Role of Milk-Derived Opioid Peptides and Proline Dipeptidyl Peptidase-4 in Autism Spectrum Disorders
Abstract
1. Introduction
2. Materials and Method
2.1. Patients and Control Group Characteristic
2.2. Examined Substances
2.3. Biological Material
2.4. Measurement of BCM7 Concentration
2.5. Measurement of DPPIV Activity and Concentration in Serum
2.6. Analysis of the DPPIV Gene Expression under the Influence of Peptide Extract Obtained from Hydrolysed Bovine Milk
2.7. Statistical Data
3. Results
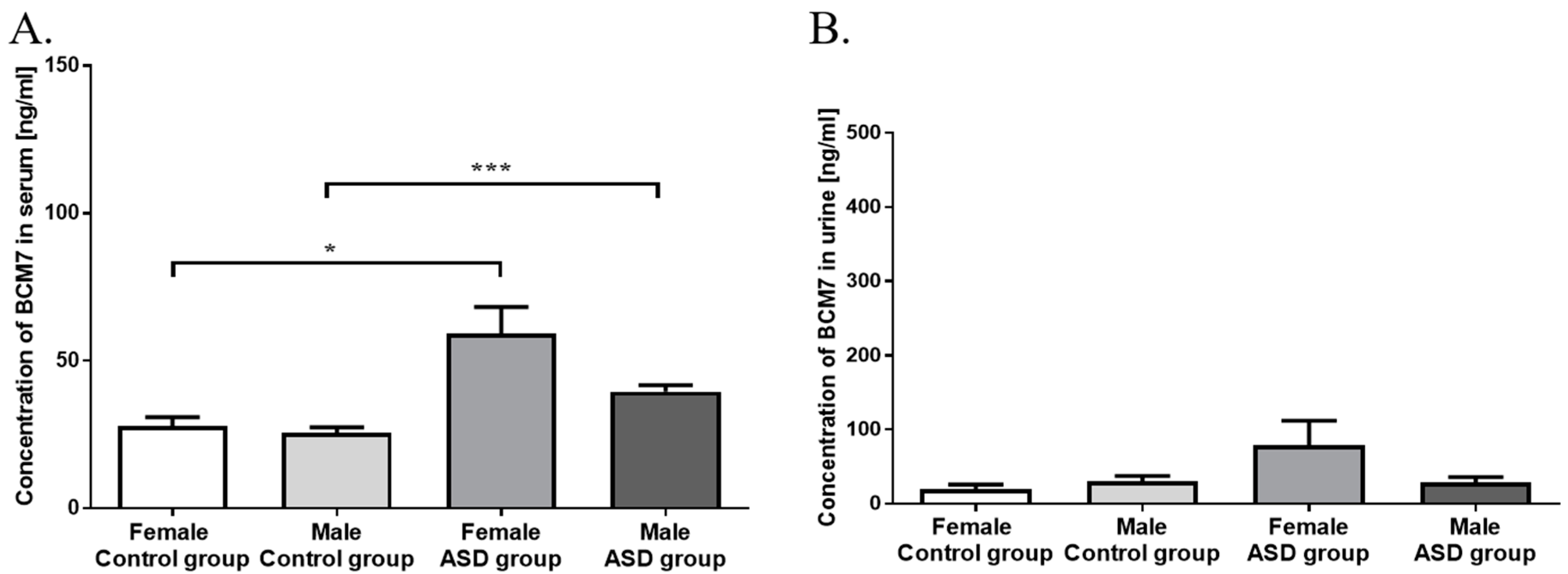
3.1. Measurement of BCM7 Concentration in Hydrolysed Bovine Milk and Patients’ Serum and Urine
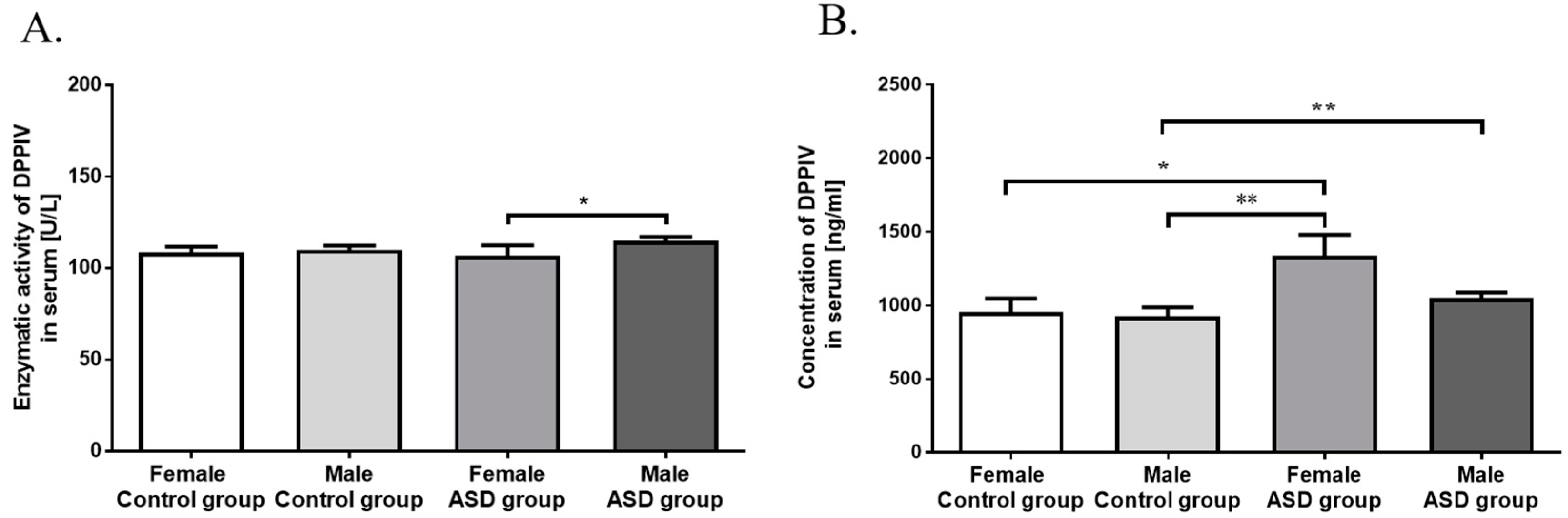
3.2. Measurement of DPPIV Activity and Concentration in Serum
3.3. DPPIV Gene Expression in PBMCs
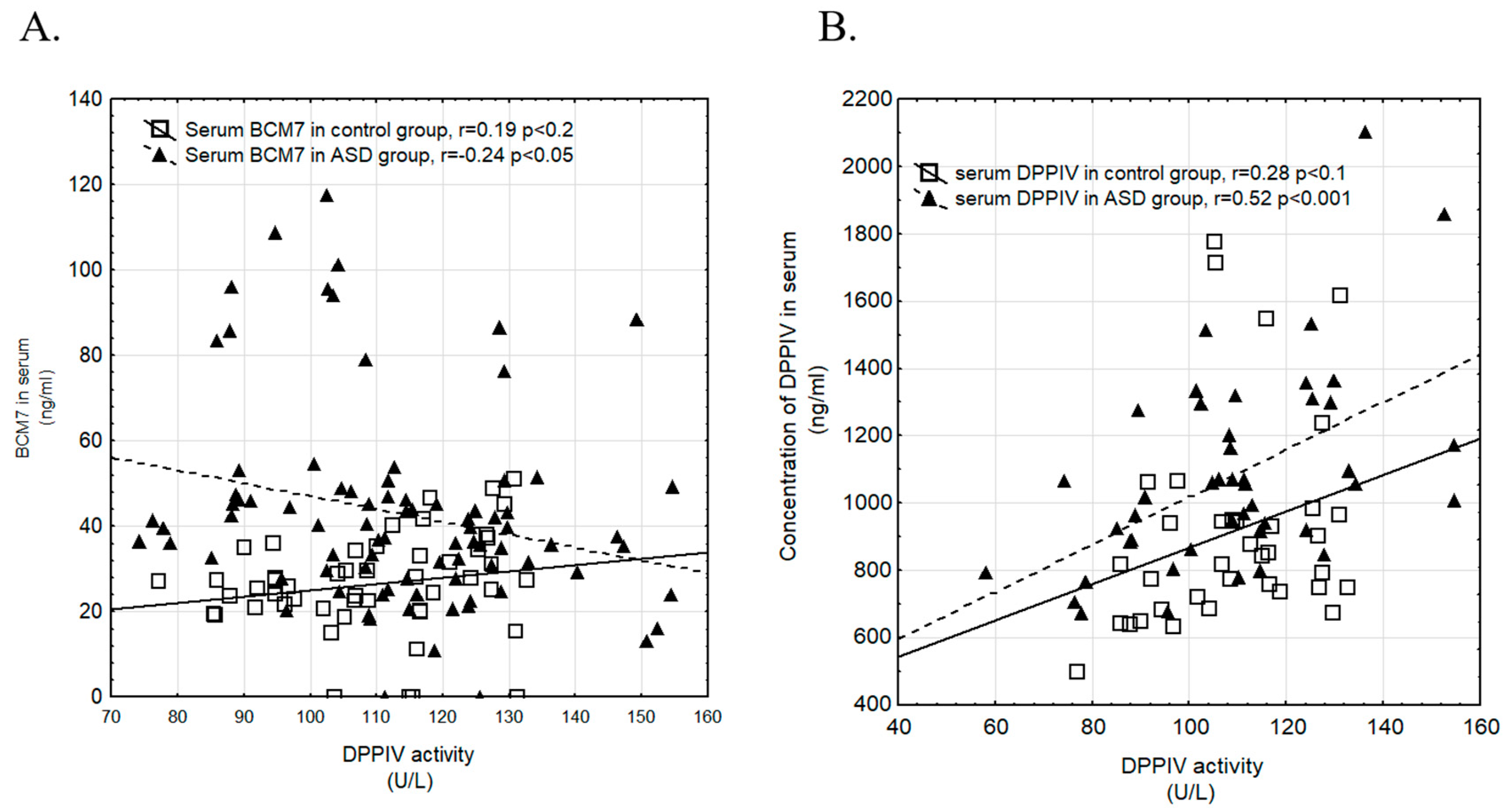
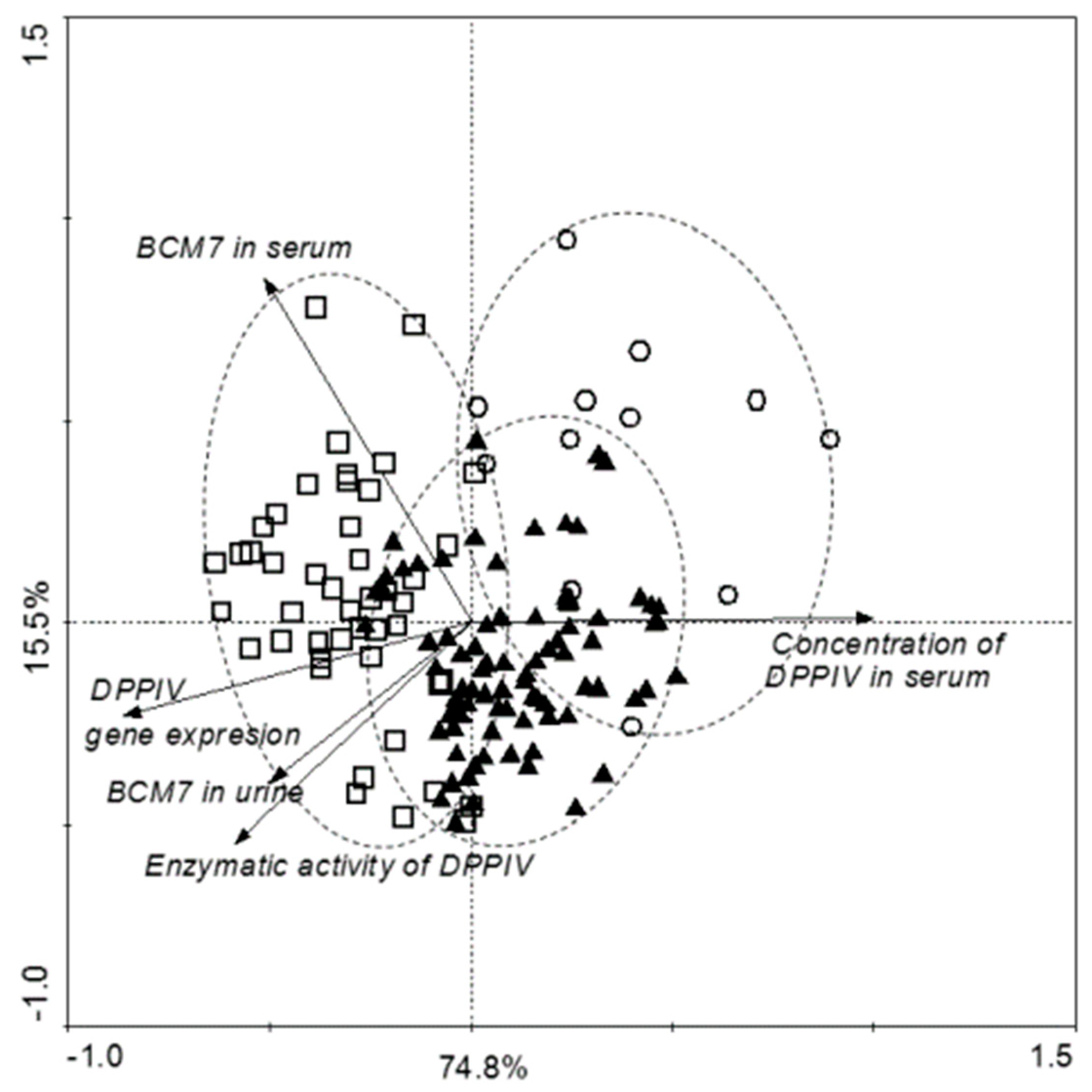
3.4. Correlation of Examined Parameters between ASD and Control Groups, and with Gender
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Piskorz-Ogórek, K.; Ogórek, S.; Cieślińska, A.; Kostyra, E. Autism in Poland in comparison to other countries. Pol. Ann. Med. 2015, 22, 35–40. [Google Scholar] [CrossRef]
- Skonieczna-Żydecka, K.; Gorzkowska, I.; Pierzak-Sominka, J.; Adler, G. The prevalence of autism spectrum disorders in West Pomeranian and Pomeranian regions of Poland. J. Appl. Res. Intellect. Disabil. 2017, 30, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Baio, J. Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilitiesmonitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014, 63, 1–21. [Google Scholar]
- Lai, M.; Lombardo, M.V.; Auyeung, B.; Chakrabarti, B.; Baron-Cohen, S. Sex/gender differences and autism: Setting the scene for future research. J. Am. Acad. Child. Adolesc. Psychiat. 2015, 54, 11–24. [Google Scholar] [CrossRef] [PubMed]
- De Theije, C.G.; Wu, J.; Da Silva, S.L.; Kamphuis, P.J.; Garssen, J.; Korte, S.M.; Kraneveld, A.D. Pathways underlying the gut-to-brain connection in autism spectrum disorders as future targets for disease management. Eur. J. Pharmacol. 2011, 668, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Hallmayer, J.; Cleveland, S.; Torres, A.; Phillips, J.; Cohen, B.; Torigoe, T.; Miller, J.; Fedele, A.; Collins, J.; Smith, K.; et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch. Gen. Psychiatry 2011, 68, 1095–1102. [Google Scholar] [CrossRef]
- Elder, J.H.; Kreider, C.M.; Schaefer, N.M.; de Laosa, M.B. A review of gluten-and casein-free diets for treatment of autism: 2005–2015. Nutr. Diet. Suppl. 2015, 7, 87–101. [Google Scholar] [CrossRef]
- Panksepp, J. A neurochemical theory of autism. Trends Neurosci. 1979, 2, 174–177. [Google Scholar] [CrossRef]
- Leboyer, M.; Bouvard, M.P.; Launay, J.M.; Recasens, C.; Plumet, M.H.; Waller-Perotte, D. Opiate hypothesis in infantile autism? Therapeutic trials with naltrexone. Encephale 1993, 9, 95–102. [Google Scholar]
- Brantl, V.; Teschemacher, H.; Henschen, A.; Lottspeich, F. Novel opioid peptides derived from casein (β-casomorphins). I. Isolation from bovine casein peptone. Hoppe Seylers Z Physiol. Chem. 1979, 360, 1211–1224. [Google Scholar] [CrossRef]
- Cieślińska, A.; Kostyra, E.; Kostyra, H.; Oleński, K.; Fiedorowicz, E.; Kamiński, S. Milk from cows of different β-casein genotypes as a source of β-casomorphin-7. Int. J. Food Sci. Nutr. 2012, 63, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Fiedorowicz, E.; Kaczmarski, M.; Cieślińska, A.; Sienkiewicz-Szłapka, E.; Jarmołowska, B.; Chwała, B.; Kostyra, E. β-casomorphin-7 alters μ-opioid receptor and dipeptidyl peptidase IV genes expression in children with atopic dermatitis. Peptides 2014, 62, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz-Szłapka, E.; Jarmołowska, B.; Krawczuk, S.; Kostyra, E.; Kostyra, H.; Iwan, M. Contents of agonistic and antagonistic opioid peptides in different cheese varieties. Int. Dairy J. 2009, 19, 258–263. [Google Scholar] [CrossRef]
- Pal, S.; Woodford, K.; Kukuljan, S.; Ho, S. Milk intolerance, beta-casein and lactose. Nutrients 2015, 7, 7285–7297. [Google Scholar] [CrossRef] [PubMed]
- Fiedorowicz, E.; Jarmołowska, B.; Iwan, M.; Kostyra, E.; Obuchowicz, R.; Obuchowicz, M. The influence of μ-opioid receptor agonist and antagonist peptides on peripheral blood mononuclear cells (PBMCs). Peptides 2011, 32, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, K.L.; Knivsberg, A.M. Can the pathophysiology of autism be explained by the nature of the discovered urine peptides? Nutr. Neurosci. 2003, 6, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, O.; Kost, N.; Andreeva, O.; Korneeva, E.; Meshavkin, V.; Tarakanova, Y.; Dadayan, A.; Zolotarev, Y.; Grachev, S.; Mikheeva, I.; et al. Autistic children display elevated urine levels of bovine casomorphin-7 immunoreactivity. Peptides 2014, 56, 68–71. [Google Scholar] [CrossRef] [PubMed]
- De Noni, I.; Cattaneo, S. Occurrence of β-casomorphins 5 and 7 in commercial dairy products and in their digests following in vitro simulated gastro-intestinal digestion. Food Chem. 2010, 119, 560–566. [Google Scholar] [CrossRef]
- De Noni, I. Release of β-casomorphins 5 and 7 during simulated gastro-intestinal digestion of bovine β-casein variants and milk-based infant formulas. Food Chem. 2008, 110, 897–903. [Google Scholar] [CrossRef]
- Jarmołowska, B.; Bielikowicz, K.; Iwan, M.; Sidor, K.; Kostyra, E.; Kaczmarski, M. Serum activity of dipeptidyl peptidase IV (DPPIV; EC 3.4. 14.5) in breast-fed infants with symptoms of allergy. Peptides 2007, 28, 678–682. [Google Scholar] [CrossRef]
- Jarmołowska, B.; Kostyra, E.; Krawczuk, S.; Kostyra, H. β-Casomorphin-7 isolated from Brie cheese. J. Sci. Food Agric. 1999, 79, 1788–1792. [Google Scholar] [CrossRef]
- Wociór, A.; Iwan, M.; Kostyra, H.; Kostyra, E. Opioid activity of high-protein supplement for sportsmen. Pol. J. Food Nutr. Sci. 2008, 58, 247. [Google Scholar]
- Reichelt, K.L.; Knivsberg, A.M.; Lind, G.; Nødland, M. Probable etiology and possible treatment of childhood autism. Brain Dysfunct. 1991, 4, 308–319. [Google Scholar]
- Cieślińska, A.; Kostyra, E.; Savelkoul, H.F.J. Treating autism spectrum disorder with gluten-free and casein-free diet: The underlying microbiota-gut-brain axis mechanisms. J. Clin. Immunol. Immunother. 2017, 3, 9. [Google Scholar]
- Erić-Nikolić, A.; Matić, I.Z.; Ðorđević, M.; Milovanović, Z.; Marković, I.; Džodić, R.; Inić, M.; Srdić-Rajić, T.; Jevrić, M.; Gavrilović, D.; et al. Serum DPPIV activity and CD26 expression on lymphocytes in patients with benign or malignant breast tumors. Immunobiology 2011, 216, 942–946. [Google Scholar] [CrossRef]
- Whiteley, P.; Shattock, P. Biochemical aspects in autism spectrum disorders: Updating the opioid-excess theory and presenting new opportunities for biomedical intervention. Expert. Opin. Ther. Targets 2002, 6, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Elder, J.H.; Shankar, M.; Shuster, J.; Theriaque, D.; Burns, S.; Sherrill, L. The gluten-free, casein-free diet in autism: Results of a preliminary double blind clinical trial. J. Autism Dev. Disord. 2006, 36, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.W.; Hauser, J.; Reissmann, A. Gluten-free and casein-free diets in the therapy of autism. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 572–575. [Google Scholar] [CrossRef]
- Pedersen, L.; Parlar, S.; Kvist, K.; Whiteley, P.; Shattock, P. Data mining the ScanBrit study of a gluten-and casein-free dietary intervention for children with autism spectrum disorders: Behavioural and psychometric measures of dietary response. Nutr. Neurosci. 2014, 17, 207–213. [Google Scholar] [CrossRef]
- Herbert, M.; Weintraub, K. The Autism Revolution: Whole-Body Strategies for Making Life All It Can Be, 1st ed.; Ballantine Books: New York, NY, USA, 2013; pp. 1095–1102. ISBN 9780345527219. [Google Scholar]
- Kordulewska, N.K.; Kostyra, E.; Cieślińska, A.; Matysiewicz, M.; Fiedorowicz, E.; Sienkiewicz-Szłapka, E. Changes in gene expression induced by histamine, fexofenadine and osthole: Expression of histamine H1 receptor, COX-2, NF-κB, CCR1, chemokine CCL5/RANTES and interleukin-1β in PBMC allergic and non-allergic patients. Immunobiology 2017, 222, 571–581. [Google Scholar] [CrossRef]
- Pfaffl, M.W. Live Stock Transcriptomics: Quantitative mRNA Analytics in Molecular Endocrinology and Physiology; Technische Universität München–Weihenstephan: Freising, Germany, 2003. [Google Scholar]
- Cieślińska, A.; Sienkiewicz-Szłapka, E.; Wasilewska, J.; Fiedorowicz, E.; Chwała, B.; Moszyńska-Dumara, M.; Cieśliński, T.; Bukało, M.; Kostyra, E. Influence of candidate polymorphisms on the dipeptidyl peptidase IV and μ-opioid receptor genes expression in aspect of the β-casomorphin-7 modulation functions in autism. Peptides 2015, 65, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, J.; Sienkiewicz-Szłapka, E.; Kuźbida, E.; Jarmołowska, B.; Kaczmarski, M.; Kostyra, E. The exogenous opioid peptides and DPPIV serum activity in infants with apnoea expressed as apparent life threatening events (ALTE). Neuropeptides 2011, 45, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Teschemacher, H. Opioid receptor ligands derived from food proteins. Curr. Pharm. Des. 2003, 9, 1331–1344. [Google Scholar] [CrossRef] [PubMed]
- Haq, M.R.U.; Kapila, R.; Sharma, R.; Saliganti, V.; Kapila, S. Comparative evaluation of cow β-casein variants (A1/A2) consumption on Th 2-mediated inflammatory response in mouse gut. Eur. J. Nutr. 2014, 53, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Kurek, M.; Rueff, F.; Czerwionka-Szaflarska, M.; Doroszewska, G.; Przybilla, B. Exorphins derived from cow’s milk casein elicit pseudo-allergic wheal-and-flare reactions in healthy children. French Rev. Allergol. Clin. Immunol. 1996, 36, 191–196. [Google Scholar] [CrossRef]
- Reddi, S.; Kapila, R.; Ajay Kumar Dang, A.K.; Kapila, S. Evaluation of allergenic response of milk bioactive peptides using mouse mast cell. Milchwissenschaft-Milk Sci. Internat. 2012, 67, 189. [Google Scholar]
- Lázaro, C.P.; Pondé, M.P.; Rodrigues, L.E. Opioid peptides and gastrointestinal symptoms in autism spectrum disorders. Rev. Bras. Psiquiatr. 2016, 38, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.S.; Hodgson, N.W.; Walker, S.J.; Trooskens, G.; Nair, V.; Deth, R.C. Epigenetic effects of casein-derived opioid peptides in SH-SY5Y human neuroblastoma cells. Nutr. Metab. 2015, 12, 54. [Google Scholar] [CrossRef]
- Pennesi, C.M.; Klein, L.C. Effectiveness of the gluten-free, casein-free diet for children diagnosed with autism spectrum disorder: Based on parental report. Nutr. Neurosci. 2012, 15, 85–91. [Google Scholar] [CrossRef]
- Reichelt, K.L.; Knivsberg, A.M. The possibility and probability of a gut-to-brain connection in autism. Ann. Clin. Psychiat. 2009, 21, 205–211. [Google Scholar]
- Kieffer, B.L.; Gavériaux-Ruff, C. Exploring the opioid system by gene knockout. Prog. Neurobiol. 2002, 66, 285–306. [Google Scholar] [CrossRef]
- Durinx, C.; Neels, H.; Van Der Auwera, J.C.; Naelaerts, K.; Scharpé, S.; De Meester, I. Reference values for plasma dipeptidyl peptidase IV activity and their association with other laboratory parameters. Clin. Chem. Lab. Med. 2001, 39, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Osawa, S.; Kawamori, D.; Katakami, N.; Takahara, M.; Sakamoto, F.; Katsura, T.; Yasuda, T.; Kaneto, H.; Matsuhisa, M.; Matsuoka, T.A.; et al. Significant elevation of serum dipeptidyl peptidase-4 activity in young-adult type 1 diabetes. Diabetes Res. Clin. Pract. 2016, 113, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Laila, A.A. Alterations in plasma dipeptidyl peptidase IV in autism: A. pilot study. Neurol. Psychiat. Brain Res. 2014, 20, 41–44. [Google Scholar] [CrossRef]
- Hunter, L.C.; O’hare, A.; Herron, W.J.; Fisher, L.A.; Jones, G.E. Opioid peptides and dipeptidyl peptidase in autism. Dev. Med. Child. Neurol. 2003, 45, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, D.W.; Rapin, I.; Goldman, S. Male predominance in autism: Neuroendocrine influences on arousal and social anxiety. Autism Res. 2011, 4, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Lombardo, M.V.; Auyeung, B.; Ashwin, E.; Chakrabarti, B.; Knickmeyer, R. Why are autism spectrum conditions more prevalent in males? PLoS Biol. 2011, 9, 1001–1081. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.; Miller, M.; Taylor, S.L.; Hinshaw, S.P.; Carter, C.S. Autism symptoms and internalizing psychopathology in girls and boys with autism spectrum disorders. J. Autism Dev. Disord. 2012, 42, 48–59. [Google Scholar] [CrossRef]
- Singh, S.; Yazdani, U.; Gadad, B.; Zaman, S.; Hynan, L.S.; Roatch, N.; Schutte, C.; Marti, C.N.; Hewitson, L.; German, D.C. Serum thyroid-stimulating hormone and interleukin-8 levels in boys with autism spectrum disorder. J. Neuroinflammat. 2017, 14, 113. [Google Scholar] [CrossRef]
- Anderson, G.W.; Schoonover, C.M.; Jones, S.A. Control of thyroid hormone action in the developing rat brain. Thyroid 2003, 13, 1039–1056. [Google Scholar] [CrossRef]

| Gene | Forward Primer | Revers Primer | Annealing Temperature | Base Pairs |
|---|---|---|---|---|
| ACTB NM-001101.3 | 5′-TCC CTG GAG GAA GAG CTA CGA-3′ | 5′-AGC ACT GTG TTG GCG TAC G-3 | 60 °C | 194 bp |
| DPPIV NM-001935 | 5′-GAA TTA TCC GGT CGG GTT TT-3′ | 5′-GTG ACA TCA CTG CCC ACA TC-3′ | 60 °C | 189 bp |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarmołowska, B.; Bukało, M.; Fiedorowicz, E.; Cieślińska, A.; Kordulewska, N.K.; Moszyńska, M.; Świątecki, A.; Kostyra, E. Role of Milk-Derived Opioid Peptides and Proline Dipeptidyl Peptidase-4 in Autism Spectrum Disorders. Nutrients 2019, 11, 87. https://doi.org/10.3390/nu11010087
Jarmołowska B, Bukało M, Fiedorowicz E, Cieślińska A, Kordulewska NK, Moszyńska M, Świątecki A, Kostyra E. Role of Milk-Derived Opioid Peptides and Proline Dipeptidyl Peptidase-4 in Autism Spectrum Disorders. Nutrients. 2019; 11(1):87. https://doi.org/10.3390/nu11010087
Chicago/Turabian StyleJarmołowska, Beata, Marta Bukało, Ewa Fiedorowicz, Anna Cieślińska, Natalia Karolina Kordulewska, Małgorzata Moszyńska, Aleksander Świątecki, and Elżbieta Kostyra. 2019. "Role of Milk-Derived Opioid Peptides and Proline Dipeptidyl Peptidase-4 in Autism Spectrum Disorders" Nutrients 11, no. 1: 87. https://doi.org/10.3390/nu11010087
APA StyleJarmołowska, B., Bukało, M., Fiedorowicz, E., Cieślińska, A., Kordulewska, N. K., Moszyńska, M., Świątecki, A., & Kostyra, E. (2019). Role of Milk-Derived Opioid Peptides and Proline Dipeptidyl Peptidase-4 in Autism Spectrum Disorders. Nutrients, 11(1), 87. https://doi.org/10.3390/nu11010087








