The Effects of Quercetin Supplementation on Eccentric Exercise-Induced Muscle Damage
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
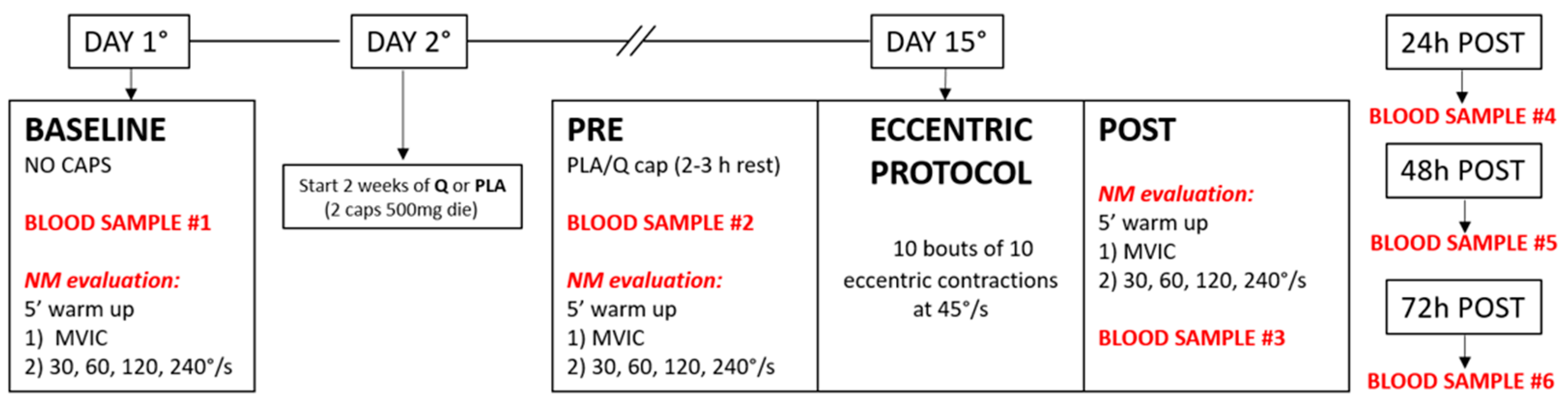
2.2. Experimental Overview
2.3. Treatment
2.4. NM Evaluation
- 1
- MVIC: The joint angle was fixed at 90° (0°, full extension). The task consisted of rapidly increasing the force “as hard as possible” to produce a maximal contraction with the elbow flexors. All participants were verbally encouraged to exceed a visual target and to maintain the MVIC for 2–3 s before relaxing. Three attempts separated by 5 min were performed.
- 2
2.5. Eccentric Protocol
2.6. Indices of EEIMD
2.7. Blood Samples Collection
2.8. Data Analysis
2.8.1. NM Data
2.8.2. Biochemical Analyses
2.9. Statistical Analysis
3. Results
3.1. Eccentric Protocol
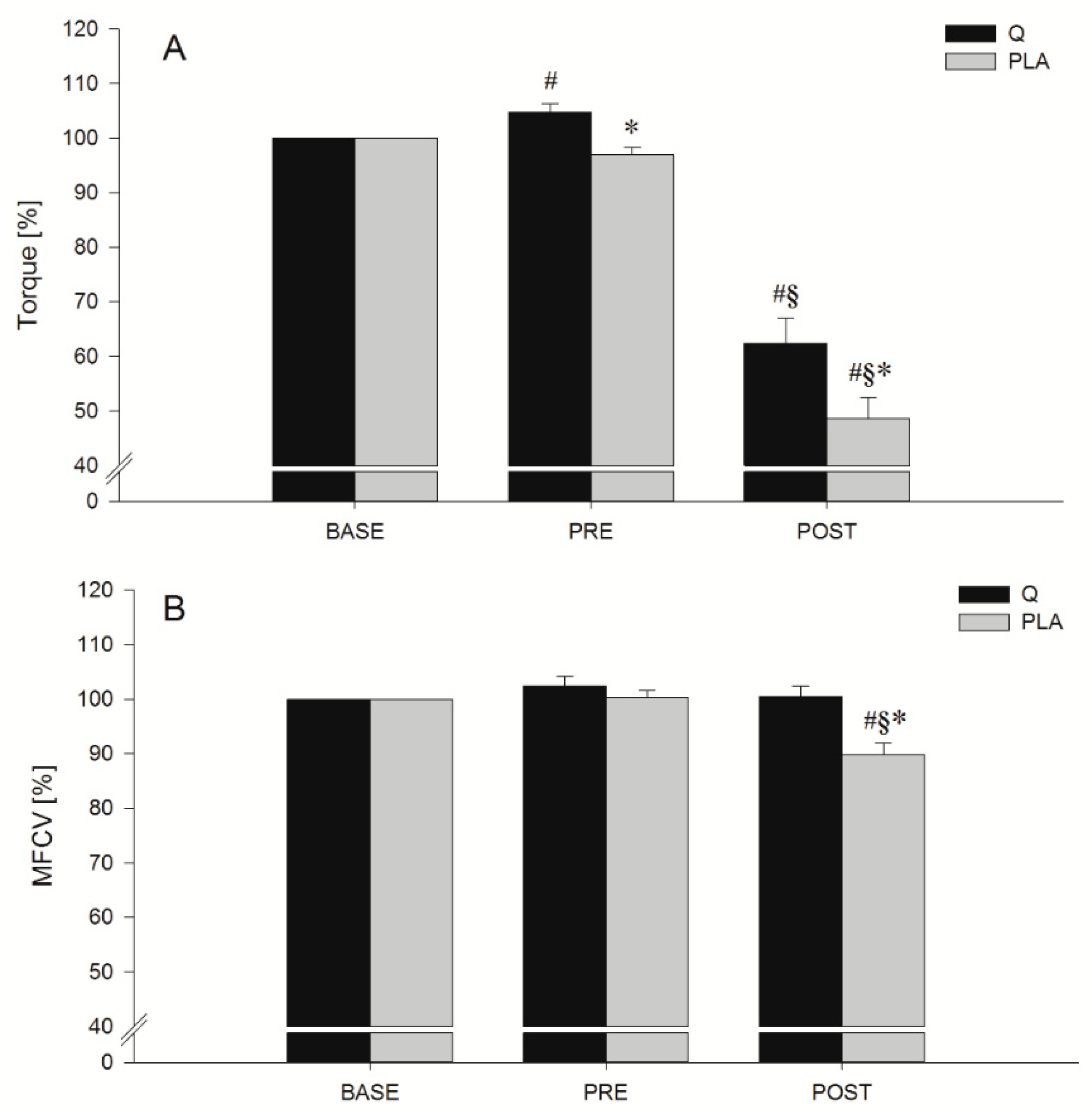
3.2. MVIC
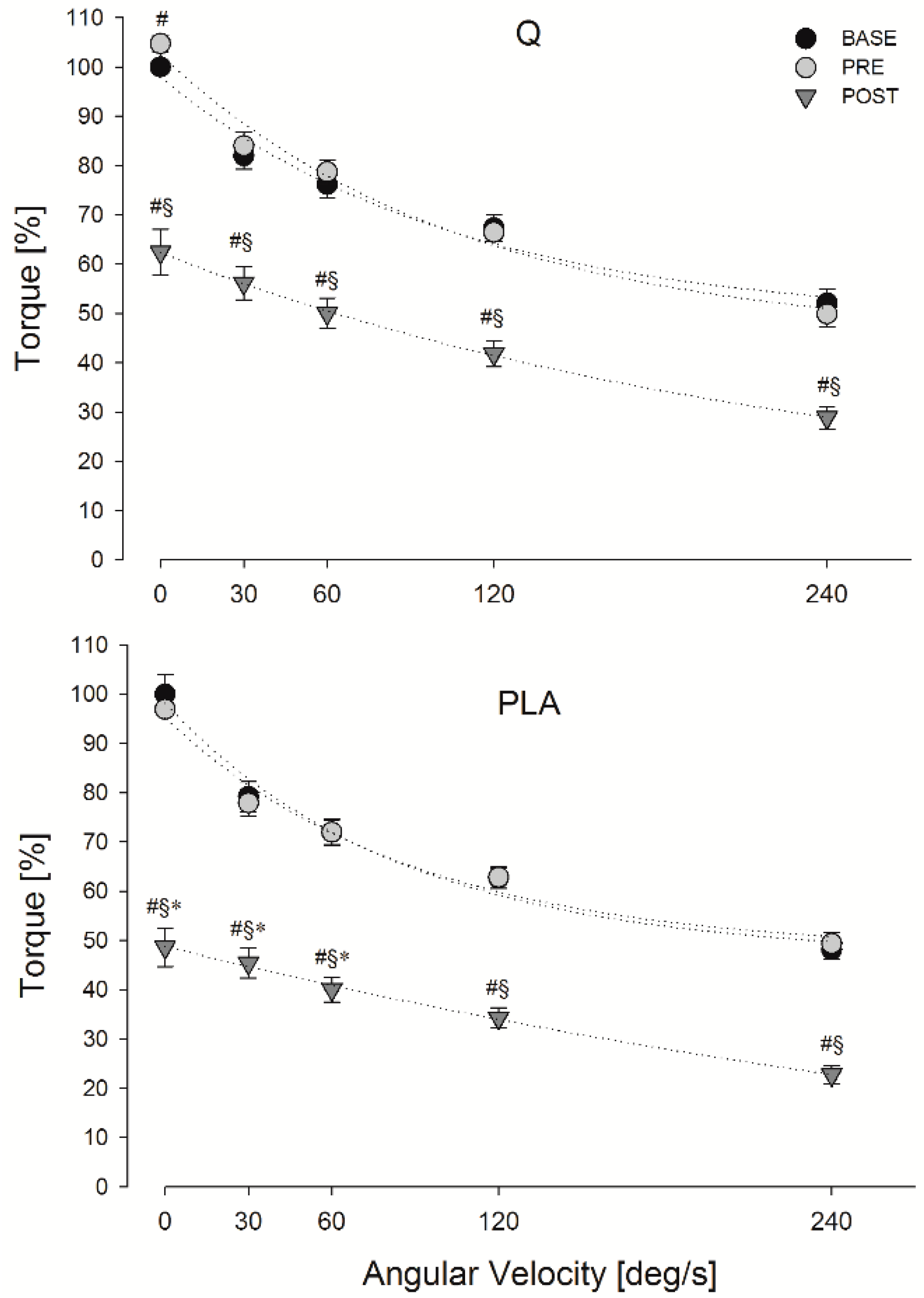
3.3. FV Relationship Task
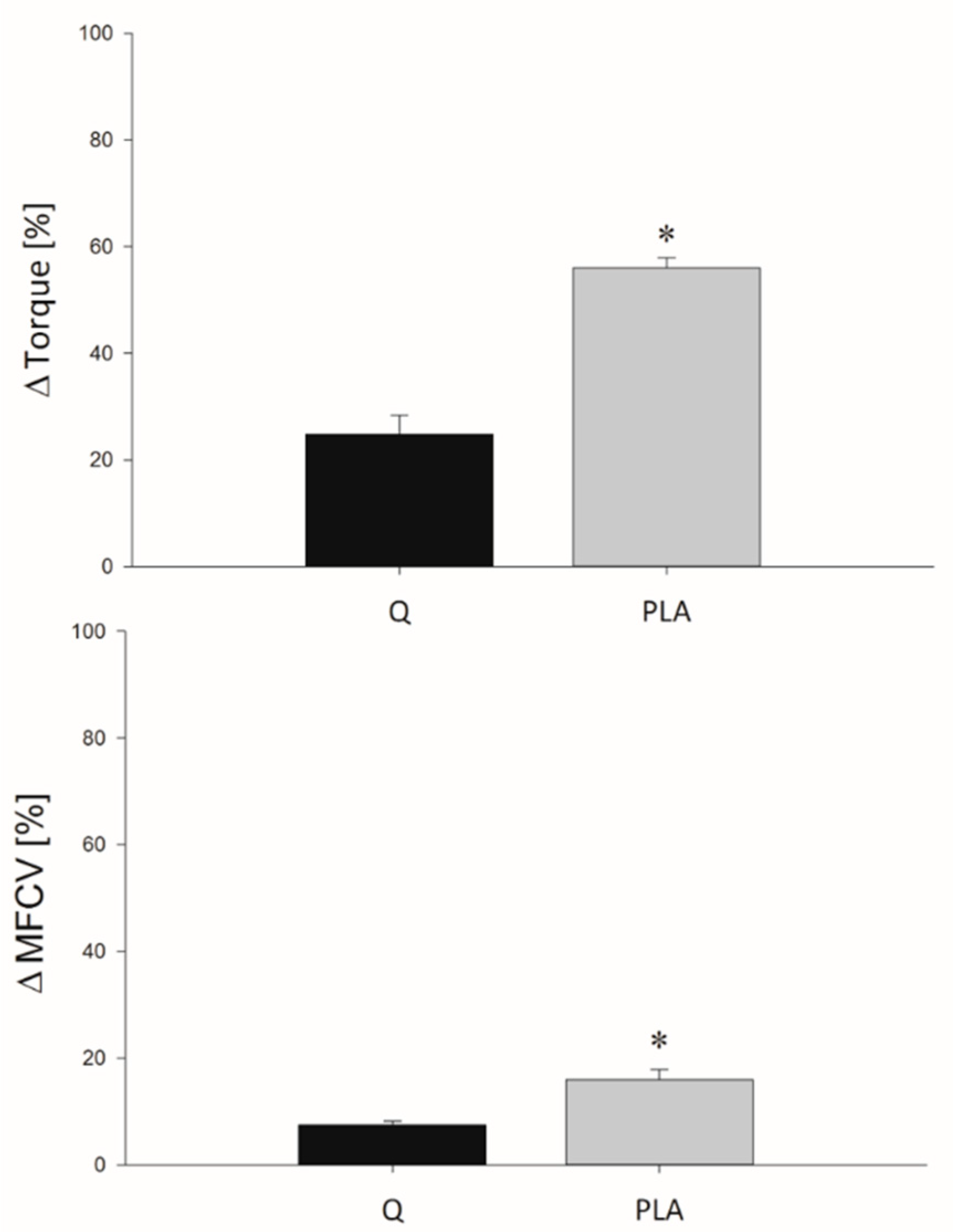
3.4. Indices of EEIMD
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Proske, U.; Morgan, D.L. Muscle damage from eccentric exercise: Mechanism, mechanical signs, adaptations and clinical applications. J. Physiol. 2001, 537, 333–345. [Google Scholar] [CrossRef] [PubMed]
- McGinley, C.; Shafat, A.; Donnelly, A.E. Does antioxidant vitamin supplementation protect against muscle damage? Sports Med. 2009, 39, 1011–1032. [Google Scholar] [CrossRef] [PubMed]
- Pittaluga, M.; Sgadari, A.; Tavazzi, B.; Fantini, C.; Sabatini, S.; Ceci, R.; Amorini, A.M.; Parisi, P.; Caporossi, D. Exercise-induced oxidative stress in elderly subjects: The effect of red orange supplementation on the biochemical and cellular response to a single bout of intense physical activity. Free Radic. Res. 2013, 47, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Abbey, E.L.; Rankin, J.W. Effect of quercetin supplementation on repeated-sprint performance, xanthine oxidase activity, and inflammation. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Bigelman, K.A.; Fan, E.H.; Chapman, D.P.; Freese, E.C.; Trilk, J.L.; Cureton, K.J. Effects of six weeks of quercetin supplementation on physical performance in ROTC cadets. Mil. Med. 2010, 175, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Cureton, K.J.; Tomporowski, P.D.; Singhal, A.; Pasley, J.D.; Bigelman, K.A.; Lambourne, K.; Trilk, J.L.; McCully, K.K.; Arnaud, M.J.; Zhao, Q. Dietary quercetin supplementation is not ergogenic in untrained men. J. Appl. Physiol. 2009, 107, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Duranti, G.; Ceci, R.; Patrizio, F.; Sgrò, P.; Di Luigi, L.; Sabatini, S.; Felici, F.; Bazzucchi, I. Chronic consumption of quercetin reduces erythrocytes oxidative damage: Evaluation at resting and after eccentric exercise in humans. Nutr. Res. 2018, 50, 73–81. [Google Scholar] [CrossRef]
- Konrad, M.; Nieman, D.C.; Henson, D.A.; Kennerly, K.M.; Jin, F.; Wallner-Liebmann, S.J. The acute effect of ingesting a quercetin-based supplement on exercise-induced inflammation and immune changes in runners. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 338–346. [Google Scholar] [CrossRef]
- O’Fallon, K.S.; Kaushik, D.; Michniak-Kohn, B.; Dunne, C.P.; Zambraski, E.J.; Clarkson, P.M. Effects of quercetin supplementation on markers of muscle damage and inflammation after eccentric exercise. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 430–437. [Google Scholar] [CrossRef]
- Bazzucchi, I.; De Vito, G.; Felici, F.; Dewhurst, S.; Sgadari, A.; Sacchetti, M. Effect of exercise training on neuromuscular function of elbow flexors and knee extensors of type 2 diabetic patients. J. Electromyogr. Kinesiol. 2015, 25, 815–823. [Google Scholar] [CrossRef]
- Bazzucchi, I.; Felici, F.; Montini, M.; Figura, F.; Sacchetti, M. Caffeine improves neuromuscular function during maximal dynamic exercise. Muscle Nerve 2011, 43, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Bazzucchi, I.; Felici, F.; Sacchetti, M. Effect of Short-Term Creatine Supplementation on Neuromuscular Function. Med. Sci. Sport. Exerc. 2009, 41, 1934–1942. [Google Scholar] [CrossRef] [PubMed]
- Prasartwuth, O.; Taylor, J.L.; Gandevia, S.C. Maximal force, voluntary activation and muscle soreness after eccentric damage to human elbow flexor muscles. J. Physiol. 2005, 567, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Hübscher, M.; Tu, S.; Stanton, T.; Moseley, G.L.; Wand, B.M.; Booth, J.; McAuley, J.H. Movement restriction does not modulate sensory and perceptual effects of exercise-induced arm pain. Eur. J. Appl. Physiol. 2015, 115, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, K.; Clarkson, P.M. Muscle damage following repeated bouts of high force eccentric exercise. Med. Sci. Sports Exerc. 1995, 27, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Ceci, R.; Duranti, G.; Sgrò, P.; Sansone, M.; Guidetti, L.; Baldari, C.; Sabatini, S.; Di Luigi, L. Effects of tadalafil administration on plasma markers of exercise-induced muscle damage, IL6 and antioxidant status capacity. Eur. J. Appl. Physiol. 2014, 115, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Ganio, M.S.; Armstrong, L.E.; Johnson, E.C.; Klau, J.F.; Ballard, K.D.; Michniak-Kohn, B.; Kaushik, D.; Maresh, C.M. Effect of quercetin supplementation on maximal oxygen uptake in men and women. J. Sports Sci. 2010, 28, 201–208. [Google Scholar] [CrossRef]
- Davis, J.M.; Carlstedt, C.J.; Chen, S.; Carmichael, M.D.; Murphy, E.A. The dietary flavonoid quercetin increases VO2max and endurance capacity. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 56–62. [Google Scholar] [CrossRef]
- Di Luigi, L.; Baldari, C.; Pigozzi, F.; Emerenziani, G.P.; Gallotta, M.C.; Iellamo, F.; Ciminelli, E.; Sgrò, P.; Romanelli, F.; Lenzi, A.; et al. The long-acting phosphodiesterase inhibitor tadalafil does not influence athletes’ VO2max, aerobic, and anaerobic thresholds in normoxia. Int. J. Sports Med. 2008, 29, 110–115. [Google Scholar] [CrossRef]
- Kressler, J.; Millard-Stafford, M.; Warren, G.L. Quercetin and endurance exercise capacity: A systematic review and meta-analysis. Med. Sci. Sports Exerc. 2011, 43, 2396–2404. [Google Scholar] [CrossRef]
- Pelletier, D.M.; Lacerte, G.; Goulet, E.D.B. Effects of quercetin supplementation on endurance performance and maximal oxygen consumption: A meta-analysis. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Davis, B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am. J. Physiol. 2009, 296, 1071–1077. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; Maxwell, K.R.; Williams, A.S.; Mcanulty, S.R.; Jin, F.; Shanely, R.A.; Lines, T.C. Effects of quercetin and egcg on mitochondrial biogenesis and immunity. Med. Sci. Sports Exerc. 2009, 41, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Ohnishi, S.T.; Ikemoto, N. Kinetic studies of calcium release from sarcoplasmic reticulum in vitro. J. Biol. Chem. 1983, 258, 9662–9668. [Google Scholar] [PubMed]
- Lee, E.H.; Meissner, G.; Kim, D.H. Effects of Quercetin on Single Ca2+ Release Channel Behavior of Skeletal Muscle. Biophys. J. 2002, 82, 1266–1277. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef]
- Reid, M.B.; Khawli, F.A.; Moody, M.R. Reactive oxygen in skeletal muscle. III. Contractility of unfatigued muscle. J. Appl. Physiol. 1993, 75, 1081–1087. [Google Scholar] [CrossRef]
- Warren, G.; Lowe, D.; Armstrong, R. Measurement tools used in the study of eccentric contraction—Induced injury. Sport. Med. 1999, 27, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Merry, T.L.; Ristow, M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J. Physiol. 2016, 594, 5135–5147. [Google Scholar] [CrossRef]
- Paulsen, G.; Hamarsland, H.; Cumming, K.T.; Johansen, R.E.; Hulmi, J.J.; Børsheim, E.; Wiig, H.; Garthe, I.; Raastad, T. Vitamin C and E supplementation alters protein signalling after a strength training session, but not muscle growth during 10 weeks of training. J. Physiol. 2014, 592, 5391–5408. [Google Scholar] [CrossRef]
- Çelik, N.; Vurmaz, A.; Kahraman, A. Protective effect of quercetin on homocysteine-induced oxidative stress. Nutrition 2017, 33, 291–296. [Google Scholar] [CrossRef]
- Scholten, S.D.; Sergeev, I.N. Long-term quercetin supplementation reduces lipid peroxidation but does not improve performance in endurance runners. Open Access J. Sport. Med. 2013, 4, 53–61. [Google Scholar] [CrossRef]
- Boots, A.W.; Wilms, L.C.; Swennen, E.L.R.; Kleinjans, J.C.S.; Bast, A.; Haenen, G.R.M.M. In vitro and ex vivo anti-inflammatory activity of quercetin in healthy volunteers. Nutrition 2008, 24, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.S. Quercetin. Altern. Med. Rev. 2011, 16, 172–194. [Google Scholar]
- Santos, M.R.; Mira, L. Protection by flavonoids against the peroxynitrite-mediated oxidation of dihydrorhodamine. Free Radic. Res. 2004, 38, 1011–1018. [Google Scholar] [CrossRef]
- Wai, M.L.; Proudfoot, J.M.; Mckinley, A.J.; Needs, P.W.; Kroon, P.A.; Hodgson, J.M.; Croft, K.D. Quercetin and its in vivo metabolites inhibit neutrophil-mediated low-density lipoprotein oxidation. J. Agric. Food Chem. 2008, 56, 3609–3615. [Google Scholar]
- Dorta, D.J.; Pigoso, A.A.; Mingatto, F.E.; Rodrigues, T.; Prado, I.M.R.; Helena, A.F.C.; Uyemura, S.A.; Santos, A.C.; Curti, C. The interaction of flavonoids with mitochondria: Effects on energetic processes. Chem. Biol. Interact. 2005, 152, 67–78. [Google Scholar] [CrossRef]
- Dorta, D.J.; Pigoso, A.A.; Mingatto, F.E.; Rodrigues, T.; Pestana, C.R.; Uyemura, S.A.; Santos, A.C.; Curti, C. Antioxidant activity of flavonoids in isolated mitochondria. Phyther. Res. 2008, 22, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D. Effects of the dietary flavonoid quercetin upon performance and health. Curr. Sports Med. Rep. 2009, 8, 206–213. [Google Scholar] [CrossRef]
- Shi, Y.; Williamson, G. Quercetin lowers plasma uric acid in pre-hyperuricaemic males: A randomised, double-blinded, placebo-controlled, cross-over trial. Br. J. Nutr. 2016, 115, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Semmler, J.G. Motor unit activity after eccentric exercise and muscle damage in humans. Acta Physiol. 2014, 210, 754–767. [Google Scholar] [CrossRef]
- Solomonow, M.; Baten, C.; Smit, J.; Baratta, R.; Hermens, H.; D’Ambrosia, R.; Shoji, H. Electromyogram power spectra frequencies associated with motor unit recruitment strategies. J. Appl. Physiol. 1990, 68, 1177–1185. [Google Scholar] [CrossRef]
- Cermak, N.M.; Snijders, T.; McKay, B.R.; Parise, G.; Verdijk, L.B.; Tarnopolsky, M.A.; Gibala, M.J.; Van Loon, L.J.C. Eccentric exercise increases satellite cell content in type II muscle fibers. Med. Sci. Sports Exerc. 2013, 45, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Hirose, L.; Nosaka, K.; Newton, M.; Laveder, A.; Kano, M.; Peake, J.; Suzuki, K. Changes in inflammatory mediators following eccentric exercise of the elbow flexors. Exerc. Immunol. Rev. 2004, 10, 75–90. [Google Scholar]
- Patrizio, F.; Ditroilo, M.; Felici, F.; Duranti, G.; De Vito, G.; Sabatini, S.; Sacchetti, M.; Bazzucchi, I. The acute effect of Quercetin on muscle performance following a single resistance training session. Eur. J. Appl. Physiol. 2018, 118, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
| MFCV (%) | ||||||
|---|---|---|---|---|---|---|
| 0 deg/s | 30 deg/s | 60 deg/s | 120 deg/s | 240 deg/s | ||
| Baseline | Q | 100 | 97.7 ± 1.7 | 100.3 ± 1.8 | 103.2 ± 2.0 | 105.8 ± 2.2 |
| PLA | 100 | 91.7 ± 1.9 | 94.7 ± 1.6 | 96.9 ± 1.8 | 99.6 ± 2.2 | |
| PRE | Q | 102.4 ± 1.8 | 98.7 ± 1.8 | 102.3 ± 1.8 | 105.6 ± 2.3 | 109.1 ± 2.3 |
| PLA | 100.2 ± 1.4 | 93.6 ± 1.7 | 96.5 ± 1.6 | 98.7 ± 1.6 | 101.4 ± 1.8 | |
| POST | Q | 100.5 ± 1.9 | 96.4 ± 2.1 | 98.9 ± 2.2 | 101.7 ± 2.3 | 105.1 ± 2.6 |
| PLA | 89.8 ± 2.1 #§ | 89.8 ± 1.9 | 92.2 ± 1.6 | 87.4 ± 2.7 #§* | 87.0 ± 2.2#§* | |
| Arm Circumference | Elbow Angle | VAS | ||
|---|---|---|---|---|
| cm | deg | cm | ||
| Baseline | Q | 31.1 ± 0.7 | 175.4 ± 1.4 | 0 |
| PLA | 30.6 ± 0.7 | 175.0 ± 1.9 | 0 | |
| PRE | Q | 31.1 ± 0.7 | 174.9 ± 1.4 | 0 |
| PLA | 30.7 ± 0.8 | 175.1 ± 1.5 | 0 | |
| POST | Q | 32.2 ± 0.7 #§ | 167.1 ± 1.8 #§ | 0.4 ± 0.2 |
| PLA | 32.2 ± 0.8 #§ | 161.6 ± 2.3 #§* | 1.2 ± 0.4 #§ | |
| CK | LDH | ||
|---|---|---|---|
| a.u. | a.u. | ||
| Baseline | Q | 1 | 1 |
| PLA | 1 | 1 | |
| PRE | Q | 0.98 ± 0.11 | 0.99 ± 0.05 |
| PLA | 1.18 ± 0.19 | 1.11 ± 0.07 | |
| POST | Q | 0.91 ± 0.08 | 1.04 ± 0.06 |
| PLA | 1.22 ± 0.18 | 1.20 ± 0.09 | |
| 24 h | Q | 1.15 ± 0.14 | 1.07 ± 0.04 |
| PLA | 1.73 ± 0.28 | 1.33 ± 0.08 * | |
| 48 h | Q | 1.20 ± 0.17 | 1.26 ± 0.06 |
| PLA | 2.54 ± 0.49 * | 1.44 ± 0.07 * | |
| 72 h | Q | 1.26 ± 0.15 | 1.23 ± 0.06 |
| PLA | 5.89 ± 0.58 * | 1.49 ± 0.09 * | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazzucchi, I.; Patrizio, F.; Ceci, R.; Duranti, G.; Sgrò, P.; Sabatini, S.; Di Luigi, L.; Sacchetti, M.; Felici, F. The Effects of Quercetin Supplementation on Eccentric Exercise-Induced Muscle Damage. Nutrients 2019, 11, 205. https://doi.org/10.3390/nu11010205
Bazzucchi I, Patrizio F, Ceci R, Duranti G, Sgrò P, Sabatini S, Di Luigi L, Sacchetti M, Felici F. The Effects of Quercetin Supplementation on Eccentric Exercise-Induced Muscle Damage. Nutrients. 2019; 11(1):205. https://doi.org/10.3390/nu11010205
Chicago/Turabian StyleBazzucchi, Ilenia, Federica Patrizio, Roberta Ceci, Guglielmo Duranti, Paolo Sgrò, Stefania Sabatini, Luigi Di Luigi, Massimo Sacchetti, and Francesco Felici. 2019. "The Effects of Quercetin Supplementation on Eccentric Exercise-Induced Muscle Damage" Nutrients 11, no. 1: 205. https://doi.org/10.3390/nu11010205
APA StyleBazzucchi, I., Patrizio, F., Ceci, R., Duranti, G., Sgrò, P., Sabatini, S., Di Luigi, L., Sacchetti, M., & Felici, F. (2019). The Effects of Quercetin Supplementation on Eccentric Exercise-Induced Muscle Damage. Nutrients, 11(1), 205. https://doi.org/10.3390/nu11010205









