Meal Enjoyment and Tolerance in Women and Men
Abstract
:1. Introduction
2. Material and Methods
2.1. Participants
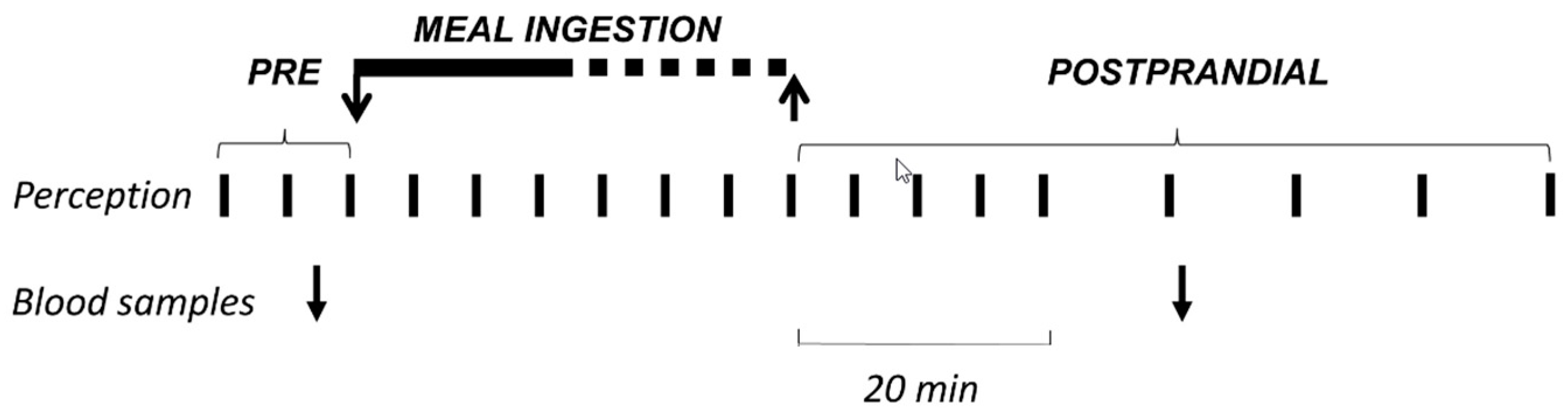
2.2. Experimental Design
2.3. Randomization and Masking
2.4. General Procedure
2.5. Probe Meal
2.6. Perception Measurements
2.7. Analytical Procedures
2.8. Statistical Analysis
3. Results
3.1. Demographics
3.2. Baseline Conditions
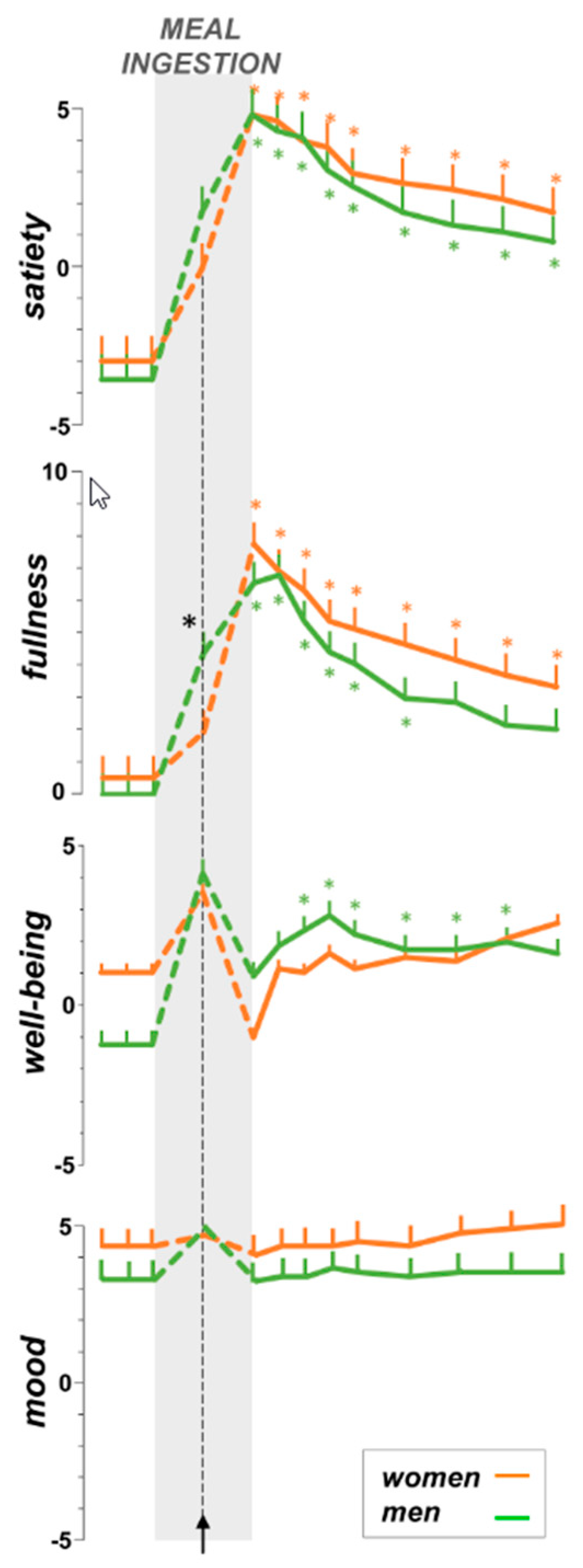
3.3. Sensations during Meal Ingestion
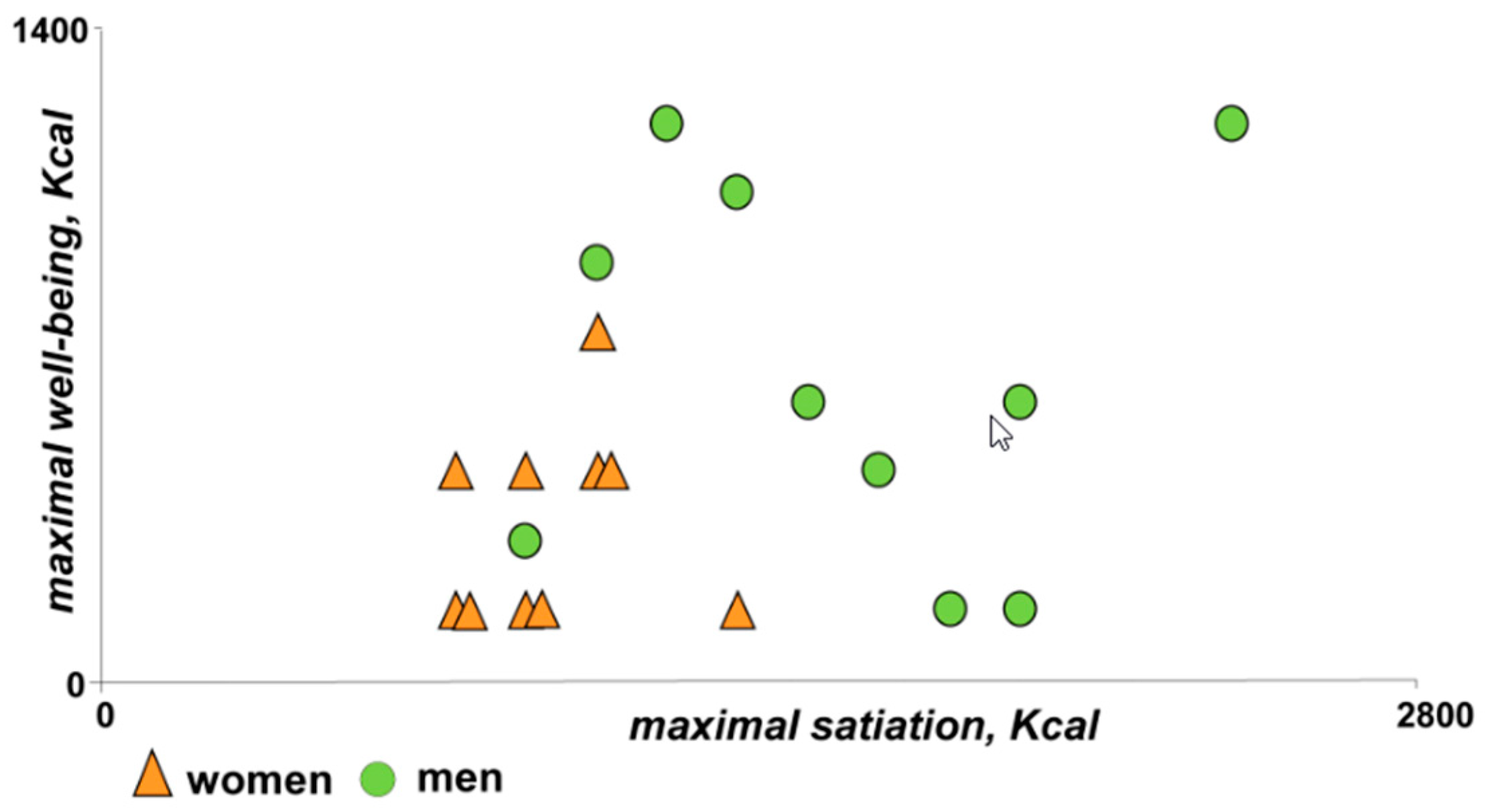
3.4. The Post-Prandial Experience
3.4.1. Homeostatic Sensations
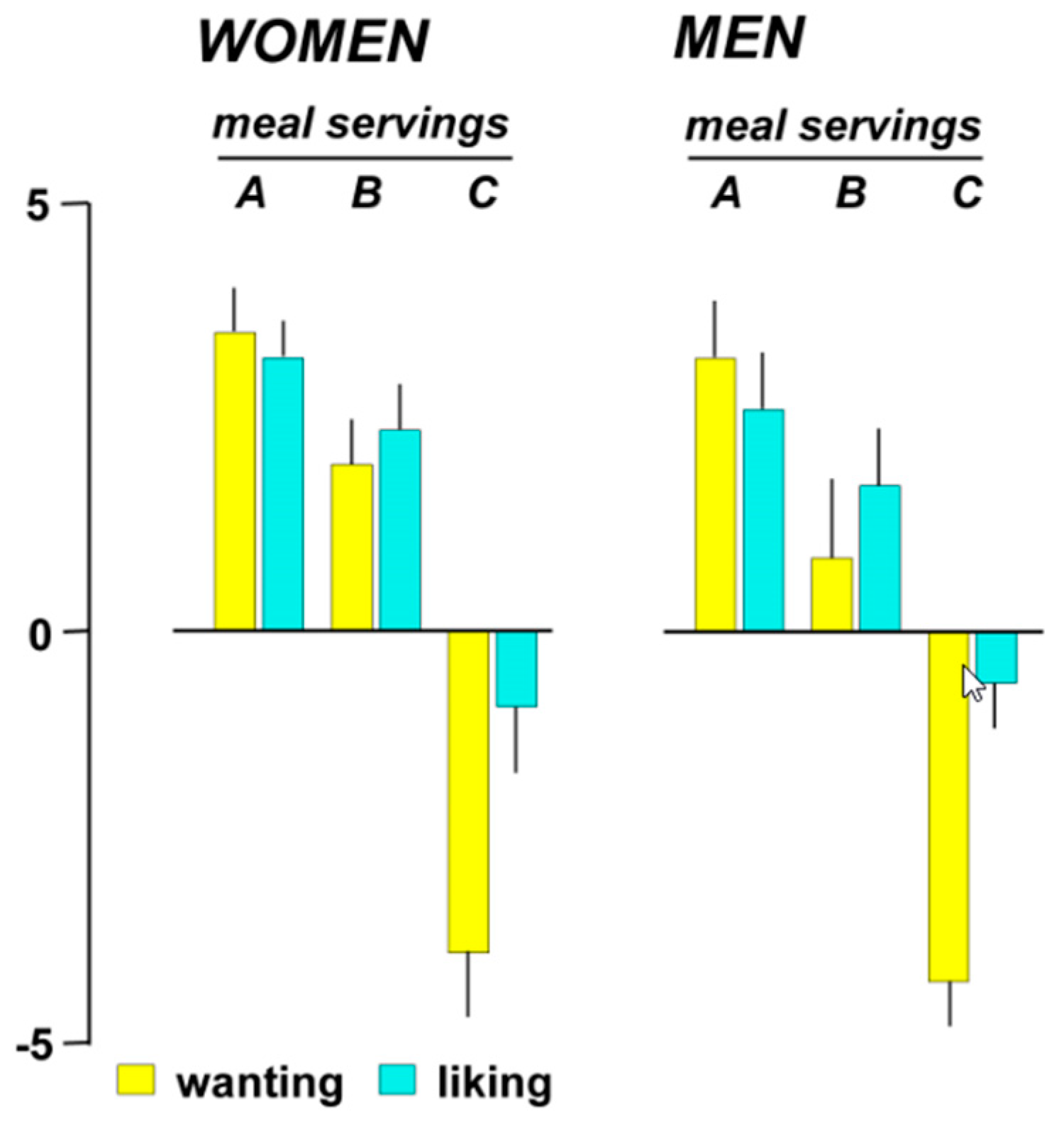
3.4.2. Hedonic Responses
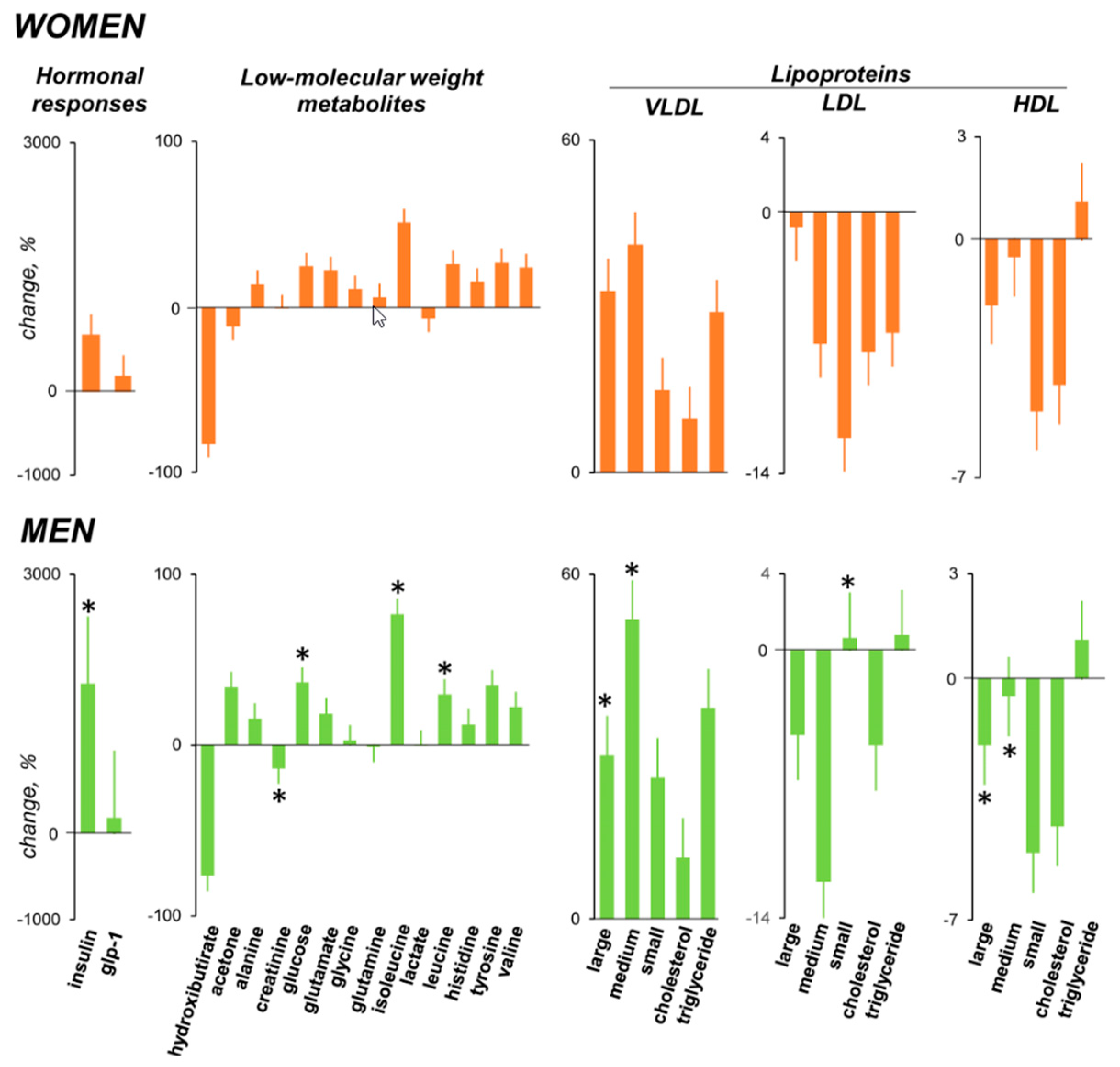
3.5. Hormonal Response
3.6. Metabolomic Response
3.6.1. Low-Molecular Weight Metabolites
3.6.2. Lipoprotein Profile
4. Discussions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Azpiroz, F.; Feinle, C.; Grundy, D.; Tack, J. Gastric sensitivity and reflexes: Basic mechanism underlying clinical problems. J. Gastroenterol. 2014, 49, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Boeckxstaens, G.; Camilleri, M.; Sifrim, D.; Houghton, L.A.; Elsenbruch, S.; Lindberg, G.; Azpiroz, F.; Parkman, H.P. Fundamentals of Neurogastroenterology: Physiology/Motility—Sensation. Gastroenterology 2016, 150, 1292–1304. [Google Scholar] [CrossRef] [PubMed]
- Pribic, T.; Azpiroz, F. Biogastronomy: Factors that determine the biological response to meal ingestion. Neurogastroenterol. Motil. 2018, 30, e13309. [Google Scholar] [CrossRef]
- Malagelada, C.; Accarino, A.; Molne, L.; Mendez, S.; Campos, E.; Gonzalez, A.; Malagelada, J.R.; Azpiroz, F. Digestive, cognitive and hedonic responses to a meal. Neurogastroenterol. Motil. 2015, 27, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Ciccantelli, B.; Pribic, T.; Malagelada, C.; Accarino, A.; Azpiroz, F. Relation between cognitive and hedonic responses to a meal. Neurogastroenterol. Motil. 2017, 29, e13011. [Google Scholar] [CrossRef] [PubMed]
- Pribic, T.; Hernandez, L.; Nieto, A.; Malagelada, C.; Accarino, A.; Azpiroz, F. Effects of meal palatability on postprandial sensations. Neurogastroenterol. Motil. 2018, 30, e13197. [Google Scholar] [CrossRef]
- Pribic, T.; Vilaseca, H.; Nieto, A.; Hernandez, L.; Monrroy, H.; Malagelada, C.; Accarino, A.; Roca, J.; Azpiroz, F. Meal composition influences postprandial sensations independently of valence and gustation. Neurogastroenterol. Motil. 2018, 30, e13337. [Google Scholar] [CrossRef]
- Pribic, T.; Nieto, A.; Hernandez, L.; Malagelada, C.; Accarino, A.; Azpiroz, F. Appetite influences the responses to meal ingestion. Neurogastroenterol. Motil. 2017, 29, e13072. [Google Scholar] [CrossRef]
- Pribic, T.; Vilaseca, H.; Nieto, A.; Hernandez, L.; Malagelada, C.; Accarino, A.; Azpiroz, F. Education of the postprandial experience by a sensory-cognitive intervention. Neurogastroenterol. Motil. 2017, 30, e13197. [Google Scholar] [CrossRef]
- Malagelada, C.; Barba, I.; Accarino, A.; Molne, L.; Mendez, S.; Campos, E.; González, A.; Alonso-Cotoner, C.; Santos, J.; Malagelada, J.R.; et al. Cognitive and hedonic responses to meal ingestion correlate with changes in circulating metabolites. Neurogastroenterol. Motil. 2016, 28, 1806–1814. [Google Scholar] [CrossRef]
- Malagelada, C.; Pribic, T.; Ciccantelli, B.; Cañellas, N.; Gomez, J.; Amigo, N.; Accarino, A.; Correig, X.; Azpiroz, F. Metabolomic signature of the postprandial experience. Neurogastroenterol. Motil. 2018, 30, e13447. [Google Scholar] [CrossRef] [PubMed]
- Pribic, T.; Kilpatrick, L.; Ciccantelli, B.; Malagelada, C.; Accarino, A.; Rovira, A.; Parto, D.; Mayer, E.; Azpiroz, F. Brain networks associated with cognitive and hedonic responses to a meal. Neurogastroenterol. Motil. 2017, 29, e13031. [Google Scholar] [CrossRef] [PubMed]
- Mallol, R.; Amigó, N.; Rodríguez, M.A.; Heras, M.; Vinaixa, M.; Plana, N.; Rock, E.; Ribalta, J.; Yanes, O.; Masana, L.; et al. Liposcale: A novel advanced lipoprotein test based on 2D diffusion-ordered 1H NMR spectroscopy. J. Lipid Res. 2015, 56, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.; Brezmes, J.; Mallol, R.; Rodríguez, M.A.; Vinaixa, M.; Salek, R.M.; Correig, X.; Cañellas, N. Dolphin: A tool for automatic targeted metabolite profiling using 1D and 2D 1H-NMR data. Anal. Bioanal. Chem. 2014, 406, 7967–7976. [Google Scholar] [CrossRef] [PubMed]
- Nagana Gowda, G.A.; Gowda, Y.N.; Raftery, D. Expanding the limits of human blood metabolite quantitation using NMR spectroscopy. Anal. Chem. 2015, 87, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Cloarec, O.; Dumas, M.E.; Craig, A.; Barton, R.H.; Trygg, J.; Hudson, J.; Blancher, C.; Gauguier, D.; Lindon, J.C.; Holmes, E.; et al. Statistical total correlation spectroscopy: An exploratory approach for latent biomarker identification from metabolic 1H NMR data sets. Anal. Chem. 2005, 77, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]
- Blundell, J.; de Graaf, C.; Hulshof, T.; Jebb, S.; Livingstone, B.; Lluch, A.; Mela, D.; Salah, S.; Schuring, E.; van der Knaap, H.; et al. Appetite control: Methodological aspects of the evaluation of foods. Obes. Rev. 2010, 11, 251–270. [Google Scholar] [CrossRef]
- Bedard, A.; Hudon, A.M.; Drapeau, V.; Corneau, L.; Dodin, S.; Lemieux, S. Gender Differences in the Appetite Response to a Satiating Diet. J. Obes. 2015, 2015, 140139. [Google Scholar] [CrossRef]
- Gregersen, N.T.; Moller, B.K.; Raben, A.; Kristensen, S.T.; Holm, L.; Flint, A.; Astrup, A. Determinants of appetite ratings: The role of age, gender, BMI, physical activity, smoking habits, and diet/weight concern. Food Nutr. Res. 2011, 55, 7028. [Google Scholar] [CrossRef]
- Zylan, K.D. Gender differences in the reasons given for meal termination. Appetite 1996, 26, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Arciero, P.J.; Goran, M.I.; Poehlman, E.T. Resting metabolic rate is lower in women than in men. J. Appl. Physiol. 1993, 75, 2514–2520. [Google Scholar] [CrossRef]
- Wu, B.N.; O’Sullivan, A.J. Sex differences in energy metabolism need to be considered with lifestyle modifications in humans. J. Nutr. Metab. 2011, 2011, 391809. [Google Scholar] [CrossRef]
- Redman, L.M.; Kraus, W.E.; Bhapkar, M.; Das, S.K.; Racette, S.B.; Martin, C.K.; Fontana, L.; Wong, W.W.; Roberts, S.B.; Ravussin, E.; et al. Energy requirements in nonobese men and women: Results from CALERIE. Am. J. Clin. Nutr. 2014, 99, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Asarian, L.; Geary, N. Sex differences in the physiology of eating. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1215–R1267. [Google Scholar] [CrossRef] [PubMed]
- Chial, H.J.; Camilleri, C.; Delgado-Aros, S.; Burton, D.; Thomforde, G.; Ferber, I.; Camilleri, M. A nutrient drink test to assess maximum tolerated volume and postprandial symptoms: Effects of gender, body mass index and age in health. Neurogastroenterol. Motil. 2002, 14, 249–253. [Google Scholar] [CrossRef]
- Boeckxstaens, G.E.; Hirsch, D.P.; van den Elzen, B.D.; Heisterkamp, S.H.; Tytgat, G.N. Impaired drinking capacity in patients with functional dyspepsia: Relationship with proximal stomach function. Gastroenterology 2001, 121, 1054–1063. [Google Scholar] [CrossRef]
- Abid, S.; Anis, M.K.; Azam, Z.; Jafri, W.; Lindberg, G. Satiety drinking tests: Effects of caloric content, drinking rate, gender, age, and body mass index. Scand. J. Gastroenterol. 2009, 44, 551–556. [Google Scholar] [CrossRef]
- Mearadji, B.; Penning, C.; Vu, M.K.; van der Schaar, P.J.; van Petersen, A.S.; Kamerling, I.M.; Masclee, A.A. Influence of gender on proximal gastric motor and sensory function. Am. J. Gastroenterol. 2001, 96, 2066–2073. [Google Scholar] [CrossRef]
- Azpiroz, F.; Malagelada, J.R. Gastric tone measured by an electronic barostat in health and postsurgical gastroparesis. Gastroenterology 1987, 92, 934–943. [Google Scholar] [CrossRef]
- Moragas, G.; Azpiroz, F.; Pavía, J.; Malagelada, J.R. Relations among intragastric pressure, postcibal perception and gastric emptying. Am. J. Physiol. 1993, 264, G1112–G1117. [Google Scholar] [CrossRef] [PubMed]
- Hutson, W.; Roehrkasse, L. Influence of gender and menopause on gastric emptying and motility. Gastroenterology 1989, 96, 11–17. [Google Scholar] [CrossRef]
- Bennink, R.; Peeters, M.; Van den Maegdenbergh, V.; Geypens, B.; Rutgeerts, P.; De Roo, M.; Mortelmans, L. Comparison of total and compartmental gastric emptying and antral motility between healthy men and women. Eur. J. Nucl. Med. 1998, 25, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
| Total | Total | FAT | PROT | CHO | |
|---|---|---|---|---|---|
| g | kcal | g | g | g | |
| fatty liver duck + | 10 | 53.1 | 5.5 | 0.8 | 0.1 |
| toast | 3 | 12.2 | 0.1 | 0.4 | 2.3 |
| cheese | 10 | 34.7 | 2.7 | 2.6 | 0.0 |
| potato chips | 5 | 25.6 | 1.6 | 0.3 | 2.5 |
| peanuts | 2 | 12.8 | 1.1 | 0.6 | 0.2 |
| cola drink | 28 | 11.8 | 0.0 | 0.0 | 3.0 |
| per serving | 58 | 150.0 | 11.0 | 4.6 | 8.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monrroy, H.; Pribic, T.; Galan, C.; Nieto, A.; Amigo, N.; Accarino, A.; Correig, X.; Azpiroz, F. Meal Enjoyment and Tolerance in Women and Men. Nutrients 2019, 11, 119. https://doi.org/10.3390/nu11010119
Monrroy H, Pribic T, Galan C, Nieto A, Amigo N, Accarino A, Correig X, Azpiroz F. Meal Enjoyment and Tolerance in Women and Men. Nutrients. 2019; 11(1):119. https://doi.org/10.3390/nu11010119
Chicago/Turabian StyleMonrroy, Hugo, Teodora Pribic, Carmen Galan, Adoracion Nieto, Nuria Amigo, Anna Accarino, Xavier Correig, and Fernando Azpiroz. 2019. "Meal Enjoyment and Tolerance in Women and Men" Nutrients 11, no. 1: 119. https://doi.org/10.3390/nu11010119
APA StyleMonrroy, H., Pribic, T., Galan, C., Nieto, A., Amigo, N., Accarino, A., Correig, X., & Azpiroz, F. (2019). Meal Enjoyment and Tolerance in Women and Men. Nutrients, 11(1), 119. https://doi.org/10.3390/nu11010119






