched Roux-en-Y gastric bypass (RYGB) participants in the Longitudinal Assessment of Bariatric Surgery (LABS) study. Surgery 2018, 164, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Peterli, R.; Wölnerhanssen, B.K.; Vetter, D.; Nett, P.; Gass, M.; Borbély, Y.; Peters, T.; Schiesser, M.; Schultes, B.; Beglinger, C.; et al. Laparoscopic sleeve gastrectomy versus Roux-Y-Gastric bypass for morbid obesity-3-year outcomes of the prospective randomized Swiss Multicenter Bypass Or Sleeve Study (SM-BOSS). Ann. Surg. 2017, 265, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Salminen, P.; Helmiö, M.; Ovaska, J.; Juuti, A.; Leivonen, M.; Peromaa-Haavisto, P.; Hurme, S.; Soinio, M.; Nuutila, P.; Victorzon, M. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss at 5 Years Among Patients With Morbid Obesity: The SLEEVEPASS Randomized Clinical Trial. JAMA 2018, 319, 241–254. [Google Scholar] [CrossRef]
- Rogula, T.G.; Schauer, P.R.; Fouse, T. (Eds.) Prevention and Management of Complications in Bariatric Surgery; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Tversky, A.; Kahneman, D. Judgment under uncertainty: Heuristics and biases. Biases in judgments reveal some heuristics of thinking under uncertainty. Science 1974, 185, 1124–1131. [Google Scholar] [CrossRef]
- Gluud, L.L. Bias in clinical intervention research. Am. J. Epidemiol. 2006, 163, 493–501. [Google Scholar] [CrossRef]
- Contreras, J.E.; Santander, C.; Court, I.; Bravo, J. Correlation between age and weight loss after bariatric surgery. Obes. Surg. 2013, 23, 1286–1289. [Google Scholar] [CrossRef] [PubMed]
- Pascot, A.; Lemieux, S.; Lemieux, I.; Prud’homme, D.; Tremblay, A.; Bouchard, C.; Nadeau, A.; Couillard, C.; Tchernof, A.; Bergeron, J.; et al. Age-related increase in visceral adipose tissue and body fat and the metabolic risk profile of premenopausal women. Diabetes Care 1999, 22, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Reeves, M.J.; Lisabeth, L.D. The confounding issue of sex and stroke. Neurology 2010, 74, 947–948. [Google Scholar] [CrossRef] [PubMed]
- Groenwold, R.H.H.; Klungel, O.H.; Grobbee, D.E.; Hoes, A.W. Selection of confounding variables should not be based on observed associations with exposure. Eur. J. Epidemiol. 2011, 26, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Zareba, W.; Moss, A.J.; Locati, E.H.; Lehmann, M.H.; Peterson, D.R.; Hall, W.J.; Schwartz, P.J.; Vincent, G.M.; Priori, S.G.; Benhorin, J.; et al. Modulating effects of age and gender on the clinical course of long QT syndrome by genotype. J. Am. Coll. Cardiol. 2003, 42, 103–109. [Google Scholar] [CrossRef]
| Metabolic | Anthropometric |
|---|---|
| 4-point glucose, OGTT | Waist-to-hip ratio |
| 4-point insulin, OGTT | Total body mass |
| Glycated hemoglobin (HbA1c) | Lean body mass (DXA) |
| HOMA-β | Visceral adipose tissue mass (DXA) |
| HOMA-IR | Fat mass (DXA) |
| Matsuda index | Fat mass (bioimpedance) |
| Total cholesterol | Skeletal muscle mass (bioimpedance) |
| Triglycerides | BMI |
| HDL cholesterol | Nutritional/Lifestyle |
| LDL cholesterol | Total Daily Calories |
| Aspartate transaminase (AST) | Carbohydrate mass-consumed |
| Alanine transaminase (ALT) | Fat mass-consumed |
| Protein mass-consumed | |
| Physical activity (IPAQ) |
| Parameter (Unit) | Male | Female | p |
|---|---|---|---|
| N | 73 (47) | 81 (53) | NA |
| Age | 44 (34–54) | 48 (40–55) | 0.109 |
| Never Smoked | 25 (34) | 37 (46) | NA |
| Positive History of Smoking | 37 (51) | 32 (40) | NA |
| Currently Smoking | 11 (15) | 12 (15) | NA |
| FH T2D | 27 (37) | 28 (35) | NA |
| FH Obesity | 52 (71) | 70 (86) | NA |
| Dysglycemia diagnosis: non-diabetic | 7 (10) | 9 (11) | NA |
| Dysglycemia diagnosis: IFG | 29 (40) | 29 (36) | NA |
| Dysglycemia diagnosis: IFG + IGT | 11 (15) | 15 (19) | NA |
| Dysglycemia diagnosis: untreated T2D | 11 (15) | 9 (11) | NA |
| Dysglycemia diagnosis: treated T2D | 11 (15) | 16 (20) | NA |
| Total body mass (kg) | 145 (135.6–160.6) | 121.5 (107.95–139.4) | <0.001 |
| Fat mass (DXA) (kg) | 66.2 (56.3–77.6) | 63.6 (53.2–71.4) | 0.249 |
| Lean body mass (DXA) (kg) | 77.0 (72.9–83.8) | 56.5 (51.5–62.6) | <0.001 |
| Visceral adipose tissue mass (DXA) (kg) | 5.1 (4.2–5.6) | 2.5 (2.1–3.4) | <0.001 |
| Muscle mass (bioimpedance) (kg) | 46.65 (43.68–51.12) | 33.6 (30.3–37.3) | <0.001 |
| Fat mass (bioimpedance) (kg) | 67.7 (57.82–79.38) | 63.9 (53.8–72.7) | 0.230 |
| BMI (kg/m2) | 46.18 (43.38–51.49) | 44.54 (39.76–49.62) | 0.295 |
| Daily kcal intake (kcal) | 2072.37 (1465.93–2461.11) | 1477.02 (1204–1935.54) | 0.023 |
| Daily protein intake (g) | 89.9 (67.94–115.95) | 67.47 (53.49–80.18) | <0.001 |
| Daily fat intake (g) | 65.79 (45.23–85.01) | 50.41 (35.36–67.46) | 0.027 |
| Daily carbs intake (g) | 260.13 (187.69–357.19) | 211.38 (163.08–261.59) | 0.012 |
| Glucose at 0 min of OGTT (mg/dL | 114 (107–134) | 114 (106–127) | 0.766 |
| Insulin at 0 min of OGTT (U/mL) | 37.42 (27.31–51.44) | 23.86 (16.68–31.89) | <0.001 |
| Glucose at 120 min of OGTT (mg/dL) | 133 (112.5–188.5) | 140 (112–182.5) | 0.485 |
| Insulin at 120 min of OGTT (U/mL) | 107.8 (60.7–170.4) | 94.4 (52.7–158.2) | 0.201 |
| Glycated hemoglobin (HbA1c) (%) | 5.9 (5.5–6.5) | 5.8 (5.5–6.35) | 0.509 |
| Mean insulin concentration during OGTT (U/mL) | 116.08 (64.24–159.83) | 91.81 (66.8–123.84) | 0.076 |
| Mean glucose concentration during OGTT (mg/dL) | 156 (138–193) | 160.5 (138.25–196.25) | 0.259 |
| Matsuda index | 1.16 (0.73–1.94) | 1.6 (1–2.31) | 0.002 |
| Glucose AUC | 339.5 (307–422.5) | 344.25 (296.75–422.25) | 0.864 |
| Insulin AUC | 272.88 (205.3–392.15) | 231.01 (162.66–319.8) | 0.021 |
| HOMA- β | 236.35 (168.95–350.78) | 160.16 (112.94–223.94) | <0.001 |
| HOMA-IR | 11.38 (7.8–16.09) | 6.76 (4.54–9.83) | <0.001 |
| Total cholesterol (mg/dL) | 192 (160–219) | 191 (165–223) | 0.872 |
| Triglycerides (mg/dL) | 143 (114–189) | 135 (99–167) | 0.259 |
| HDL cholesterol (mg/dL) | 39 (34–45) | 49 (41–57) | <0.001 |
| LDL cholesterol (mg/dL) | 122 (95–143) | 120 (97–145) | 0.872 |
| Aspartate transaminase (U/L) | 27.5 (22.1–35.8) | 20.5 (17.2–26.2) | <0.001 |
| Alanine transaminase (U/L) | 42 (32.6–55.3) | 25.2 (19–31.9) | <0.001 |
| Physical activity (METs- min/week) | 5772 (2590–10,314) | 4227 (2292–11,257) | 0.167 |
| Time | Time 2 | Time | Time 2 | ||
|---|---|---|---|---|---|
| [glucose] 0′ (OGTT) | −2.624 *** | 0.152 ** | waist circumference | −5.246 *** | 0.240 *** |
| [glucose] 30′ (OGTT) | −1.931 | 0.073 | hip circumference | −4.312 *** | 0.195 *** |
| [glucose] 60′ (OGTT) | −7.784 ** | 0.440 * | waist-to-hip ratio | −0.011 *** | 0.000 * |
| [glucose] 120′ (OGTT) | −12.152 *** | 0.673 *** | total body mass | −6.212 *** | 0.304 *** |
| [insulin] 0′ (OGTT) | −0.812 | 0.037 | fat mass (DXA) | −4924.935 *** | 229.342 *** |
| [insulin] 30′ (OGTT) | 5.864 | −0.267 | lean body mass (DXA) | −794.795 *** | 36.214 *** |
| [insulin] 60′ (OGTT) | 1.407 | −0.245 | visceral adipose (DXA) | −277.855 *** | 13.527 *** |
| [insulin] 120′ (OGTT) | −13.138 ** | 0.593 | muscle mass (bio.) | −0.758 *** | 0.038 *** |
| HbA1c | −0.082 *** | 0.005 ** | fat mass (bio.) | −5.330 *** | 0.262 *** |
| HOMA- β | −0.593 | 0.031 | BMI | −2.328 *** | 0.115 *** |
| HOMA-IR | −0.353 * | 0.018 | BMI change | 2.057 *** | −0.100 *** |
| mean [insulin] (OGTT) | 7.904 ** | −0.706 *** | EBMIL | 10.142 *** | −0.492 *** |
| mean [glucose] (OGTT) | −0.61 | −0.081 | total weight loss | 4.456 *** | −0.216 *** |
| Matsuda index | 0.631 ** | −0.026 | excess weight loss | 10.093 *** | −0.490 *** |
| glucose AUC (OGTT) | −13.797 *** | 0.783 ** | HDL cholesterol | 0.419 | 0.053 |
| insulin AUC (OGTT) | −3.591 | 0.112 | LDL cholesterol | 1.804 | −0.118 |
| total cholesterol | 0.421 | −0.019 | Aspartate transaminase | −1.421 *** | 0.080 * |
| triglycerides | −5.441 * | 0.256 | Alanine transaminase | −3.256 ** | 0.184 * |
| Time × Sex | Time2 × Sex | Time × Sex | Time2 × Sex | ||
|---|---|---|---|---|---|
| [glucose] 0′ (OGTT) | −1.323 | 0.114 | waist circumference | 0.117 | −0.009 |
| [glucose] 30′ (OGTT) | −4.457 * | 0.383 * | hip circumference | 0.321 | 0.002 |
| [glucose] 60′ (OGTT) | −2.46 | 0.203 | waist-to-hip ratio | 0.001 | 0 |
| [glucose] 120′ (OGTT) | −2.588 | 0.188 | total body mass | −1.149 * | 0.073 |
| [insulin] 0′ (OGTT) | −3.128 * | 0.179 | fat mass (DXA) | −1105.802 * | 69.935 |
| [insulin] 30′ (OGTT) | −2.464 | −0.072 | lean body mass (DXA) | −355.734 | 30.562 |
| [insulin] 60′ (OGTT) | −5.767 | 0.449 | visceral adipose (DXA) | −307.185 *** | 17.030 *** |
| [insulin] 120′ (OGTT) | −16.033 ** | 1.170 * | muscle mass (bio.) | −0.051 | 0.006 |
| HbA1c | −0.025 | 0.001 | fat mass (bio.) | −1.263 ** | 0.074 * |
| HOMA-beta | −10.117 * | 0.515 | BMI | 0.06 | 0 |
| HOMA-IR | −1.061 *** | 0.070 *** | BMI change | −0.136 | 0.005 |
| mean [insulin] (OGTT) | 1.055 | 0.026 | EBMIL | −1.382 | 0.058 |
| mean [glucose] (OGTT) | 1.365 | 0.006 | total weight loss | −0.383 | 0.013 |
| Matsuda index | −0.672 ** | 0.031 | excess weight loss | −1.475 | 0.056 |
| glucose AUC (OGTT) | −5.272 | 0.419 | HDL cholesterol | 1.198 * | −0.086 |
| insulin AUC (OGTT) | −13.561 | 0.816 | LDL cholesterol | 0.011 | 0.012 |
| Total cholesterol | −1.602 | 0.107 | Aspartate transaminase | −1.383 * | 0.093 * |
| triglycerides | −6.03 | 0.374 | Alanine transaminase | −3.789 * | 0.219 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, M.A.; Szczerbinski, L.; Citko, A.; Niemira, M.; Gorska, M.; Hady, H.R.; Kretowski, A. Sex-Specific Glucose Homeostasis and Anthropometric Responses to Sleeve Gastrectomy in Obese Patients. Nutrients 2019, 11, 2408. https://doi.org/10.3390/nu11102408
Taylor MA, Szczerbinski L, Citko A, Niemira M, Gorska M, Hady HR, Kretowski A. Sex-Specific Glucose Homeostasis and Anthropometric Responses to Sleeve Gastrectomy in Obese Patients. Nutrients. 2019; 11(10):2408. https://doi.org/10.3390/nu11102408
Chicago/Turabian StyleTaylor, Mark A., Lukasz Szczerbinski, Anna Citko, Magdalena Niemira, Maria Gorska, Hady Razak Hady, and Adam Kretowski. 2019. "Sex-Specific Glucose Homeostasis and Anthropometric Responses to Sleeve Gastrectomy in Obese Patients" Nutrients 11, no. 10: 2408. https://doi.org/10.3390/nu11102408
APA StyleTaylor, M. A., Szczerbinski, L., Citko, A., Niemira, M., Gorska, M., Hady, H. R., & Kretowski, A. (2019). Sex-Specific Glucose Homeostasis and Anthropometric Responses to Sleeve Gastrectomy in Obese Patients. Nutrients, 11(10), 2408. https://doi.org/10.3390/nu11102408





