Transporters in the Mammary Gland—Contribution to Presence of Nutrients and Drugs into Milk
Abstract
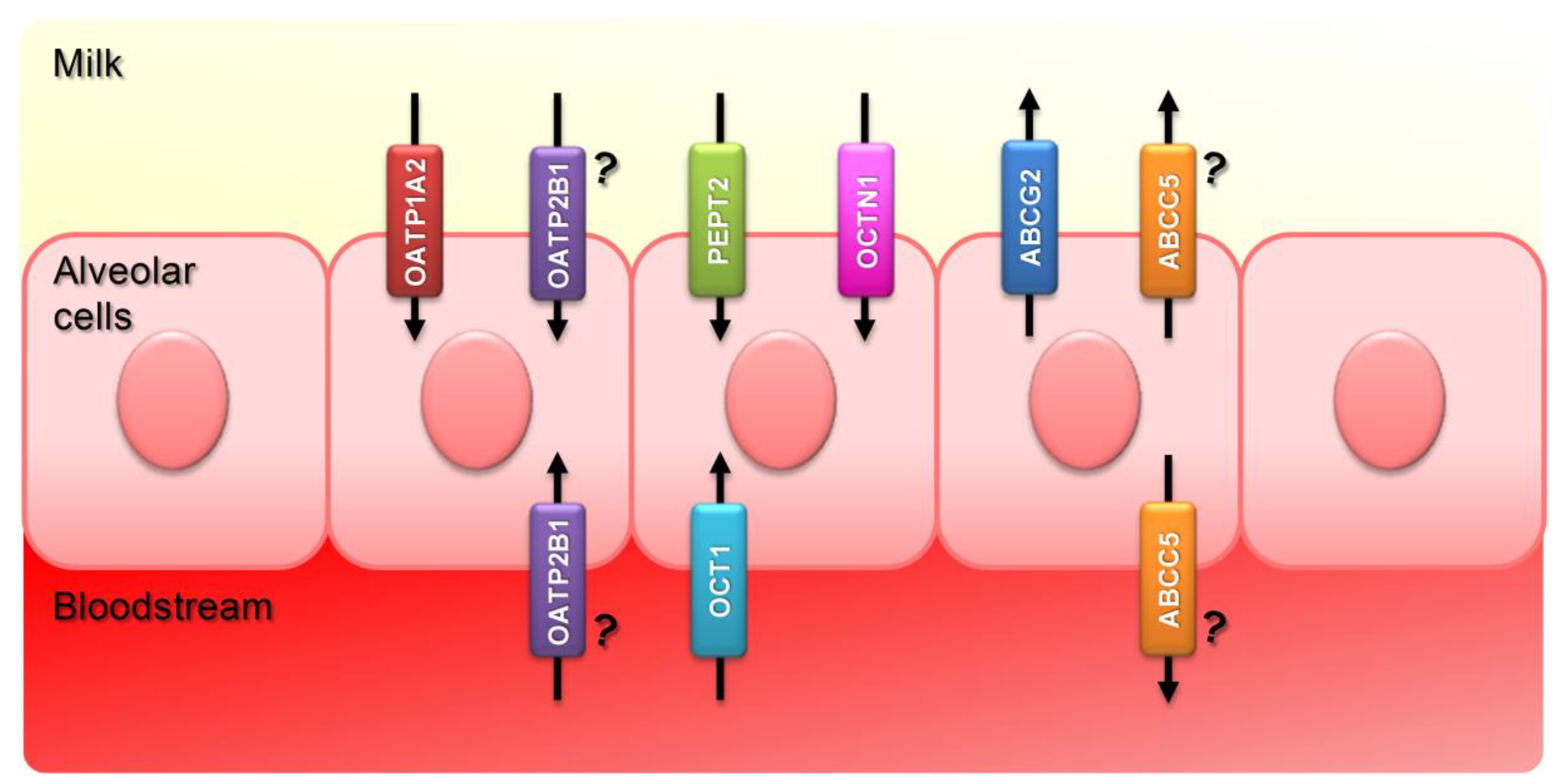
1. Introduction
2. Transporters with Increased Expression in Lactating Mammary Gland
2.1. Influx Transporters: SLCs
2.1.1. OATPs
2.1.2. OCTs
2.1.3. CNTs
2.1.4. PEPTs
2.1.5. SVCTs
2.2. Efflux Transporters: ABCs
3. Polymorphisms in Transporters with Increased Expression in Lactating Mammary Gland: Potential Effect on Breast-Fed Infants and Dairy Consumers
3.1. OATP1A2 and OATP2B1 Polymorphisms
3.2. OCT1 and OCTN1 Polymorphisms
3.3. PEPT2 Polymorphisms
3.4. ABCG2 Polymorphisms
4. Modulation of the Genes Encoding Transporters with Increased Expression in Lactating Mammary Gland
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neville, M.C.; Anderson, S.M.; McManaman, J.L.; Badger, T.M.; Bunik, M.; Contractor, N.; Crume, T.; Dabelea, D.; Donovan, S.M.; Forman, N.; et al. Lactation and neonatal nutrition: Defining and refining the critical questions. J. Mammary Gland Biol. Neoplasia 2012, 17, 167–188. [Google Scholar] [CrossRef] [PubMed]
- Andreas, N.J.; Kampmann, B.; Mehring Le-Doare, K. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Truchet, S.; Honvo-Houéto, E. Physiology of milk secretion. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 367–384. [Google Scholar] [CrossRef] [PubMed]
- Nauta, A.J.; Garssen, J. Nutritional Programming of Immune Defense Against Infections in Early Life. In Pharma-Nutrition: An Overview; Folkerts, G., Garssen, J., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 113–126. [Google Scholar]
- Maertens, K.; De Schutter, S.; Braeckman, T.; Baerts, L.; Van Damme, P.; De Meester, I.; Leuridan, E. Breastfeeding after maternal immunisation during pregnancy: Providing immunological protection to the newborn: A review. Vaccine 2014, 32, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Cacho, N.T.; Lawrence, R.M. Innate Immunity and Breast Milk. Front. Immunol. 2017, 8, 584. [Google Scholar] [CrossRef]
- Molès, J.-P.; Tuaillon, E.; Kankasa, C.; Bedin, A.-S.; Nagot, N.; Marchant, A.; McDermid, J.M.; Van de Perre, P. Breastmilk cell trafficking induces microchimerism-mediated immune system maturation in the infant. Pediatr. Allergy Immunol. 2018, 29, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Jakaitis, B.M.; Denning, P.W. Human breast milk and the gastrointestinal innate immune system. Clin. Perinatol. 2014, 41, 423–435. [Google Scholar] [CrossRef]
- Telang, S. Lactoferrin: A Critical Player in Neonatal Host Defense. Nutrients 2018, 10, 1228. [Google Scholar] [CrossRef]
- Kosaka, N.; Izumi, H.; Sekine, K.; Ochiya, T. microRNA as a new immune-regulatory agent in breast milk. Silence 2010, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Zempleni, J.; Sukreet, S.; Zhou, F.; Wu, D.; Mutai, E. Milk-Derived Exosomes and Metabolic Regulation. Annu. Rev. Anim. Biosci. 2019, 7, 245–262. [Google Scholar] [CrossRef]
- Leiferman, A.; Shu, J.; Upadhyaya, B.; Cui, J.; Zempleni, J. Storage of Extracellular Vesicles in Human Milk, and MicroRNA Profiles in Human Milk Exosomes and Infant Formulas. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Boix-Amorós, A.; Collado, M.C.; Van’t Land, B.; Calvert, A.; Le Doare, K.; Garssen, J.; Hanna, H.; Khaleva, E.; Peroni, D.G.; Geddes, D.T.; et al. Reviewing the evidence on breast milk composition and immunological outcomes. Nutr. Rev. 2019, 77, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Shennan, D.B.; Peaker, M. Transport of Milk Constituents by the Mammary Gland. Physiol. Rev. 2000, 80, 925–951. [Google Scholar] [CrossRef] [PubMed]
- Montalbetti, N.; Dalghi, M.G.; Albrecht, C.; Hediger, M.A. Nutrient transport in the mammary gland: Calcium, trace minerals and water soluble vitamins. J. Mammary Gland Biol. Neoplasia 2014, 19, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Ventrella, D.; Forni, M.; Bacci, M.L.; Annaert, P. Non-clinical Models to Determine Drug Passage into Human Breast Milk. Curr. Pharm. Des. 2019, 25, 534–548. [Google Scholar] [CrossRef]
- Groneberg, D.A.; Döring, F.; Theis, S.; Nickolaus, M.; Fischer, A.; Daniel, H. Peptide transport in the mammary gland: Expression and distribution of PEPT2 mRNA and protein. Am. J. Physiol. Metab. 2015, 282, E1172–E1179. [Google Scholar] [CrossRef] [PubMed]
- Alcorn, J.; Lu, X.; Moscow, J.A.; McNamara, P.J. Transporter Gene Expression in Lactating and Nonlactating Human Mammary Epithelial Cells Using Real-Time Reverse Transcription-Polymerase Chain Reaction. J. Pharmacol. Exp. Ther. 2002, 303, 487–496. [Google Scholar] [CrossRef]
- Jonker, J.W.; Merino, G.; Musters, S.; Van Herwaarden, A.E.; Bolscher, E.; Wagenaar, E.; Mesman, E.; Dale, T.C.; Schinkel, A.H. The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat. Med. 2005, 11, 127–129. [Google Scholar] [CrossRef]
- Kwok, B.; Yamauchi, A.; Rajesan, R.; Chan, L.; Dhillon, U.; Gao, W.; Xu, H.; Wang, B.; Takahashi, S.; Semple, J.; et al. Carnitine/xenobiotics transporters in the human mammary gland epithelia, MCF12A. Am. J. Physiol. Integr. Comp. Physiol. 2006, 290, R793–R802. [Google Scholar] [CrossRef]
- Gilchrist, S.E.; Alcorn, J. Lactation stage-dependent expression of transporters in rat whole mammary gland and primary mammary epithelial organoids. Fundam. Clin. Pharmacol. 2010, 24, 205–214. [Google Scholar] [CrossRef]
- Kindla, J.; Rau, T.T.; Jung, R.; Fasching, P.A.; Strick, R.; Stoehr, R.; Hartmann, A.; Fromm, M.F.; König, J. Expression and localization of the uptake transporters OATP2B1, OATP3A1 and OATP5A1 in non-malignant and malignant breast tissue. Cancer Biol. Ther. 2011, 11, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Ito, K.; Ikebuchi, Y.; Kito, T.; Miyata, H.; Toyoda, Y.U.; Takada, T.; Hisaka, A.; Honma, M.; Oka, A.; et al. Organic cation transporter/solute carrier family 22a is involved in drug transfer into milk in mice. J. Pharm. Sci. 2014, 103, 3342–3348. [Google Scholar] [CrossRef] [PubMed]
- Lindner, S.; Halwachs, S.; Wassermann, L.; Honscha, W. Expression and subcellular localization of efflux transporter ABCG2/BCRP in important tissue barriers of lactating dairy cows, sheep and goats. J. Vet. Pharmacol. Ther. 2013, 36, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.H. B Vitamins in Breast Milk: Relative Importance of Maternal Status and Intake, and Effects on Infant Status and Function. Adv. Nutr. 2012, 3, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Shamir, R. Thiamine-deficient infant formula: What happened and what have we learned? Ann. Nutr. Metab. 2012, 60, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Harel, Y.; Zuk, L.; Guindy, M.; Nakar, O.; Lotan, D.; Fattal-Valevski, A. The effect of subclinical infantile thiamine deficiency on motor function in preschool children. Matern. Child Nutr. 2017, 13, e12397. [Google Scholar] [CrossRef] [PubMed]
- Sinai, T.; Goldberg, M.R.; Nachshon, L.; Amitzur-Levy, R.; Yichie, T.; Katz, Y.; Monsonego-Ornan, E.; Elizur, A. Reduced Final Height and Inadequate Nutritional Intake in Cow’s Milk-Allergic Young Adults. J. Allergy Clin. Immunol. Pract. 2019, 7, 509–515. [Google Scholar] [CrossRef]
- Ito, N.; Ito, K.; Ikebuchi, Y.; Toyoda, Y.; Takada, T.; Hisaka, A.; Oka, A.; Suzuki, H. Prediction of drug transfer into milk considering breast cancer resistance protein (BCRP)-mediated transport. Pharm. Res. 2015, 32, 2527–2537. [Google Scholar] [CrossRef]
- Yagdiran, Y.; Oskarsson, A.; Knight, C.H.; Tallkvist, J. ABC- and SLC-transporters in murine and bovine mammary epithelium—Effects of prochloraz. PLoS ONE 2016, 11, e0151904. [Google Scholar] [CrossRef]
- Leslie, E.M.; Deeley, R.G.; Cole, S.P.C. Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol. Appl. Pharmacol. 2005, 204, 216–237. [Google Scholar] [CrossRef]
- Van Herwaarden, A.E.; Schinkel, A.H. The function of breast cancer resistance protein in epithelial barriers, stem cells and milk secretion of drugs and xenotoxins. Trends Pharmacol. Sci. 2006, 27, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Schrickx, J.A.; Fink-Gremmels, J. Implications of ABC transporters on the disposition of typical veterinary medicinal products. Eur. J. Pharmacol. 2008, 585, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Virkel, G.; Ballent, M.; Lanusse, C.; Lifschitz, A. Role of ABC Transporters in Veterinary Medicine: Pharmaco- Toxicological Implications. Curr. Med. Chem. 2018, 26, 1251–1269. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Wentrup, F.; Karbach, U.; Gorboulev, V.; Arndt, P.; Koepsell, H. Membrane localization of the electrogenic cation transporter rOCT1 in rat liver. Biochem. Biophys. Res. Commun. 1998, 248, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Karbach, U.; Kricke, J.; Meyer-Wentrup, F.; Gorboulev, V.; Volk, C.; Loffing-Cueni, D.; Kaissling, B.; Bachmann, S.; Koepsell, H. Localization of organic cation transporters OCT1 and OCT2 in rat kidney. Am. J. Physiol. Physiol. 2000, 279, F679–F687. [Google Scholar] [CrossRef] [PubMed]
- Tamai, I.; Nakanishi, T.; Kobayashi, D.; China, K.; Kosugi, Y.; Nezu, J.; Sai, Y.; Tsuji, A. Involvement of OCTN1 (SLC22A4) in pH-dependent transport of organic cations. Mol. Pharm. 2004, 1, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Lips, K.S.; Metzner, L.; Neubert, R.H.H.; Koepsell, H.; Brandsch, M. Drug specificity and intestinal membrane localization of human organic cation transporters (OCT). Biochem. Pharmacol. 2005, 70, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Glaeser, H.; Smith, L.H.; Roberts, R.L.; Moeckel, G.W.; Gervasini, G.; Leake, B.F.; Kim, R.B. Polymorphisms in human organic anion-transporting polypeptide 1A2 (OATP1A2): Implications for altered drug disposition and central nervous system drug entry. J. Biol. Chem. 2005, 280, 9610–9617. [Google Scholar] [CrossRef]
- Kobayashi, D.; Nozawa, T.; Imai, K.; Nezu, J.; Tsuji, A.; Tamai, I. Involvement of human organic anion transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport across intestinal apical membrane. J. Pharmacol. Exp. Ther. 2003, 306, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Nies, A.T.; Jedlitschky, G.; König, J.; Herold-Mende, C.; Steiner, H.H.; Schmitt, H.-P.; Keppler, D. Expression and immunolocalization of the multidrug resistance proteins, MRP1-MRP6 (ABCC1-ABCC6), in human brain. Neuroscience 2004, 129, 349–360. [Google Scholar] [CrossRef]
- Grube, M.; Köck, K.; Oswald, S.; Draber, K.; Meissner, K.; Eckel, L.; Böhm, M.; Felix, S.B.; Vogelgesang, S.; Jedlitschky, G.; et al. Organic anion transporting polypeptide 2B1 is a high-affinity transporter for atorvastatin and is expressed in the human heart. Clin. Pharmacol. Ther. 2006, 80, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Aye, I.L.M.H.; Paxton, J.W.; Evseenko, D.A.; Keelan, J.A. Expression, localisation and activity of ATP binding cassette (ABC) family of drug transporters in human amnion membranes. Placenta 2007, 28, 868–877. [Google Scholar] [CrossRef]
- Aleksunes, L.M.; Cui, Y.; Klaassen, C.D. Prominent Expression of Xenobiotic Efflux Transporters in Mouse Extraembryonic Fetal Membranes Compared with Placenta. Drug Metab. Dispos. 2008, 36, 1960–1970. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.M.; Black, D.S.; Raman, C.; Woodford, K.; Zhou, M.; Haggerty, J.E.; Yan, A.T.; Cwirla, S.E.; Grindstaff, K.K. Subcellular localization of transporters along the rat blood–brain barrier and blood–cerebral-spinal fluid barrier by in vivo biotinylation. Neuroscience 2008, 155, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, C.D.; Aleksunes, L.M. Xenobiotic, Bile Acid, and Cholesterol Transporters: Function and Regulation. Pharmacol. Rev. 2010, 62, 1–96. [Google Scholar] [CrossRef] [PubMed]
- McNamara, P.J.; Abbassi, M. Neonatal exposure to drugs in breast milk. Pharm. Res. 2004, 21, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.O. Drugs in Lactation. Pharm. Res. 2018, 35, 45. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, H.C.; Begg, E.J. Relationship between human milk lipid-ultrafiltrate and octanol-water partition coefficients. J. Pharm. Sci. 1988, 77, 796–798. [Google Scholar] [CrossRef] [PubMed]
- Verstegen, R.H.J.; Ito, S. Drugs in lactation. J. Obstet. Gynaecol. Res. 2019, 45, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Fleishaker, J.C. Models and methods for predicting drug transfer into human milk. Adv. Drug Deliv. Rev. 2003, 55, 643–652. [Google Scholar] [CrossRef]
- Lepist, E.I.; Ray, A.S. Beyond drug-drug interactions: Effects of transporter inhibition on endobiotics, nutrients and toxins. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA) Multicriteria-Based Ranking Model for Risk Management of Animal Drug Residues in Milk and Milk Products. Available online: https://www.fda.gov/downloads/Food/FoodScienceResearch/RiskSafetyAssessment/UCM444035.pdf (accessed on 16 July 2019).
- European Food Safety Authority (EFSA). Report for 2016 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. EFSA Support. Publ. 2018, 15, 1358. [Google Scholar]
- Davanzo, R.; Bua, J.; De Cunto, A.; Farina, M.L.; De Ponti, F.; Clavenna, A.; Mandrella, S.; Sagone, A.; Clementi, M. Advising Mothers on the Use of Medications during Breastfeeding: A Need for a Positive Attitude. J. Hum. Lact. 2016, 32, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Ling, B.; Alcorn, J. Lactation stage influences drug milk-to-serum values and neonatal exposure risk. Int. J. Toxicol. 2010, 29, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Poppenga, R.H. Commercial and Industrial Chemical Hazards for Ruminants. Vet. Clin. North Am. Food Anim. Pract. 2011, 27, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, C.Y.-T.; Kong, A.-N.T. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharm. Res. 2005, 28, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Phillips, D.H. Mammary expression of xenobiotic metabolizing enzymes and their potential role in breast cancer. Cancer Res. 2000, 60, 4667–4677. [Google Scholar] [PubMed]
- Muskhelishvili, L.; Thompson, P.A.; Kusewitt, D.F.; Wang, C.; Kadlubar, F.F. In situ hybridization and immunohistochemical analysis of cytochrome P450 1B1 expression in human normal tissues. J. Histochem. Cytochem. 2001, 49, 229–236. [Google Scholar] [CrossRef]
- Bieche, I.; Asselah, T.; Vacher, S.; Marcellin, P.; Lidereau, R.; Beaune, P.; Waziers, I. De Reverse transcriptase-PCR quantification of mRNA levels from cytochrome (CYP)1, CYP2 and CYP3 families in 22 different human tissues. Pharmacogenet. Genom. 2007, 17, 731–742. [Google Scholar] [CrossRef]
- Lin, Y.; Yao, Y.; Liu, S.; Wang, L.; Moorthy, B.; Xiong, D.; Cheng, T.; Ding, X.; Gu, J. Role of mammary epithelial and stromal P450 enzymes in the clearance and metabolic activation of 7,12- dimethylbenz(a)anthracene in mice. Toxicol. Lett. 2012, 212, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Ebert, B.; Seidel, A.; Lampen, A. Identification of BCRP as transporter of benzo[a]pyrene conjugates metabolically formed in Caco-2 cells and its induction by Ah-receptor agonists. Carcinogenesis 2005, 26, 1754–1763. [Google Scholar] [CrossRef] [PubMed]
- Anapolsky, A.; Teng, S.; Dixit, S.; Piquette-Miller, M. The role of pregnane X receptor in 2-acetylaminofluorene-mediated induction of drug transport and -metabolizing enzymes in mice. Drug Metab. Dispos. 2006, 34, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wang, Q.; Bircsak, K.M.; Wen, X.; Aleksunes, L.M. In Vitro Screening of Environmental Chemicals Identifies Zearalenone as a Novel Substrate of the Placental BCRP/ABCG2 Transporter. Toxicol. Res. 2015, 4, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Hu, D.; Li, Y. Effects of zearalenone on mRNA expression and activity of cytochrome P450 1A1 and 1B1 in MCF-7 cells. Ecotoxicol. Environ. Saf. 2004, 58, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Akamine, Y.; Yasui-Furukori, N.; Uno, T. Drug-Drug Interactions of P-gp Substrates Unrelated to CYP Metabolism. Curr. Drug Metab. 2019, 20, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Nies, A.T.; Schwab, M.; Keppler, D. Interplay of conjugating enzymes with OATP uptake transporters and ABCC/MRP efflux pumps in the elimination of drugs. Expert Opin. Drug Metab. Toxicol. 2008, 4, 545–568. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.V.; El-Kattan, A.F. Transporter-Enzyme Interplay: Deconvoluting Effects of Hepatic Transporters and Enzymes on Drug Disposition Using Static and Dynamic Mechanistic Models. J. Clin. Pharmacol. 2016, S99–S109. [Google Scholar] [CrossRef]
- Fritz, A.; Busch, D.; Lapczuk, J.; Ostrowski, M.; Drozdzik, M.; Oswald, S. Expression of clinically relevant drug-metabolizing enzymes along the human intestine and their correlation to drug transporters and nuclear receptors: An intra-subject analysis. Basic Clin. Pharmacol. Toxicol. 2019, 124, 245–255. [Google Scholar] [CrossRef]
- Tamai, I.; Nezu, J.; Uchino, H.; Sai, Y.; Oku, A.; Shimane, M.; Tsuji, A. Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem. Biophys. Res. Commun. 2000, 273, 251–260. [Google Scholar] [CrossRef]
- Kullak-Ublick, G.A.; Ismair, M.G.; Stieger, B.; Landmann, L.; Huber, R.; Pizzagalli, F.; Fattinger, K.; Meier, P.J.; Hagenbuch, B. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology 2001, 120, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, F.; Varga, Z.; Huber, R.D.; Folkers, G.; Meier, P.J.; St.-Pierre, M.V. Identification of steroid sulfate transport processes in the human mammary gland. J. Clin. Endocrinol. Metab. 2003, 88, 3902–3912. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.; Zhu, L.; Madigan, M.C.; Wang, K.; Shen, W.; Gillies, M.C.; Zhou, F. Human organic anion transporting polypeptide 1A2 (OATP1A2) mediates cellular uptake of all-trans-retinol in human retinal pigmented epithelial cells. Br. J. Pharmacol. 2015, 172, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.S.R.; da Silva Ribeiro, K.D.; Pires, J.F.; Bezerra, D.F.; Bellot, P.E.N.R.; de Oliveira Weigert, L.P.; Dimenstein, R. Breast milk retinol concentration in mothers of preterm newborns. Early Hum. Dev. 2017, 106–107, 41–45. [Google Scholar] [CrossRef]
- Kullak-Ublick, G.A.; Fisch, T.; Oswald, M.; Hagenbuch, B.; Meier, P.J.; Beuers, U.; Paumgartner, G. Dehydroepiandrosterone sulfate (DHEAS): Identification of a carrier protein in human liver and brain. FEBS Lett. 1998, 424, 173–176. [Google Scholar] [CrossRef]
- Grube, M.; Hagen, P.; Jedlitschky, G. Neurosteroid transport in the brain: Role of ABC and SLC transporters. Front. Pharmacol. 2018, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Grube, M.; Kock, K.; Karner, S.; Reuther, S.; Ritter, C.A.; Jedlitschky, G.; Kroemer, H.K. Modification of OATP2B1-Mediated Transport by Steroid Hormones. Mol. Pharmacol. 2006, 70, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Vavricka, S.R.; Meier, P.J.; Stieger, B. Differential cellular expression of organic anion transporting peptides OATP1A2 and OATP2B1 in the human retina and brain: Implications for carrier-mediated transport of neuropeptides and neurosteriods in the CNS. Pflugers Arch. Eur. J. Physiol. 2015, 467, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Kullak-Ublick, G.A.; Hagenbuch, B.; Stieger, B.; Schteingart, C.D.; Hofmann, A.F.; Wolkoff, A.W.; Meier, P.J. Molecular and functional characterization of an organic anion transporting polypeptide cloned from human liver. Gastroenterology 1995, 109, 1274–1282. [Google Scholar] [CrossRef]
- Hagenbuch, B.; Stieger, B. The SLCO (former SLC21) superfamily of transporters. Mol. Asp. Med. 2013, 34, 396–412. [Google Scholar] [CrossRef]
- Kovacsics, D.; Patik, I.; Özvegy-Laczka, C. The role of organic anion transporting polypeptides in drug absorption, distribution, excretion and drug-drug interactions. Expert Opin. Drug Metab. Toxicol. 2017, 13, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Wagenaar, E.; Xu, W.; Huang, K.; Schinkel, A.H. Ochratoxin A transport by the human breast cancer resistance protein (BCRP), multidrug resistance protein 2 (MRP2), and organic anion-transporting polypeptides 1A2, 1B1 and 2B1. Toxicol. Appl. Pharmacol. 2017, 329, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.; Obaidat, A.; Hagenbuch, B. OATPs, OATs and OCTs: The organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br. J. Pharmacol. 2012, 165, 1260–1287. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Moriyama, C.; Ito, N.; Zhang, X.; Hachiuma, K.; Hagima, N.; Iwata, K.; Yamaguchi, J.I.; Maeda, K.; Ito, K.; et al. Involvement of organic cation transporters in the clearance and milk secretion of thiamine in mice. Pharm. Res. 2015, 32, 2192–2204. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.E.; Quester, S.; Ulzheimer, J.C.; Gorboulev, V.; Akhoundova, A.; Waldegger, S.; Lang, F.; Koepsell, H. Monoamine neurotransmitter transport mediated by the polyspecific cation transporter rOCT1. FEBS Lett. 1996, 395, 153–156. [Google Scholar] [CrossRef]
- Breidert, T.; Spitzenberger, F.; Gründemann, D.; Schömig, E. Catecholamine transport by the organic cation transporter type 1 (OCT1). Br. J. Pharmacol. 1998, 125, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Sala-Rabanal, M.; Li, D.C.; Dake, G.R.; Kurata, H.T.; Inyushin, M.; Skatchkov, S.N.; Nichols, C.G. Polyamine Transport by the Polyspecific Organic Cation Transporters OCT1, OCT2 and OCT3. Mol. Pharm. 2013, 10, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Wessler, I.; Herschel, S.; Bittinger, F.; Kirkpatrick, C.J. Release of non-neuronal acetylcholine from the isolated human placenta is affected by antidepressants. Life Sci. 2007, 80, 2210–2213. [Google Scholar] [CrossRef]
- Lips, K.S.; Volk, C.; Schmitt, B.M.; Pfeil, U.; Arndt, P.; Miska, D.; Ermert, L.; Kummer, W.; Koepsell, H. Polyspecific cation transporters mediate luminal release of acetylcholine from bronchial epithelium. Am. J. Respir. Cell Mol. Biol. 2005, 33, 79–88. [Google Scholar] [CrossRef]
- Kimura, H.; Takeda, M.; Narikawa, S.; Enomoto, A.; Ichida, K. Human Organic Anion Transporters and Human Organic Cation Transporters Mediate Renal Transport of Prostaglandins. J. Pharmacol. Exp. Ther. 2002, 301, 293–298. [Google Scholar] [CrossRef]
- Harlfinger, S.; Fork, C.; Lazar, A.; Schömig, E.; Gründemann, D. Are organic cation transporters capable of transporting prostaglandins? Naunyn-Schmiedebergs Arch. Pharmacol. 2005, 372, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Nelson, J.A. Role of organic cation transporters in the renal secretion of nucleosides. Biochem. Pharmacol. 2000, 60, 215–219. [Google Scholar] [CrossRef]
- Oo, C.Y.; Kuhn, R.J.; Desai, N.; McNamara, P.J. Active transport of cimetidine into human milk. Clin. Pharmacol. Ther. 1995, 58, 548–555. [Google Scholar] [CrossRef]
- Gerk, P.M.; Oo, C.Y.; Paxton, E.W.; Moscow, J.A.; McNamara, P.J. Interactions between cimetidine, nitrofurantoin, and probenecid active transport into rat milk. J. Pharmacol. Exp. Ther. 2001, 296, 175–180. [Google Scholar] [PubMed]
- Alcorn, J.; McNamara, P.J. Acyclovir, Ganciclovir, and Zidovudine Transfer into Rat Milk. Antimicrob. Agents Chemother. 2002, 46, 1831–1836. [Google Scholar] [CrossRef] [PubMed]
- Elimrani, I.; Lahjouji, K.; Seidman, E.; Roy, M.-J.; Mitchell, G.A.; Qureshi, I. Expression and localization of organic cation/carnitine transporter OCTN2 in Caco-2 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G863–G871. [Google Scholar] [CrossRef] [PubMed]
- Lahjouji, K.; Elimrani, I.; Lafond, J.; Leduc, L.; Qureshi, I.A.; Mitchell, G.A. l-Carnitine transport in human placental brush-border membranes is mediated by the sodium-dependent organic cation transporter OCTN2. Am. J. Physiol. Cell Physiol. 2004, 287, C263–C269. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lamhonwah, A.-M.; Mai, L.; Chung, C.; Lamhonwah, D.; Ackerley, C.; Tein, I. Upregulation of mammary gland OCTNs maintains carnitine homeostasis in suckling infants. Biochem. Biophys. Res. Commun. 2011, 404, 1010–1015. [Google Scholar] [CrossRef]
- Shennan, D.B.; Grant, A.; Ramsay, R.R.; Burns, C.; Zammit, V.A. Characteristics of l-carnitine transport by lactating rat mammary tissue. Biochim. Biophys. Acta 1998, 1393, 49–56. [Google Scholar] [CrossRef]
- Grundemann, D.; Harlfinger, S.; Golz, S.; Geerts, A.; Lazar, A.; Berkels, R.; Jung, N.; Rubbert, A.; Schomig, E.; Gründemann, D.; et al. Discovery of the ergothioneine transporter. Proc. Natl. Acad. Sci. USA 2005, 102, 5256–5261. [Google Scholar] [CrossRef]
- Drenberg, C.D.; Gibson, A.A.; Pounds, S.B.; Shi, L.; Rhinehart, D.P.; Li, L.; Hu, S.; Du, G.; Nies, A.T.; Schwab, M.; et al. OCTN1 is a high-affinity carrier of nucleoside analogs. Cancer Res. 2017, 77, 2102–2111. [Google Scholar] [CrossRef]
- Tschirka, J.; Kreisor, M.; Betz, J.; Gründemann, D. Substrate selectivity check of the ergothioneine transporter. Drug Metab. Dispos. 2018, 46, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Young, J.D.; Yao, S.Y.M.; Baldwin, J.M.; Cass, C.E.; Baldwin, S.A. The human concentrative and equilibrative nucleoside transporter families, SLC28 and SLC29. Mol. Asp. Med. 2013, 34, 529–547. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Anglada, M.; Cano-Soldado, P.; Errasti-Murugarren, E.; Casado, F.J. SLC28 genes and concentrative nucleoside transporter (CNT) proteins. Xenobiotica 2008, 38, 972–994. [Google Scholar] [CrossRef] [PubMed]
- Shennan, D.B.; Calvert, D.T.; Backwell, F.R.C.; Boyd, C.A.R. Peptide aminonitrogen transport by the lactating rat mammary gland. Biochim. Biophys. Acta—Biomembr. 1998, 1373, 252–260. [Google Scholar] [CrossRef]
- Bürzle, M.; Suzuki, Y.; Ackermann, D.; Miyazaki, H.; Maeda, N.; Clémençon, B.; Burrier, R.; Hediger, M.A. The sodium-dependent ascorbic acid transporter family SLC23. Mol. Asp. Med. 2013, 34, 436–454. [Google Scholar] [CrossRef] [PubMed]
- Tsukaguchi, H.; Tokui, T.; Mackenzie, B.; Berger, U.V.; Chen, X.-Z.; Wang, Y.; Brubaker, R.F.; Hediger, M.A. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature 1999, 399, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Farke, C.; Meyer, H.H.D.; Bruckmaier, R.M.; Albrecht, C. Differential expression of ABC transporters and their regulatory genes during lactation and dry period in bovine mammary tissue. J. Dairy Res. 2008, 75, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Mealey, K.L. ABCG2 transporter: Therapeutic and physiologic implications in veterinary species. J. Vet. Pharmacol. Ther. 2012, 35, 105–112. [Google Scholar] [CrossRef]
- Jani, M.; Ambrus, C.; Magnan, R.; Tauberné, K.; Erzsébet, J.; Zolnerciks, J.K.; Krajcsi, P. Structure and function of BCRP, a broad specificity transporter of xenobiotics and endobiotics. Arch. Toxicol. 2014, 88, 1205–1248. [Google Scholar] [CrossRef] [PubMed]
- Jedlitschky, G.; Burchell, B.; Keppler, D. The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J. Biol. Chem. 2000, 275, 30069–30074. [Google Scholar] [CrossRef] [PubMed]
- Laue, S.; Winterhoff, M.; Kaever, V.; van den Heuvel, J.J.; Russel, F.G.; Seifert, R. cCMP is a substrate for MRP5. Naunyn-Schmiedebergs. Arch. Pharmacol. 2014, 387, 893–895. [Google Scholar] [CrossRef] [PubMed]
- Wielinga, P.R.; van der Heijden, I.; Reid, G.; Beijnen, J.H.; Wijnholds, J.; Borst, P. Characterization of the MRP4- and MRP5-mediated transport of cyclic nucleotides from intact cells. J. Biol. Chem. 2003, 278, 17664–17671. [Google Scholar] [CrossRef] [PubMed]
- Schulz, T.; Schumacher, U.; Prehm, P. Hyaluronan export by the ABC transporter MRP5 and its modulation by intracellular cGMP. J. Biol. Chem. 2007, 282, 20999–21004. [Google Scholar] [CrossRef]
- van Herwaarden, A.E.; Wagenaar, E.; Merino, G.; Jonker, J.W.; Rosing, H.; Beijnen, J.H.; Schinkel, A.H. Multidrug Transporter ABCG2/Breast Cancer Resistance Protein Secretes Riboflavin (Vitamin B2) into Milk. Mol. Cell. Biol. 2007, 27, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Volk, E.L.; Rohde, K.; Rhee, M.; McGuire, J.J.; Doyle, L.A.; Ross, D.D.; Schneider, E. Methotrexate cross-resistance in a mitoxantrone-selected multidrug—Resistant MCF7 breast cancer cell line is attributable to enhanced energy- dependent drug efflux. Cancer Res. 2000, 60, 3514–3521. [Google Scholar]
- Chen, Z.S.; Robey, R.W.; Belinsky, M.G.; Shchaveleva, I.; Ren, X.Q.; Sugimoto, Y.; Ross, D.D.; Bates, S.E.; Kruh, G.D. Transport of methotrexate, methotrexate polyglutamates, and 17β-estradiol 17-(β-D-glucuronide) by ABCG2: Effects of acquired mutations at R482 on methotrexate transport. Cancer Res. 2003, 63, 4048–4054. [Google Scholar] [PubMed]
- Wielinga, P.; Hooijberg, J.H.; Gunnarsdottir, S.; Kathmann, I.; Reid, G.; Zelcer, N.; van der Born, K.; de Haas, M.; van der Heijden, I.; Kaspers, G.; et al. The human multidrug resistance protein MRP5 transports folates and can mediate cellular resistance against antifolates. Cancer Res. 2005, 65, 4425–4430. [Google Scholar] [CrossRef]
- Shukla, S.; Wu, C.-P.; Nandigama, K.; Ambudkar, S.V. The naphthoquinones, vitamin K3 and its structural analog plumbagin, are substrates of the multidrug resistance-linked ABC drug transporter ABCG2. Mol. Cancer Ther. 2007, 6, 3279–3286. [Google Scholar] [CrossRef]
- Imai, Y.; Asada, S.; Tsukahara, S.; Ishikawa, E.; Tsuruo, T.; Sugimoto, Y. Breast Cancer Resistance Protein Exports Sulfated Estrogens but Not Free Estrogens. Mol. Pharmacol. 2003, 64, 610–618. [Google Scholar] [CrossRef]
- Suzuki, M.; Suzuki, H.; Sugimoto, Y.; Sugiyama, Y. ABCG2 transports sulfated conjugates of steroids and xenobiotics. J. Biol. Chem. 2003, 278, 22644–22649. [Google Scholar] [CrossRef] [PubMed]
- Grube, M.; Reuther, S.; Meyer Zu Schwabedissen, H.; Köck, K.; Draber, K.; Ritter, C.A.; Fusch, C.; Jedlitschky, G.; Kroemer, H.K. Organic anion transporting polypeptide 2B1 and breast cancer resistance protein interact in the transepithelial transport of steroid sulfates in human placenta. Drug Metab. Dispos. 2007, 35, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, E.; Deng, F.; Kidron, H.; Finel, M. Efflux transport of estrogen glucuronides by human MRP2, MRP3, MRP4 and BCRP. J. Steroid Biochem. Mol. Biol. 2018, 178, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Blazquez, A.M.G.; Macias, R.I.R.; Cives-Losada, C.; De La Iglesia, A.; Marin, J.J.G.; Monte, M.J. Lactation during cholestasis: Role of ABC proteins in bile acid traffic across the mammary gland. Sci. Rep. 2017, 7, 7475. [Google Scholar] [CrossRef] [PubMed]
- Parikh, M.; Maddaford, T.G.; Austria, J.A.; Aliani, M.; Netticadan, T.; Pierce, G.N. Dietary Flaxseed as a Strategy for Improving Human Health. Nutrients 2019, 11, 1171. [Google Scholar] [CrossRef]
- Miguel, V.; Otero, J.A.; García-Villalba, R.; Tomás-Barberán, F.; Espín, J.C.; Merino, G.; Álvarez, A.I. Role of ABCG2 in transport of the mammalian lignan enterolactone and its secretion into milk in abcg2 knockout mice. Drug Metab. Dispos. 2014, 42, 943–946. [Google Scholar] [CrossRef]
- García-Mateos, D.; García-Villalba, R.; Marañón, J.A.; Espín, J.C.; Merino, G.; Álvarez, A.I.; Angel, J.; Carlos, J.; Merino, G.; Álvarez, A.I. The Breast Cancer Resistance Protein (BCRP/ABCG2) influences the levels of enterolignans and their metabolites in plasma, milk and mammary gland. J. Funct. Foods 2017, 35, 648–654. [Google Scholar] [CrossRef]
- Merino, G.; Álvarez, A.I.; Pulido, M.M.; Molina, A.J.; Schinkel, A.H.; Prieto, J.G. Breast cancer resistance protein (BCRP/ABCG2) transports fluoroquinolone antibiotics and affects their oral availability, pharmacokinetics, and milk secretion. Drug Metab. Dispos. 2006, 34, 690–695. [Google Scholar] [CrossRef]
- Real, R.; Egido, E.; Pérez, M.; González-Lobato, L.; Barrera, B.; Prieto, J.G.; Álvarez, A.I.; Merino, G. Involvement of breast cancer resistance protein (BCRP/ABCG2) in the secretion of danofloxacin into milk: Interaction with ivermectin. J. Vet. Pharmacol. Ther. 2011, 34, 313–321. [Google Scholar] [CrossRef]
- Pulido, M.M.; Molina, A.J.; Merino, G.; Mendoza, G.; Prieto, J.G.; Alvarez, A.I. Interaction of enrofloxacin with breast cancer resistance protein (BCRP/ABCG2): Influence of flavonoids and role in milk secretion in sheep. J. Vet. Pharmacol. Ther. 2006, 29, 279–287. [Google Scholar] [CrossRef]
- Perez, M.; Otero, J.A.; Barrera, B.; Prieto, J.G.; Merino, G.; Alvarez, A.I. Inhibition of ABCG2/BCRP transporter by soy isoflavones genistein and daidzein: Effect on plasma and milk levels of danofloxacin in sheep. Vet. J. 2013, 196, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Otero, J.A.; García-Mateos, D.; Alvarez-Fernández, I.; García-Villalba, R.; Espín, J.C.; Álvarez, A.I.; Merino, G. Flaxseed-enriched diets change milk concentration of the antimicrobial danofloxacin in sheep. BMC Vet. Res. 2018, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Merino, G. The Breast Cancer Resistance Protein (BCRP/ABCG2) Affects Pharmacokinetics, Hepatobiliary Excretion, and Milk Secretion of the Antibiotic Nitrofurantoin. Mol. Pharmacol. 2005, 67, 1758–1764. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Leggas, M.; Goswami, M.; Empey, P.E.; McNamara, P.J. N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]-phenyl)-9, 10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide (GF120918) as a chemical ATP-binding cassette transporter family G member 2 (Abcg2) knockout model to study nitrofurantoin transf. Drug Metab. Dispos. 2008, 36, 2591–2596. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.; Real, R.; Mendoza, G.; Merino, G.; Prieto, J.G.; Alvarez, A.I. Milk secretion of nitrofurantoin, as a specific BCRP/ABCG2 substrate, in assaf sheep: Modulation by isoflavones1. J. Vet. Pharmacol. Ther. 2009, 32, 498–502. [Google Scholar] [CrossRef]
- Perez, M.; Blazquez, A.G.; Real, R.; Mendoza, G.; Prieto, J.G.; Merino, G.; Alvarez, A.I. In vitro and in vivo interaction of moxidectin with BCRP/ABCG2. Chem. Biol. Interact. 2009, 180, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Barrera, B.; González-Lobato, L.; Otero, J.A.; Real, R.; Prieto, J.G.; Álvarez, A.I.; Merino, G. Effects of triclabendazole on secretion of danofloxacin and moxidectin into the milk of sheep: Role of triclabendazole metabolites as inhibitors of the ruminant ABCG2 transporter. Vet. J. 2013, 198, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Lagas, J.S.; van der Kruijssen, C.M.; van de Wetering, K.; Beijnen, J.H.; Schinkel, A.H. Transport of Diclofenac by Breast Cancer Resistance Protein (ABCG2) and Stimulation of Multidrug Resistance Protein 2 (ABCC2)-Mediated Drug Transport by Diclofenac and Benzbromarone. Drug Metab. Dispos. 2009, 37, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mateos, D.; Garcia-Lino, A.M.; Alvarez-Fernandez, I.; Blanco-Paniagua, E.; de la Fuente, A.; Alvarez, A.I.; Merino, G. Role of ABCG2 in secretion into milk of the anti-inflammatory flunixin and its main metabolite: In vitro-in vivo correlation in mice and cows. Drug Metab. Dispos. 2019, 47, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; McNamara, P.J. Stereoselective interaction of pantoprazole with ABCG2. I. Drug accumulation in rat milk. Drug Metab. Dispos. 2012, 40, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Van Herwaarden, A.E.; Wagenaar, E.; Karnekamp, B.; Merino, G.; Jonker, J.W.; Schinkel, A.H. Breast cancer resistance protein (Bcrp1/Abcg2) reduces systemic exposure of the dietary carcinogens aflatoxin B1, IQ and Trp-P-1 but also mediates their secretion into breast milk. Carcinogenesis 2006, 27, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Dornetshuber, R.; Heffeter, P.; Sulyok, M.; Schumacher, R.; Chiba, P.; Kopp, S.; Koellensperger, G.; Micksche, M.; Lemmens-Gruber, R.; Berger, W. Interactions between ABC-transport proteins and the secondary Fusarium metabolites enniatin and beauvericin. Mol. Nutr. Food Res. 2009, 53, 904–920. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, J.T.; Gorczyca, L.; Brinker, A.; Buckley, B. Placental BCRP/ABCG2 transporter prevents fetal exposure to the estrogenic mycotoxi zerealenone. Toxicol. Sci. 2019, 168, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Pavek, P.; Merino, G.; Wagenaar, E.; Bolscher, E.; Novotna, M.; Jonker, J.W.; Schinkel, A.H. Human Breast Cancer Resistance Protein: Interactions with Steroid Drugs, Hormones, the Dietary Carcinogen 2-Amino-1-methyl-6-phenylimidazo(4,5-b)pyridine, and Transport of Cimetidine. J. Pharmacol. Exp. Ther. 2005, 312, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Mazur, C.S.; Marchitti, S.A.; Dimova, M.; Kenneke, J.F.; Lumen, A.; Fisher, J. Human and rat ABC transporter efflux of bisphenol a and bisphenol a glucuronide: Interspecies comparison and implications for pharmacokinetic assessment. Toxicol. Sci. 2012, 128, 317–325. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dankers, A.C.A.; Roelofs, M.J.E.; Piersma, A.H.; Sweep, F.C.G.J.; Russel, F.G.M.; van den Berg, M.; van Duursen, M.B.M.; Masereeuw, R. Endocrine disruptors differentially target ATP-binding cassette transporters in the blood-testis barrier and affect leydig cell testosterone secretion in vitro. Toxicol. Sci. 2013, 136, 382–391. [Google Scholar] [CrossRef]
- Halwachs, S.; Schäfer, I.; Kneuer, C.; Seibel, P.; Honscha, W. Assessment of ABCG2-mediated transport of pesticides across the rabbit placenta barrier using a novel MDCKII in vitro model. Toxicol. Appl. Pharmacol. 2016, 27, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Otero, J.A.; Miguel, V.; González-Lobato, L.; García-Villalba, R.; Espín, J.C.; Prieto, J.G.; Merino, G.; Álvarez, A.I. Effect of bovine ABCG2 polymorphism Y581S SNP on secretion into milk of enterolactone, riboflavin and uric acid. Animal 2015, 10, 238–247. [Google Scholar] [CrossRef]
- Otero, J.A.; García-Mateos, D.; de la Fuente, A.; Prieto, J.G.; Álvarez, A.I.; Merino, G. Effect of bovine ABCG2 Y581S polymorphism on concentrations in milk of enrofloxacin and its active metabolite ciprofloxacin. J. Dairy Sci. 2016, 99, 5731–5738. [Google Scholar] [CrossRef]
- Otero, J.A.; Barrera, B.; de la Fuente, A.; Prieto, J.G.; Marqués, M.; Álvarez, A.I.; Merino, G. Short communication: The gain-of-function Y581S polymorphism of the ABCG2 transporter increases secretion into milk of danofloxacin at the therapeutic dose for mastitis treatment. J. Dairy Sci. 2015, 98, 312–317. [Google Scholar] [CrossRef]
- Malfará, B.N.; de Lima Benzi, J.R.; de Oliveira Filgueira, G.C.; Zanelli, C.F.; Duarte, G.; de Carvalho Cavalli, R.; de Moraes, N.V. ABCG2 c.421C>A polymorphism alters nifedipine transport to breast milk in hypertensive breastfeeding women. Reprod. Toxicol. 2019, 85, 1–5. [Google Scholar]
- Zhou, F.; Zheng, J.; Zhu, L.; Jodal, A.; Cui, P.H.; Wong, M.; Gurney, H.; Church, W.B.; Murray, M. Functional Analysis of Novel Polymorphisms in the Human SLCO1A2 Gene that Encodes the Transporter OATP1A2. AAPS J. 2013, 15, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Mougey, E.B.; Feng, H.; Castro, M.; Irvin, C.G.; Lima, J.J. Absorption of montelukast is transporter mediated: A common variant of OATP2B1 is associated with reduced plasma concentrations and poor response. Pharm. Genom. 2009, 19, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Imanaga, J.; Kotegawa, T.; Imai, H.; Tsutsumi, K.; Yoshizato, T.; Ohyama, T.; Shirasaka, Y.; Tamai, I.; Tateishi, T.; Ohashi, K. The effects of the SLCO2B1 c.1457C > T polymorphism and apple juice on the pharmacokinetics of fexofenadine and midazolam in humans. Pharm. Genom. 2011, 21, 84–93. [Google Scholar] [CrossRef]
- Kashihara, Y.; Ieiri, I.; Yoshikado, T.; Maeda, K.; Fukae, M.; Kimura, M.; Hirota, T.; Matsuki, S.; Irie, S.; Izumi, N.; et al. Small-Dosing Clinical Study: Pharmacokinetic, Pharmacogenomic (SLCO2B1 and ABCG2), and Interaction (Atorvastatin and Grapefruit Juice) Profiles of 5 Probes for OATP2B1 and BCRP. J. Pharm. Sci. 2017, 106, 2688–2694. [Google Scholar] [CrossRef] [PubMed]
- Sakata, T.; Anzai, N.; Shin, H.J.; Noshiro, R.; Hirata, T.; Yokoyama, H.; Kanai, Y.; Endou, H. Novel single nucleotide polymorphisms of organic cation transporter 1 (SLC22A1) affecting transport functions. Biochem. Biophys. Res. Commun. 2004, 313, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, A.; Motohashi, H.; Okuda, M.; Inui, K.-I. Decreased function of genetic variants, Pro283Leu and Arg287Gly, in human organic cation transporter hOCT1. Drug Metab. Pharmacokinet. 2003, 18, 409–412. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Balyan, R.; Zhang, X.; Chidambaran, V.; Martin, L.J.; Mizuno, T.; Fukuda, T.; Vinks, A.A.; Sadhasivam, S. OCT1 genetic variants are associated with postoperative morphine-related adverse effects in children. Pharmacogenomics 2017, 18, 621–629. [Google Scholar] [CrossRef]
- Chang, H.H.H.; Hsueh, Y.-S.S.; Cheng, Y.W.W.; Ou, H.-T.T.; Wu, M.-H.H. Association between Polymorphisms of OCT1 and Metabolic Response to Metformin in Women with Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2019, 20, E1720. [Google Scholar] [CrossRef]
- Choi, M.-K.; Song, I.-S. Genetic variants of organic cation transporter 1 (OCT1) and OCT2 significantly reduce lamivudine uptake. Biopharm. Drug Dispos. 2012, 33, 170–178. [Google Scholar] [CrossRef]
- Meyer, M.J.; Seitz, T.; Brockmöller, J.; Tzvetkov, M.V.; Rgen Brockmö, J.; Tzvetkov, V. Effects of genetic polymorphisms on the OCT1 and OCT2-mediated uptake of ranitidine. PLoS ONE 2017, 12, e0189521. [Google Scholar] [CrossRef] [PubMed]
- Angelini, S.; Pantaleo, M.A.; Ravegnini, G.; Zenesini, C.; Cavrini, G.; Nannini, M.; Fumagalli, E.; Palassini, E.; Saponara, M.; Di Battista, M.; et al. Polymorphisms in OCTN1 and OCTN2 transporters genes are associated with prolonged time to progression in unresectable gastrointestinal stromal tumours treated with imatinib therapy. Pharmacol. Res. 2013, 68, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pinsonneault, J.; Nielsen, C.U.; Sadée, W.; Sade, W. Genetic Variants of the Human H+/Dipeptide Transporter PEPT2: Analysis of Haplotype Functions. Pharmacology 2004, 311, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Terada, T.; Irie, M.; Okuda, M.; Inui, K. Genetic variant Arg57His in human H+/peptide cotransporter 2 causes a complete loss of transport function. Biochem. Biophys. Res. Commun. 2004, 316, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Woodward, O.M.; Köttgen, A.; Coresh, J.; Boerwinkle, E.; Guggino, W.B.; Köttgen, M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc. Natl. Acad. Sci. USA 2009, 106, 10338–10342. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Zinder, M.; Seroussi, E.; Larkin, D.M.; Loor, J.J.; Everts-van der Wind, A.; Lee, J.-H.; Drackley, J.K.; Band, M.R.; Hernandez, A.G.; Shani, M.; et al. Identification of a missense mutation in the bovine ABCG2 gene with a major effect on the QTL on chromosome 6 affecting milk yield and composition in Holstein cattle. Genome Res. 2005, 15, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Real, R.; González-Lobato, L.; Baro, M.F.; Valbuena, S.; de la Fuente, A.; Prieto, J.G.; Álvarez, A.I.; Marques, M.M.; Merino, G. Analysis of the effect of the bovine adenosine triphosphate-binding cassette transporter G2 single nucleotide polymorphism Y581S on transcellular transport of veterinary drugs using new cell culture models1. J. Anim. Sci. 2011, 89, 4325–4338. [Google Scholar] [CrossRef] [PubMed]
- Brisken, C.; Park, S.; Vass, T.; Lydon, J.P.; O’Malley, B.W.; Weinberg, R.A. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc. Natl. Acad. Sci. USA 1998, 95, 5076–5081. [Google Scholar] [CrossRef] [PubMed]
- Naylor, M.J.; Oakes, S.R.; Gardiner-Garden, M.; Harris, J.; Blazek, K.; Ho, T.W.C.; Li, F.C.; Wynick, D.; Walker, A.M.; Ormandy, C.J. Transcriptional Changes Underlying the Secretory Activation Phase of Mammary Gland Development. Mol. Endocrinol. 2005, 19, 1868–1883. [Google Scholar] [CrossRef]
- Oakes, S.R.; Rogers, R.L.; Naylor, M.J.; Ormandy, C.J. Prolactin regulation of mammary gland development. J. Mammary Gland Biol. Neoplasia 2008, 13, 13–28. [Google Scholar] [CrossRef]
- Zhou, M.M.; Wu, Y.M.; Liu, H.Y.; Zhao, K.; Liu, J.X. Effects of tripeptides and lactogenic hormones on oligopeptide transporter 2 in bovine mammary gland. J. Anim. Physiol. Anim. Nutr. 2011, 95, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Robinson, G.W.; Wagner, K.U.; Garrett, L.; Wynshaw-Boris, A.; Hennighausen, L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997, 11, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Rädler, P.D.; Wehde, B.L.; Wagner, K.-U.U. Crosstalk between STAT5 activation and PI3K/AKT functions in normal and transformed mammary epithelial cells. Mol. Cell. Endocrinol. 2017, 451, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.M.L.; Dalvi, P.; Lu, X.; Yang, M.; Riddick, D.S.; Matthews, J.; Clevenger, C.V.; Ross, D.D.; Harper, P.A.; Ito, S. Induction of multidrug resistance transporter ABCG2 by prolactin in human breast cancer cells. Mol. Pharmacol. 2013, 83, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Geale, P.F.; Sheehy, P.A.; Williamson, P. The Impact of ABCG2 on Bovine Mammary Epithelial Cell Proliferation. Anim. Biotechnol. 2012, 23, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Puga, A.; Ma, C.; Marlowe, J.L. The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem. Pharmacol. 2009, 77, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.P.; Wang, B.; Yang, M.; Boutros, P.C.; Macaulay, J.; Xu, H.; Chuang, A.I.; Kosuge, K.; Yamamoto, M.; Takahashi, S.; et al. Aryl hydrocarbon receptor is a transcriptional activator of the human breast cancer resistance protein (BCRP/ABCG2). Mol. Pharmacol. 2010, 78, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Halwachs, S.; Wassermann, L.; Lindner, S.; Zizzadoro, C.; Honscha, W. Fungicide prochloraz and environmental pollutant dioxin induce the ABCG2 transporter in bovine mammary epithelial cells by the arylhydrocarbon receptor signaling pathway. Toxicol. Sci. 2013, 131, 491–501. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manzini, L.; Halwachs, S.; Girolami, F.; Badino, P.; Honscha, W.; Nebbia, C. Interaction of mammary bovine ABCG2 with AFB1 and its metabolites and regulation by PCB 126 in a MDCKII in vitro model. J. Vet. Pharmacol. Ther. 2017, 40, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.C.; Angus, W.G.R.; Brake, P.B.; Eltom, S.E.; Sukow, K.A.; Jefcoate, C.R. Characterization of CYP1B1 and CYP1A1 expression in human mammary epithelial cells: Role of the aryl hydrocarbon receptor in polycyclic aromatic hydrocarbon metabolism. Cancer Res. 1998, 58, 2366–2374. [Google Scholar] [PubMed]
- Larsen, M.C.; Brake, P.B.; Pollenz, R.S.; Jefcoate, C.R. Linked expression of Ah receptor, ARNT, CYP1A1, and CYP1B1 in rat mammary epithelia, in vitro, is each substantially elevated by specific extracellular matrix interactions that precede branching morphogenesis. Toxicol. Sci. 2004, 82, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Vanselow, J.; Yang, W.; Herrmann, J.; Zerbe, H.; Schuberth, H.-J.; Petzl, W.; Tomek, W.; Seyfert, H.-M. DNA-remethylation around a STAT5-binding enhancer in the alphaS1-casein promoter is associated with abrupt shutdown of alphaS1-casein synthesis during acute mastitis. J. Mol. Endocrinol. 2006, 37, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.; Kacevska, M.; Ingelman-Sundberg, M. Epigenomics and Interindividual Differences in Drug Response. Clin. Pharmacol. Ther. 2012, 92, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Hirota, T.; Tanaka, T.; Takesue, H.; Ieiri, I. Epigenetic regulation of drug transporter expression in human tissues. Expert Opin. Drug Metab. Toxicol. 2017, 13, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Plachot, C.; Lelièvre, S.A. DNA methylation control of tissue polarity and cellular differentiation in the mammary epithelium. Exp. Cell Res. 2004, 298, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Kikuchi, R.; Kusuhara, H.; Yagi, S.; Shiota, K.; Sugiyama, Y. Analysis of DNA methylation and histone modification profiles of liver-specific transporters. Mol. Pharmacol. 2009, 75, 568–576. [Google Scholar] [CrossRef]
- To, K.K.W.; Zhan, Z.; Bates, S.E. Aberrant Promoter Methylation of the ABCG2 Gene in Renal Carcinoma. Mol. Cell. Biol. 2006, 26, 8572–8585. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakano, H.; Nakamura, Y.; Soda, H.; Kamikatahira, M.; Uchida, K.; Takasu, M.; Kitazaki, T.; Yamaguchi, H.; Nakatomi, K.; Yanagihara, K.; et al. Methylation status of breast cancer resistance protein detected by methylation-specific polymerase chain reaction analysis is correlated inversely with its expression in drug-resistant lung cancer cells. Cancer 2008, 112, 1122–1130. [Google Scholar] [CrossRef]
- Bram, E.E.; Stark, M.; Raz, S.; Assaraf, Y.G. Chemotherapeutic drug-induced ABCG2 promoter demethylation as a novel mechanism of acquired multidrug resistance. Neoplasia 2009, 11, 1359–1370. [Google Scholar] [CrossRef]
- Nakanishi, T.; Ross, D.D.; Journal, C. Breast Cancer Resistance Protein (BCRP/ABCG2): Its role in multidrug resistance and regulation of its gene expression. Chin. J. Cancer 2012, 2, 73–99. [Google Scholar] [CrossRef]
- Schaeffeler, E.; Hellerbrand, C.; Nies, A.T.; Winter, S.; Kruck, S.; Hofmann, U.; van der Kuip, H.; Zanger, U.M.; Koepsell, H.; Schwab, M. DNA methylation is associated with downregulation of the organic cation transporter OCT1 (SLC22A1) in human hepatocellular carcinoma. Genome Med. 2011, 3, 82. [Google Scholar] [CrossRef] [PubMed]
- Hauswald, S.; Duque-Afonso, J.; Wagner, M.M.; Schertl, F.M.; Lübbert, M.; Peschel, C.; Keller, U.; Licht, T. Histone deacetylase inhibitors induce a very broad, pleiotropic anticancer drug resistance phenotype in acute myeloid leukemia cells by modulation of multiple ABC transporter genes. Clin. Cancer Res. 2009, 15, 3705–3715. [Google Scholar] [CrossRef] [PubMed]
- You, D.; Shin, H.M.; Mosaad, F.; Richardson, J.R.; Aleksunes, L.M. Brain region-specific regulation of histone acetylation and efflux transporters in mice. J. Biochem. Mol. Toxicol. 2019, 23, e22318. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, L.; Jin, J.; Xia, A.; Wang, C.; Cui, Y.; Qu, B.; Li, Q.; Sheng, C. Comparative transcriptome analysis to investigate the potential role of miRNAs in milk protein/fat quality. Sci. Rep. 2018, 8, 6250. [Google Scholar] [CrossRef]
- Garofalo, M.; Croce, C.M. MicroRNAs as therapeutic targets in chemoresistance. Drug Resist. Updat. 2013, 16, 47–59. [Google Scholar] [CrossRef]
- Peng, L.; Zhong, X. Epigenetic regulation of drug metabolism and transport. Acta Pharm. Sin. B 2015, 5, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Haenisch, S.; Werk, A.N.; Cascorbi, I. MicroRNAs and their relevance to ABC transporters. Br. J. Clin. Pharmacol. 2014, 77, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pan, Y.-Z.; Seigel, G.M.; Hu, Z.-H.; Huang, M.; Yu, A.-M. Breast cancer resistance protein BCRP/ABCG2 regulatory microRNAs (hsa-miR-328, -519c and -520h) and their differential expression in stem-like ABCG2+ cancer cells. Biochem. Pharmacol. 2011, 81, 783–792. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.W.; Zhan, Z.; Litman, T.; Bates, S.E. Regulation of ABCG2 expression at the 3’ untranslated region of its mRNA through modulation of transcript stability and protein translation by a putative microRNA in the S1 colon cancer cell line. Mol. Cell. Biol. 2008, 28, 5147–5161. [Google Scholar] [CrossRef]
- Wong, N.; Wang, X. miRDB: An online resource for microRNA target prediction and functional annotations. Nucleic Acids Res. 2015, 43, D146–D152. [Google Scholar] [CrossRef]
- Petrovic, V.; Teng, S.; Piquette-Miller, M. Regulation of drug transporters during infection and inflammation. Mol. Interv. 2007, 7, 99–111. [Google Scholar] [CrossRef]
- Gandhi, A.; Moorthy, B.; Ghose, R. Drug disposition in pathophysiological conditions. Curr. Drug Metab. 2012, 13, 1327–1344. [Google Scholar] [CrossRef] [PubMed]
- Ling, B.; Alcorn, J. LPS-induced inflammation downregulates mammary gland glucose, fatty acid, and l-carnitine transporter expression at different lactation stages. Res. Vet. Sci. 2010, 89, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Oskarsson, A.; Yagdiran, Y.; Nazemi, S.; Tallkvist, J.; Knight, C.H. Short communication: Staphylococcus aureus infection modulates expression of drug transporters and inflammatory biomarkers in mouse mammary gland. J. Dairy Sci. 2017, 100, 2375–2380. [Google Scholar] [CrossRef] [PubMed]
- Kissell, L.W.; Leavens, T.L.; Baynes, R.E.; Riviere, J.E.; Smith, G.W. Comparison of pharmacokinetics and milk elimination of flunixin in healthy cows and cows with mastitis. J. Am. Vet. Med. Assoc. 2015, 246, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.J.; Shelver, W.L.; Baynes, R.E.; Tell, L.; Gehring, R.; Li, M.; Dutko, T.; Schroeder, J.W.; Herges, G.; Riviere, J.E. Excretory, Secretory, and Tissue Residues after Label and Extra-label Administration of Flunixin Meglumine to Saline- or Lipopolysaccharide-Exposed Dairy Cows. J. Agric. Food Chem. 2015, 63, 4893–4901. [Google Scholar] [CrossRef]
- Wu, B.; Chen, M.Y.; Gao, Y.C.; Hu, J.L.; Liu, M.Z.; Zhang, W.; Huang, W.H. In vivo pharmacodynamic and pharmacokinetic effects of metformin mediated by the gut microbiota in rats. Life Sci. 2019, 226, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, E.; Bastelica, D.; Ben Amara, A.; El Filali, A.; Dutour, A.; Mege, J.L.; Alessi, M.C.; Raoult, D. An evaluation of the effects of Lactobacillus ingluviei on body weight, the intestinal microbiome and metabolism in mice. Microb. Pathog. 2012, 52, 61–68. [Google Scholar] [CrossRef]
- Kuno, T.; Hirayama-Kurogi, M.; Ito, S.; Ohtsuki, S. Effect of Intestinal Flora on Protein Expression of Drug-Metabolizing Enzymes and Transporters in the Liver and Kidney of Germ-Free and Antibiotics-Treated Mice. Mol. Pharm. 2016, 13, 2691–2701. [Google Scholar] [CrossRef] [PubMed]
| Transporter | Substrate | Species |
|---|---|---|
| OCT1 | Endogenous: | |
| Thiamine | Murine 1 [86] | |
| Drugs: | ||
| Cimetidine | Murine 1 [23] | |
| Acyclovir | Murine 1 [23] | |
| ABCG2 | Endogenous: | |
| Riboflavin | Murine 1 [117] | |
| Biotin | Murine 1 [117] | |
| Bile acids | Murine 1 [126] | |
| Uric acid | Bovine 2 [150] | |
| Dietary: | ||
| Enterolactone | Murine 1 [128,129], bovine 2 [150] | |
| Enterodiol | Murine 1 [129] | |
| Drugs: | ||
| Ciprofloxacin | Murine 1 [130], bovine 2 [151] | |
| Danofloxacin | Murine 1 [131], ovine 3 [131], bovine 2 [152] | |
| Enrofloxacin | Ovine 3 [132], bovine 2 [151] | |
| Nitrofurantoin | Murine 1 [135], rat 3 [136], ovine 3 [137] | |
| Moxidectin | Murine 1 [138], ovine 3 [139] | |
| Flunixin and 5-hydroxyflunixin | Murine 1 [141], bovine 2 [141] | |
| Topotecan | Murine 1 [19] | |
| Acyclovir | Murine 1 [19] | |
| Cimetidine | Murine 1 [19] | |
| Pantoprazole | Rat 3 [142] | |
| Nifedipine | Human 2 [153] | |
| Toxins: | ||
| Aflatoxin B1 | Murine 1 [143] | |
| Heterocyclic amines (PhIP, IQ, Trp-P-1) | Murine 1 [19,143] | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Lino, A.M.; Álvarez-Fernández, I.; Blanco-Paniagua, E.; Merino, G.; Álvarez, A.I. Transporters in the Mammary Gland—Contribution to Presence of Nutrients and Drugs into Milk. Nutrients 2019, 11, 2372. https://doi.org/10.3390/nu11102372
García-Lino AM, Álvarez-Fernández I, Blanco-Paniagua E, Merino G, Álvarez AI. Transporters in the Mammary Gland—Contribution to Presence of Nutrients and Drugs into Milk. Nutrients. 2019; 11(10):2372. https://doi.org/10.3390/nu11102372
Chicago/Turabian StyleGarcía-Lino, Alba M., Indira Álvarez-Fernández, Esther Blanco-Paniagua, Gracia Merino, and Ana I. Álvarez. 2019. "Transporters in the Mammary Gland—Contribution to Presence of Nutrients and Drugs into Milk" Nutrients 11, no. 10: 2372. https://doi.org/10.3390/nu11102372
APA StyleGarcía-Lino, A. M., Álvarez-Fernández, I., Blanco-Paniagua, E., Merino, G., & Álvarez, A. I. (2019). Transporters in the Mammary Gland—Contribution to Presence of Nutrients and Drugs into Milk. Nutrients, 11(10), 2372. https://doi.org/10.3390/nu11102372





