6-Shogaol Suppresses 2-Amino-1-Methyl-6-Phenylimidazo [4,5-b] Pyridine (PhIP)-Induced Human 786-O Renal Cell Carcinoma Osteoclastogenic Activity and Metastatic Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture and Conditioned Medium
2.3. Measurement of Secreted Factors
2.4. Isolation of CD14+ Monocytes and Osteoclast Differentiation
2.5. Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. PTHrP Knockdown
2.7. Statistical Analysis
3. Results
3.1. 2-Amino-1-Methyl-6-Phenylimidazo [4,5-b]pyridine (PhIP) Induced Parathyroid Hormone-Related Protein (PTHrP) Secretion In Human 786-O Renal Cell Carcinoma Cells
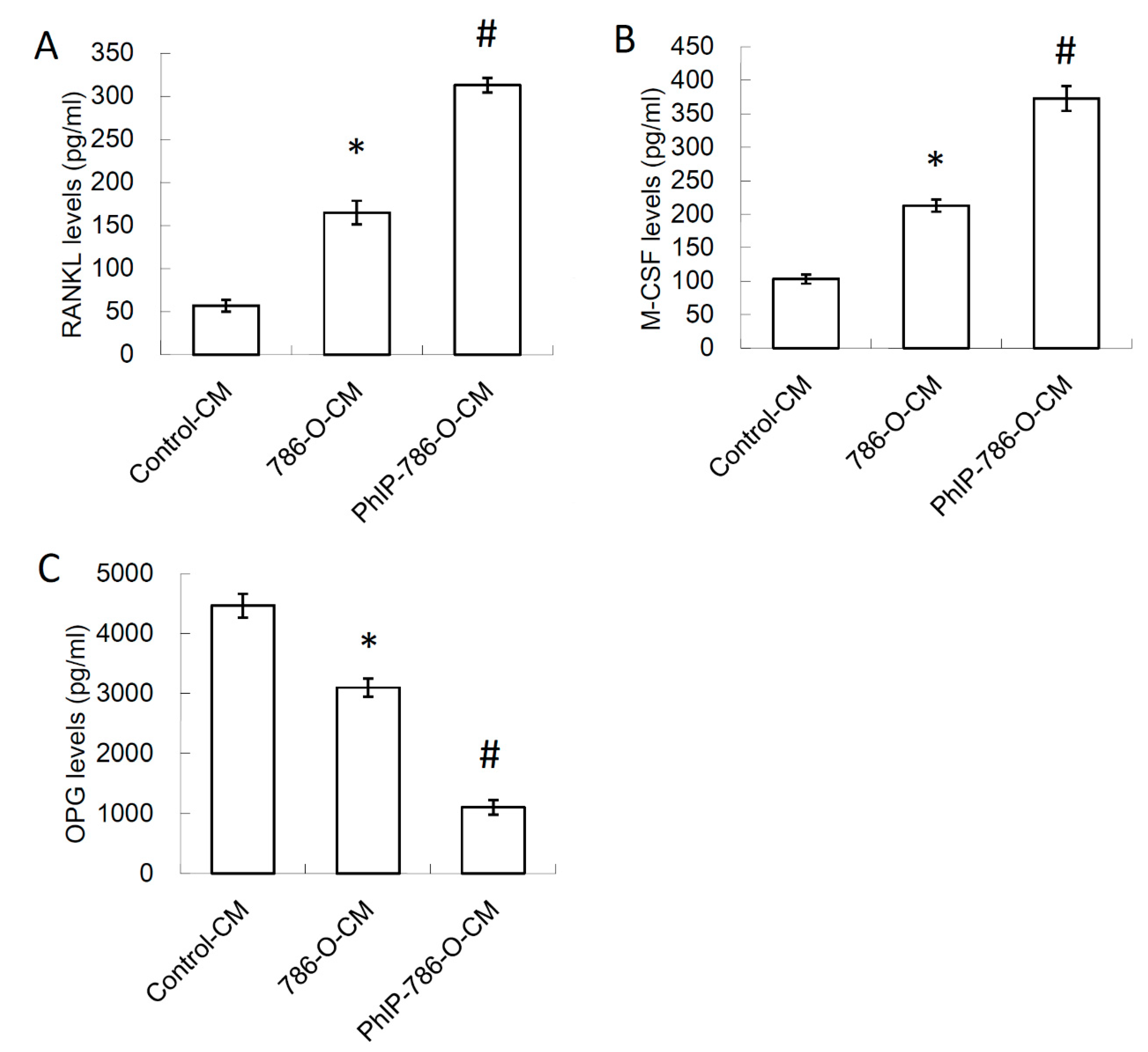
3.2. Conditioned Medium (CM) of PhIP-Treated 786-O Increased Receptor Activator of Nuclear Factor Kappa-B Ligand (RANKL) and Macrophage Colony-Stimulating Factor (M-CSF) Expression, and Decreased Osteoprotegerin (OPG) Expression in Osteoblasts
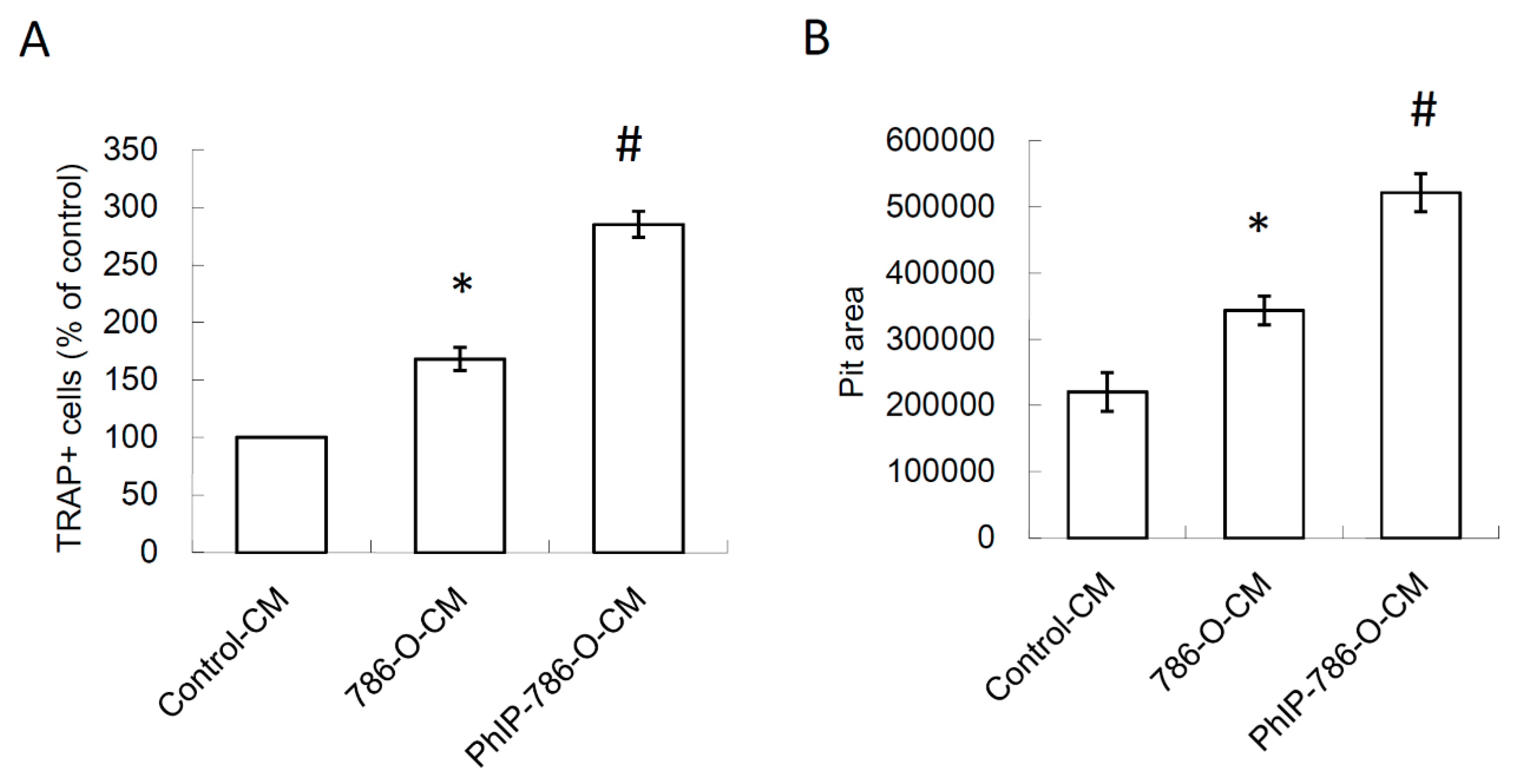
3.3. PhIP Increased Human 786-O Renal Cell Carcinoma Cell-Mediated Osteoclastogenesis and Bone Resorption
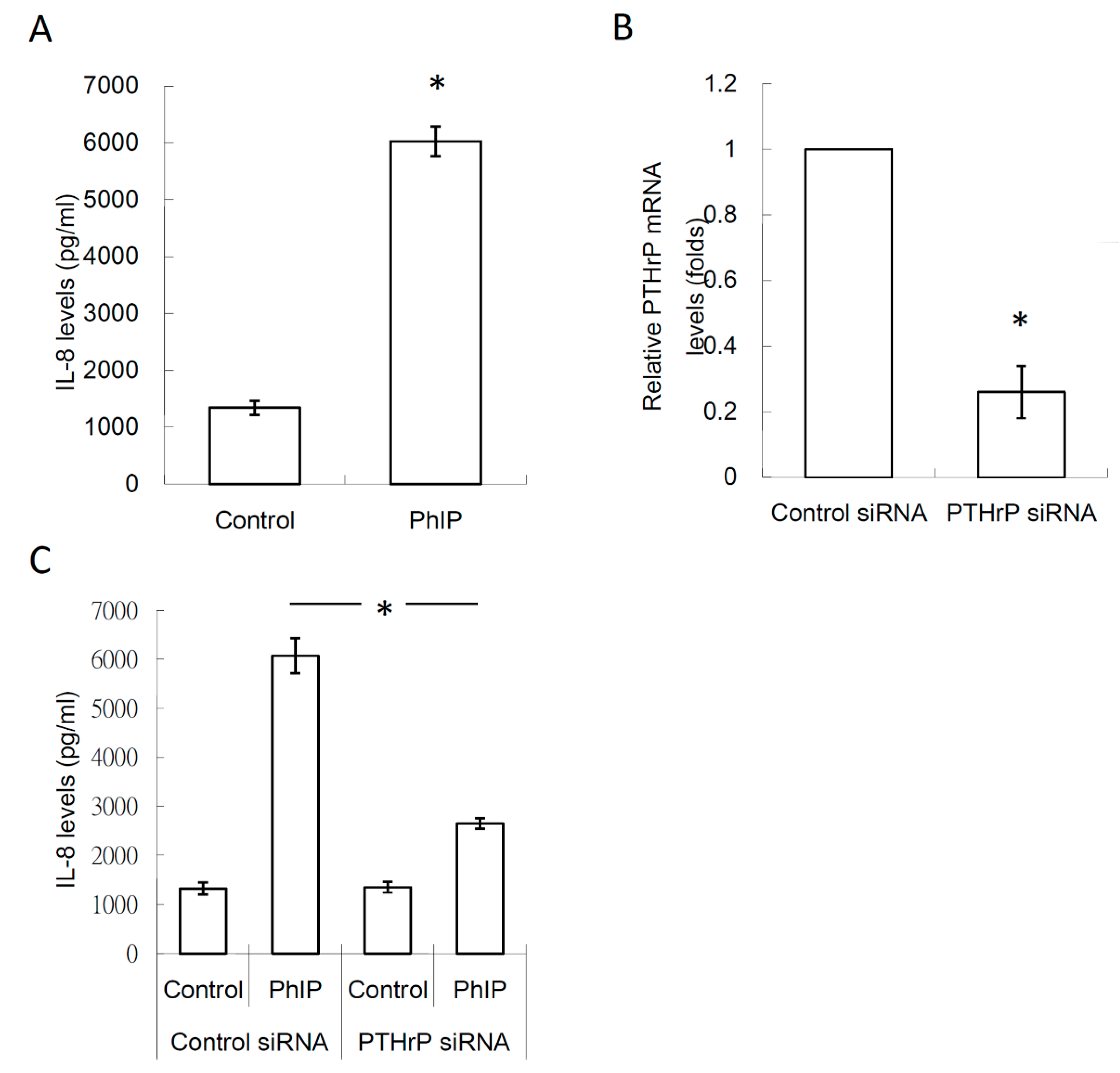
3.4. PTHrP/IL-8 Autocrine Loop was Involved in the Stimulation of PhIP on Renal Cell Carcinoma-Mediated Osteoclastogenesis
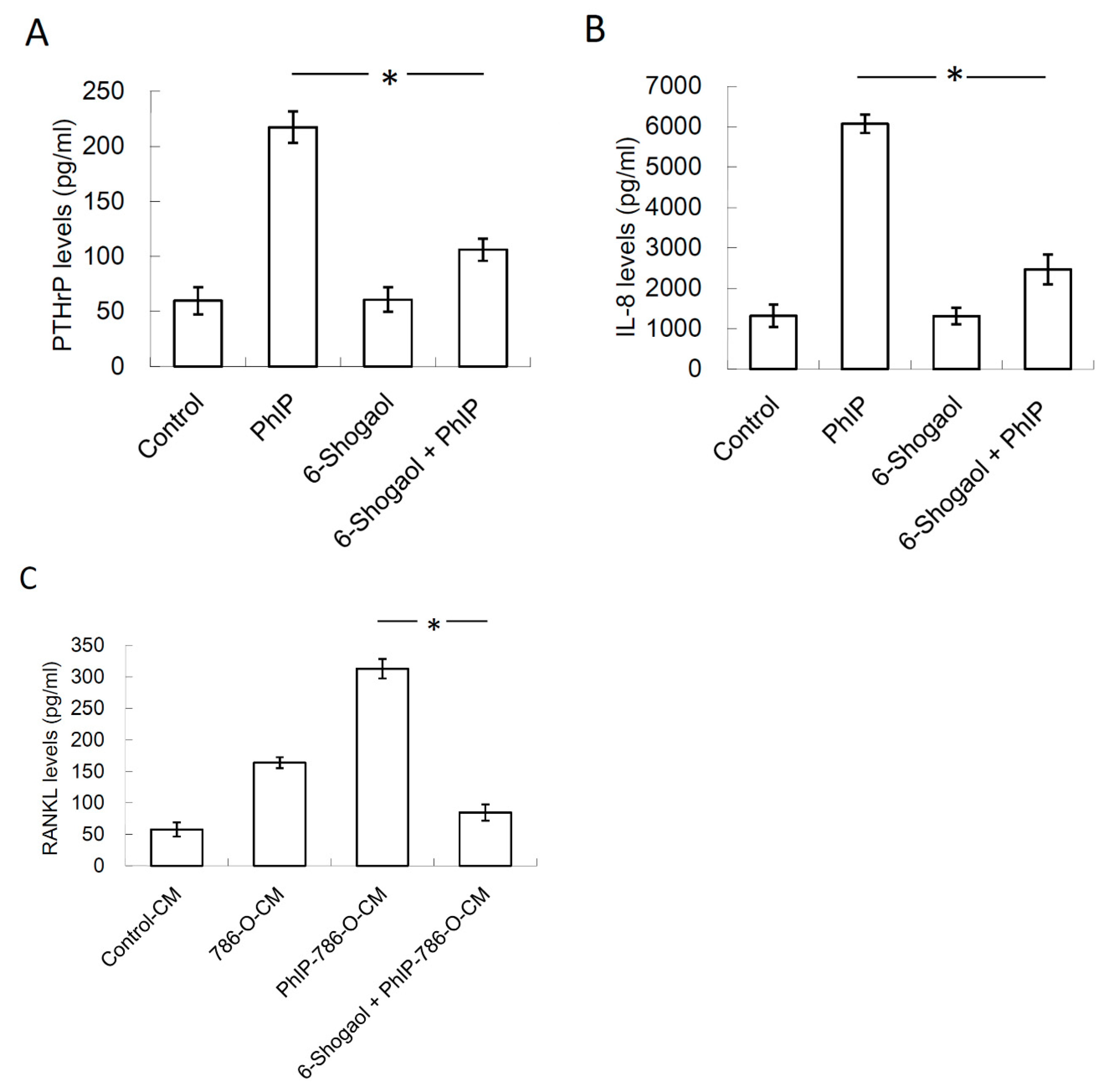
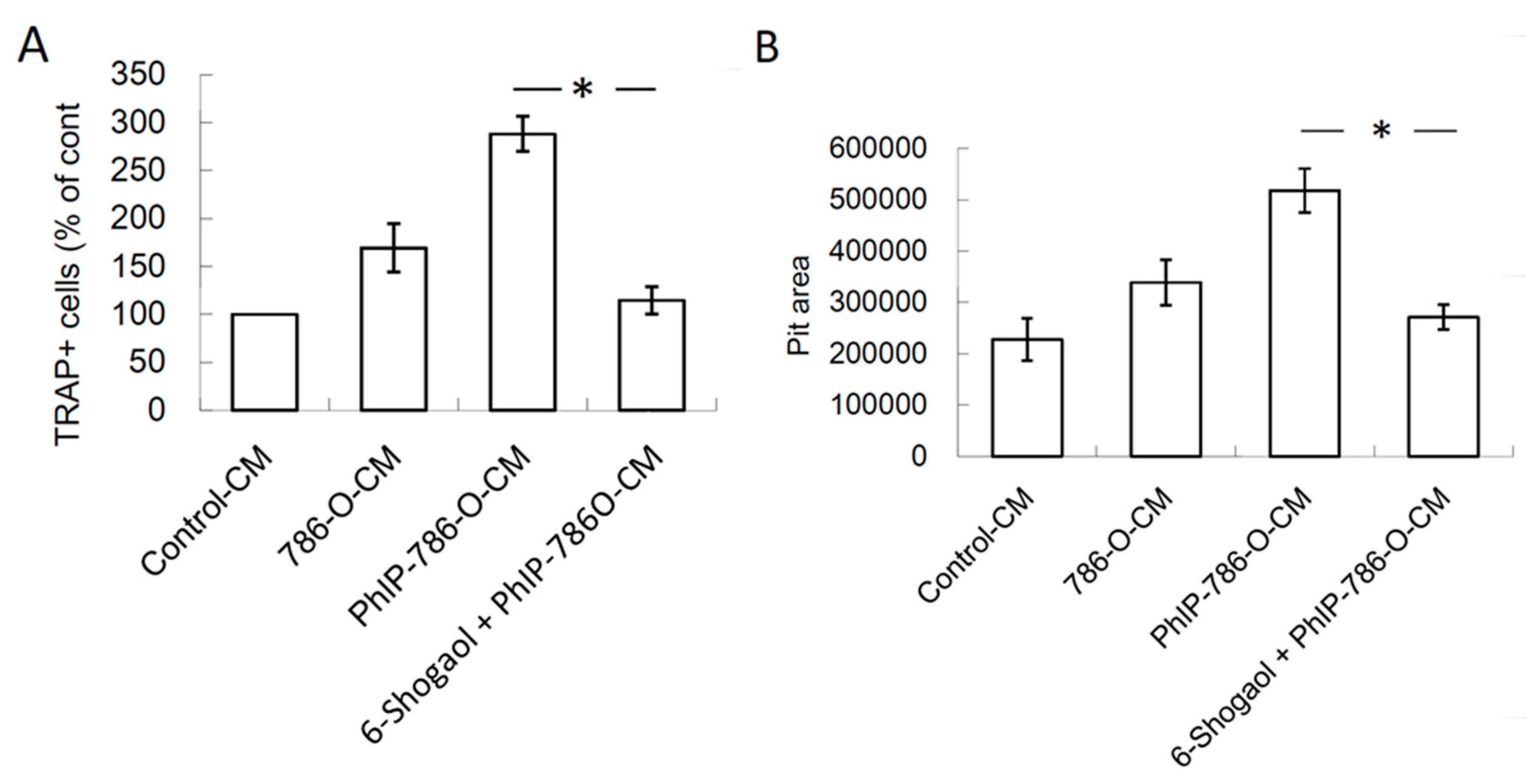
3.5. 6-Shogaol Suppressed PhIP-Mediated Bone Resorption
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rahuman, A.A.; Gopalakrishnan, G.; Venkatesan, P.; Geetha, K.; Bagavan, A. Mosquito larvicidal activity of isolated compounds from the rhizome of Zingiber officinale. Phytother. Res. 2008, 22, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Kukula-Koch, W.; Marzec, Z.; Kasperek, E.; Wyszogrodzka-Koma, L.; Szwerc, W.; Asakawa, Y. Application of Chromatographic and Spectroscopic Methods towards the Quality Assessment of Ginger (Zingiber officinale) Rhizomes from Ecological Plantations. Int. J. Mol. Sci. 2017, 18, 452. [Google Scholar] [CrossRef]
- Ali, B.H.; Blunden, G.; Tanira, M.O.; Nemmar, A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem. Toxicol. 2008, 46, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Grzanna, R.; Lindmark, L.; Frondoza, C.G. Ginger—An herbal medicinal product with broad anti-inflammatory actions. J. Med. Food 2005, 8, 125–132. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K. Ginger and its constituents: Role in prevention and treatment of gastrointestinal cancer. Gastroenterol. Res. Pract. 2015, 2015, 142979. [Google Scholar] [CrossRef]
- Pan, M.H.; Hsieh, M.C.; Hsu, P.C.; Ho, S.Y.; Lai, C.S.; Wu, H.; Sang, S.; Ho, C.T. 6-Shogaol suppressed lipopolysaccharide-induced up-expression of iNOS and COX-2 in murine macrophages. Mol. Nutr. Food Res. 2008, 52, 1467–1477. [Google Scholar] [CrossRef]
- Pan, M.H.; Hsieh, M.C.; Kuo, J.M.; Lai, C.S.; Wu, H.; Sang, S.; Ho, C.T. 6-Shogaol induces apoptosis in human colorectal carcinoma cells via ROS production, caspase activation, and GADD 153 expression. Mol. Nutr. Food Res. 2008, 52, 527–537. [Google Scholar] [CrossRef]
- Wu, C.H.; Hong, B.H.; Ho, C.T.; Yen, G.C. Targeting cancer stem cells in breast cancer: Potential anticancer properties of 6-shogaol and pterostilbene. J. Agric. Food Chem. 2015, 63, 2432–2441. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Hung, J.Y.; Tsai, Y.M.; Tsai, E.M.; Huang, M.S.; Hou, M.F.; Kuo, P.L. 6-shogaol, an active constituent of dietary ginger, impairs cancer development and lung metastasis by inhibiting the secretion of CC-chemokine ligand 2 (CCL2) in tumor-associated dendritic cells. J. Agric. Food Chem. 2015, 63, 1730–1738. [Google Scholar] [CrossRef]
- Ilic, N.M.; Dey, M.; Poulev, A.A.; Logendra, S.; Kuhn, P.E.; Raskin, I. Anti-inflammatory activity of grains of paradise (Aframomum melegueta Schum) extract. J. Agric. Food Chem. 2014, 62, 10452–10457. [Google Scholar] [CrossRef]
- Yoshida, K.; Satsu, H.; Mikubo, A.; Ogiwara, H.; Yakabe, T.; Inakuma, T.; Shimizu, M. 6-shogaol, a major compound in ginger, induces aryl hydrocarbon receptor-mediated transcriptional activity and gene expression. J. Agric. Food Chem. 2014, 62, 5492–5499. [Google Scholar] [CrossRef] [PubMed]
- Warin, R.F.; Chen, H.; Soroka, D.N.; Zhu, Y.; Sang, S. Induction of lung cancer cell apoptosis through a p53 pathway by [6]-shogaol and its cysteine-conjugated metabolite M2. J. Agric. Food Chem. 2014, 62, 1352–1362. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.Y.; Hsu, Y.L.; Li, C.T.; Ko, Y.C.; Ni, W.C.; Huang, M.S.; Kuo, P.L. 6-Shogaol, an active constituent of dietary ginger, induces autophagy by inhibiting the AKT/mTOR pathway in human non-small cell lung cancer A549 cells. J. Agric. Food Chem. 2009, 57, 9809–9816. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Liu, T.Z.; Liu, Y.W.; Tseng, W.C.; Liu, R.H.; Lu, F.J.; Lin, Y.S.; Kuo, S.H.; Chen, C.H. 6-shogaol (alkanone from ginger) induces apoptotic cell death of human hepatoma p53 mutant Mahlavu subline via an oxidative stress-mediated caspase-dependent mechanism. J. Agric. Food Chem. 2007, 55, 948–954. [Google Scholar] [CrossRef]
- Sugimura, T.; Wakabayashi, K.; Nakagama, H.; Nagao, M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004, 95, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K.; International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Ito, N.; Hasegawa, R.; Sano, M.; Tamano, S.; Esumi, H.; Takayama, S.; Sugimura, T. A new colon and mammary carcinogen in cooked food, 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine (PhIP). Carcinogenesis 1991, 12, 1503–1506. [Google Scholar] [CrossRef]
- Shirai, T.; Sano, M.; Tamano, S.; Takahashi, S.; Hirose, M.; Futakuchi, M.; Hasegawa, R.; Imaida, K.; Matsumoto, K.; Wakabayashi, K.; et al. The prostate: A target for carcinogenicity of 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine (PhIP) derived from cooked foods. Cancer Res. 1997, 57, 195–198. [Google Scholar]
- Sinha, R.; Gustafson, D.R.; Kulldorff, M.; Wen, W.Q.; Cerhan, J.R.; Zheng, W. 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine, a carcinogen in high-temperature-cooked meat, and breast cancer risk. J. Natl. Cancer Inst. 2000, 92, 1352–1354. [Google Scholar] [CrossRef]
- Daniel, C.R.; Cross, A.J.; Graubard, B.I.; Park, Y.; Ward, M.H.; Rothman, N.; Hollenbeck, A.R.; Chow, W.H.; Sinha, R. Large prospective investigation of meat intake, related mutagens, and risk of renal cell carcinoma. Am. J. Clin. Nutr. 2012, 95, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Melkonian, S.C.; Daniel, C.R.; Ye, Y.; Tannir, N.M.; Karam, J.A.; Matin, S.F.; Wood, C.G.; Wu, X. Gene-environment interaction of genome-wide association study-identified susceptibility loci and meat-cooking mutagens in the etiology of renal cell carcinoma. Cancer 2016, 122, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Murai, M.; Oya, M. Renal cell carcinoma: Etiology, incidence and epidemiology. Curr. Opin. Urol. 2004, 14, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Rathmell, W.K.; Godley, P. Renal cell carcinoma. Curr. Opin. Oncol. 2008, 20, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.; Sun, M.; Jeldres, C.; Shariat, S.F.; Trinh, Q.D.; Briganti, A.; Tian, Z.; Schmitges, J.; Graefen, M.; Perrotte, P.; et al. Distribution of metastatic sites in renal cell carcinoma: A population-based analysis. Ann. Oncol. 2012, 23, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Kuo, P.L. Bone Metastasis from Renal Cell Carcinoma. Int. J. Mol. Sci. 2016, 17, 987. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Bander, N.H.; Nanus, D.M. Renal-cell carcinoma. N. Engl. J. Med. 1996, 335, 865–875. [Google Scholar] [CrossRef]
- Woodward, E.; Jagdev, S.; McParland, L.; Clark, K.; Gregory, W.; Newsham, A.; Rogerson, S.; Hayward, K.; Selby, P.; Brown, J. Skeletal complications and survival in renal cancer patients with bone metastases. Bone 2011, 48, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Goncalves, F. Bone Metastases: An Overview. Oncol. Rev. 2017, 11, 321. [Google Scholar] [CrossRef]
- Reddington, J.A.; Mendez, G.A.; Ching, A.; Kubicky, C.D.; Klimo, P., Jr.; Ragel, B.T. Imaging characteristic analysis of metastatic spine lesions from breast, prostate, lung, and renal cell carcinomas for surgical planning: Osteolytic versus osteoblastic. Surg. Neurol. Int. 2016, 7, S361–S365. [Google Scholar] [CrossRef]
- Sourbier, C.; Massfelder, T. Parathyroid hormone-related protein in human renal cell carcinoma. Cancer Lett. 2006, 240, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Burton, P.B.; Moniz, C.; Knight, D.E. Parathyroid hormone related peptide can function as an autocrine growth factor in human renal cell carcinoma. Biochem. Biophys. Res. Commun. 1990, 167, 1134–1138. [Google Scholar] [CrossRef]
- Massfelder, T.; Lang, H.; Schordan, E.; Lindner, V.; Rothhut, S.; Welsch, S.; Simon-Assmann, P.; Barthelmebs, M.; Jacqmin, D.; Helwig, J.J. Parathyroid hormone-related protein is an essential growth factor for human clear cell renal carcinoma and a target for the von Hippel-Lindau tumor suppressor gene. Cancer Res. 2004, 64, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Roodman, G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 2004, 350, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Li, X.; Koh, A.J.; Berry, J.E.; Thudi, N.; Rosol, T.J.; Pienta, K.J.; McCauley, L.K. Tumor expressed PTHrP facilitates prostate cancer-induced osteoblastic lesions. Int. J. Cancer 2008, 123, 2267–2278. [Google Scholar] [CrossRef] [PubMed]
- Ballanti, P.; Minisola, S.; Pacitti, M.T.; Scarnecchia, L.; Rosso, R.; Mazzuoli, G.F.; Bonucci, E. Tartrate-resistant acid phosphate activity as osteoclastic marker: Sensitivity of cytochemical assessment and serum assay in comparison with standardized osteoclast histomorphometry. Osteoporos. Int. 1997, 7, 39–43. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Glass-Holmes, M.; Aguilar, B.J.; Gragg, R.D., 3rd; Darling-Reed, S.; Goodman, C.B. Characterization of 2-amino-1-methyl-6-phenylimidazo [4,5b] pyridine at androgen receptor: Mechanistic support for its role in prostate cancer. Am. J. Cancer Res. 2015, 5, 191–200. [Google Scholar]
- Bacon, J.R.; Williamson, G.; Garner, R.C.; Lappin, G.; Langouet, S.; Bao, Y. Sulforaphane and quercetin modulate PhIP-DNA adduct formation in human HepG2 cells and hepatocytes. Carcinogenesis 2003, 24, 1903–1911. [Google Scholar] [CrossRef]
- Casimiro, S.; Mohammad, K.S.; Pires, R.; Tato-Costa, J.; Alho, I.; Teixeira, R.; Carvalho, A.; Ribeiro, S.; Lipton, A.; Guise, T.A.; et al. RANKL/RANK/MMP-1 molecular triad contributes to the metastatic phenotype of breast and prostate cancer cells in vitro. PLoS ONE 2013, 8, e63153. [Google Scholar] [CrossRef]
- Krishnan, V.; Vogler, E.A.; Sosnoski, D.M.; Mastro, A.M. In vitro mimics of bone remodeling and the vicious cycle of cancer in bone. J. Cell. Physiol. 2014, 229, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Manenti, G.; Peissel, B.; Gariboldi, M.; Falvella, F.S.; Zaffaroni, D.; Allaria, B.; Pazzaglia, S.; Rebessi, S.; Covelli, V.; Saran, A.; et al. A cancer modifier role for parathyroid hormone-related protein. Oncogene 2000, 19, 5324–5328. [Google Scholar] [CrossRef] [PubMed][Green Version]
- David Roodman, G.; Silbermann, R. Mechanisms of osteolytic and osteoblastic skeletal lesions. BoneKEy Rep. 2015, 4, 753. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Kang, Y. Targeting tumor-stromal interactions in bone metastasis. Pharmacol. Ther. 2014, 141, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Mikami, S.; Oya, M.; Mizuno, R.; Kosaka, T.; Katsube, K.; Okada, Y. Invasion and metastasis of renal cell carcinoma. Med. Mol. Morphol. 2014, 47, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Yamaguchi, K.; Kinosaki, M.; Goto, M.; Mochizuki, S.I.; Tsuda, E.; Morinaga, T.; Udagawa, N.; et al. A novel molecular mechanism modulating osteoclast differentiation and function. Bone 1999, 25, 109–113. [Google Scholar] [CrossRef]
- Jansen, I.D.; Vermeer, J.A.; Bloemen, V.; Stap, J.; Everts, V. Osteoclast fusion and fission. Calcif. Tissue Int. 2012, 90, 515–522. [Google Scholar] [CrossRef]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Perut, F.; Cenni, E.; Unger, R.E.; Kirkpatrick, C.J.; Giunti, A.; Baldini, N. Immunogenic properties of renal cell carcinoma and the pathogenesis of osteolytic bone metastases. Int. J. Oncol. 2009, 34, 1387–1393. [Google Scholar] [CrossRef]
- Bendre, M.S.; Montague, D.C.; Peery, T.; Akel, N.S.; Gaddy, D.; Suva, L.J. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone 2003, 33, 28–37. [Google Scholar] [CrossRef]
- Sterling, J.A.; Edwards, J.R.; Martin, T.J.; Mundy, G.R. Advances in the biology of bone metastasis: How the skeleton affects tumor behavior. Bone 2011, 48, 6–15. [Google Scholar] [CrossRef]
- Lewiecki, E.M. Safety of long-term bisphosphonate therapy for the management of osteoporosis. Drugs 2011, 71, 791–814. [Google Scholar] [CrossRef] [PubMed]
- Papapetrou, P.D. Bisphosphonate-associated adverse events. Hormones (Athens) 2009, 8, 96–110. [Google Scholar] [CrossRef]
- Weilbaecher, K.N.; Guise, T.A.; McCauley, L.K. Cancer to bone: A fatal attraction. Nat. Rev. Cancer 2011, 11, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Yang, H.; Tan, S.H.; Chui, W.K.; Chew, E.H. 6-Shogaol, an active constituent of ginger, inhibits breast cancer cell invasion by reducing matrix metalloproteinase-9 expression via blockade of nuclear factor-kappaB activation. Br. J. Pharmacol. 2010, 161, 1763–1777. [Google Scholar] [CrossRef]
- Kakonen, S.M.; Mundy, G.R. Mechanisms of osteolytic bone metastases in breast carcinoma. Cancer 2003, 97, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Warin, R.F.; Soroka, D.N.; Chen, H.; Sang, S. Metabolites of ginger component [6]-shogaol remain bioactive in cancer cells and have low toxicity in normal cells: Chemical synthesis and biological evaluation. PLoS ONE 2013, 8, e54677. [Google Scholar] [CrossRef]
- Chrubasik, S.; Pittler, M.H.; Roufogalis, B.D. Zingiberis rhizoma: A comprehensive review on the ginger effect and efficacy profiles. Phytomedicine 2005, 12, 684–701. [Google Scholar] [CrossRef]
- Levita, J.; Syafitri, D.M.; Supu, R.D.; Mutakin, M.; Megantara, S.; Febrianti, M.; Diantini, A. Pharmacokinetics of 10-gingerol and 6-shogaol in the plasma of healthy subjects treated with red ginger (Zingiber officinale var. Rubrum) suspension. Biomed. Rep. 2018, 9, 474–482. [Google Scholar] [CrossRef]
- Zick, S.M.; Djuric, Z.; Ruffin, M.T.; Litzinger, A.J.; Normolle, D.P.; Alrawi, S.; Feng, M.R.; Brenner, D.E. Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1930–1936. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Q.; Sun, C.; Zhu, Y.; Yang, Q.; Wei, Q.; Chen, J.; Deng, W.; Adu-Frimpong, M.; Yu, J.; et al. Enhanced Oral Bioavailability, Anti-Tumor Activity and Hepatoprotective Effect of 6-Shogaol Loaded in a Type of Novel Micelles of Polyethylene Glycol and Linoleic Acid Conjugate. Pharmaceutics 2019, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Shaw, Y.A.; Williams, L.A.; Junor, G.A.; Green, C.E.; Hibbert, S.L.; Salmon, C.N.; Smith, A.M. Changes in the contents of oleoresin and pungent bioactive principles of Jamaican ginger (Zingiber officinale Roscoe.) during maturation. J. Agric. Food Chem. 2008, 56, 5564–5571. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Bernart, M.W.; Lian, L.; Lin, L. High-performance liquid chromatography–electrospray mass spectrometric analysis of pungent constituents of ginger. J. Chromatogr. A 1998, 796, 327–334. [Google Scholar] [CrossRef]
- Cheng, X.L.; Liu, Q.; Peng, Y.B.; Qi, L.W.; Li, P. Steamed ginger (Zingiber officinale): Changed chemical profile and increased anticancer potential. Food Chem. 2011, 129, 1785–1792. [Google Scholar] [CrossRef]
- Guo, J.B.; Zhang, W.J.; Wu, H.; Du, L.M. Microwave-assisted decomposition coupled with acidic food condiment as an efficient technology for ginger (Zingiber officinale Roscoe) processing. Sep. Purif. Technol. 2015, 146, 219–226. [Google Scholar] [CrossRef]
- Teng, H.; Seuseu, K.T.; Lee, W.Y.; Chen, L. Comparing the effects of microwave radiation on 6-gingerol and 6-shogaol from ginger rhizomes (Zingiber officinale Rosc). PLoS ONE 2019, 14, e0214893. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, I.-J.; Chen, S.-C.; Yen, M.-C.; Wu, Y.-H.; Hung, C.-H.; Kuo, P.-L. 6-Shogaol Suppresses 2-Amino-1-Methyl-6-Phenylimidazo [4,5-b] Pyridine (PhIP)-Induced Human 786-O Renal Cell Carcinoma Osteoclastogenic Activity and Metastatic Potential. Nutrients 2019, 11, 2306. https://doi.org/10.3390/nu11102306
Yeh I-J, Chen S-C, Yen M-C, Wu Y-H, Hung C-H, Kuo P-L. 6-Shogaol Suppresses 2-Amino-1-Methyl-6-Phenylimidazo [4,5-b] Pyridine (PhIP)-Induced Human 786-O Renal Cell Carcinoma Osteoclastogenic Activity and Metastatic Potential. Nutrients. 2019; 11(10):2306. https://doi.org/10.3390/nu11102306
Chicago/Turabian StyleYeh, I-Jeng, Szu-Chia Chen, Meng-Chi Yen, Yen-Hung Wu, Chih-Hsing Hung, and Po-Lin Kuo. 2019. "6-Shogaol Suppresses 2-Amino-1-Methyl-6-Phenylimidazo [4,5-b] Pyridine (PhIP)-Induced Human 786-O Renal Cell Carcinoma Osteoclastogenic Activity and Metastatic Potential" Nutrients 11, no. 10: 2306. https://doi.org/10.3390/nu11102306
APA StyleYeh, I.-J., Chen, S.-C., Yen, M.-C., Wu, Y.-H., Hung, C.-H., & Kuo, P.-L. (2019). 6-Shogaol Suppresses 2-Amino-1-Methyl-6-Phenylimidazo [4,5-b] Pyridine (PhIP)-Induced Human 786-O Renal Cell Carcinoma Osteoclastogenic Activity and Metastatic Potential. Nutrients, 11(10), 2306. https://doi.org/10.3390/nu11102306




