Positive Effects of Tomato Paste on Vascular Function After a Fat Meal in Male Healthy Subjects
Abstract
1. Introduction
2. Methods
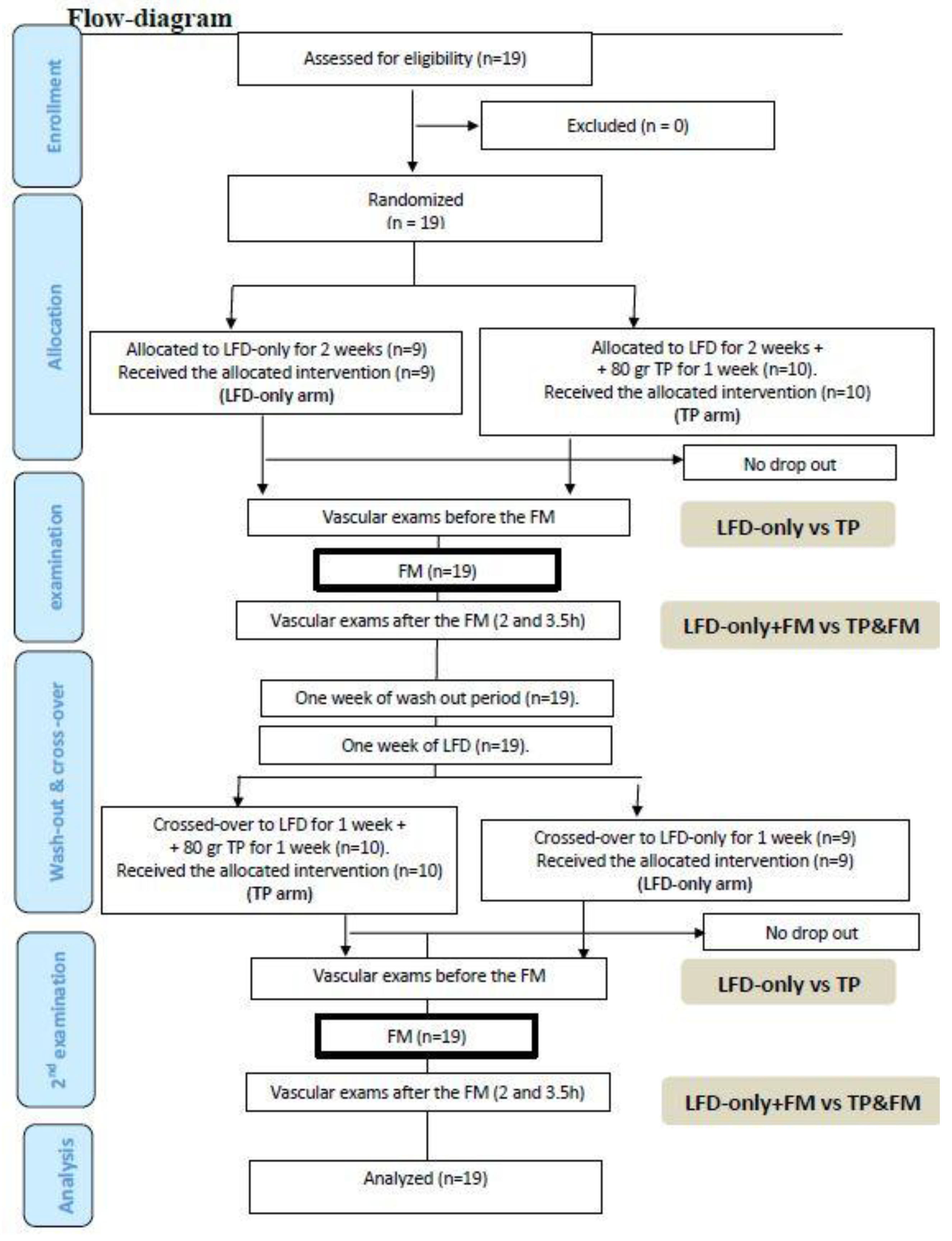
2.1. Study Design
2.2. Randomization and TOMATO Allocation
2.3. Assessments
2.4. Statistics
3. Results
Effect of the Intervention on Flow Mediated Dilatation and BP
4. Discussions
5. Conclusions
Supplementary Materials
Author contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BP | Blood pressure |
| FM | Fatty Meal |
| IMT | Intima Media Thickness |
| LFD | Low-Fiber Diet |
| NO | Nitric Oxide |
| TP | tomato paste |
| LFD | low fiber diet |
| FM | Fatty meal |
References
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Silaste, M.L.; Alfthan, G.; Aro, A.; Kesäniemi, Y.A.; Hörkkö, S. Tomato juice decreases LDL cholesterol levels and increases LDL resistance to oxidation. Br. J. Nutr. 2007, 98, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Kurata, M.; Suzuki, K.; Hamajima, N.; Hishida, H.; Aoki, K. Cardiovascular disease mortality and serum carotenoid levels: A Japanese population-based follow-up study. J. Epidemiol. 2006, 16, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Hak, A.E.; Ma, J.; Powell, C.B.; Campos, H.; Gaziano, J.M.; Willett, W.C.; Stampfer, M.J. Prospective Study of Plasma Carotenoids and Tocopherols in Relation to Risk of Ischemic Stroke. Stroke 2004, 35, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Buring, J.E.; Norkus, E.P.; Gaziano, J.M. Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in women. Am. J. Clin. Nutr. 2004, 79, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, T.H.; Voutilainen, S.; NyyssoÈnen, K.; Lakka, T.A.; Sivenius, J.; Salonen, R.; Kaplan, G.A.; Salonen, J.T. Low serum lycopene concentration is associated with an excess incidence of acute coronary events and stroke: The Kuopio Ischaemic Heart Disease Risk Factor Study. Br. J. Nutr. 2001, 85, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Karppi, J.; Laukkanen, J.A.; Sivenius, J.; Ronkainen, K.; Kurl, S. Serum lycopene decreases the risk of stroke in men: A population-based follow-up study. Neurology 2012, 79, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Hak, A.E.; Stampfer, M.J.; Campos, H.; Sesso, H.D.; Gaziano, J.M.; Willett, W.; Ma, J. Plasma carotenoids and tocopherols and risk of myocardial infarction in a low-risk population of US male physicians. Circulation 2003, 108, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Buring, J.E.; Norkus, E.P.; Gaziano, J.M. Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in men. Am. J. Clin. Nutr. 2005, 81, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.; Siervo, M.; Lara, J. Tomato and lycopene supplementation and cardiovascular risk factors: A systematic review and meta-analysis. Atherosclerosis 2017, 257, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Treggiari, D.; Dalbeni, A.; Meneguzzi, A.; Delva, P.; Fava, C.; Molesini, B.; Pandolfini, T.; Minuz, P. Lycopene inhibits endothelial cells migration induced by vascular endothelial growth factor A increasing nitric oxide bioavailability. J. Funct. Foods 2018, 42, 312–318. [Google Scholar] [CrossRef]
- Harrison, D.G.; Cai, H. Endothelial control of vasomotion and nitric oxide production. Cardiol. Clin. 2003, 21, 289–302. [Google Scholar] [CrossRef]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Vallance, P. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef]
- Millasseau, S.C.; Kelly, R.P.; Ritter, J.M.; Chowienczyk, P.J. Determination of age-related increases in large artery stiffness by digital pulse contour analysis. Clin. Sci. 2002, 103, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Westerhof, N.; Westerhof, B.E. A review of methods to determine the functional arterial parameters stiffness and resistance. J. Hypertens. 2013, 31, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Gemignani, V.; Bianchini, E.; Faita, F.; Giannarelli, C.; Plantinga, Y.; Ghiadoni, L.; Demi, M. Ultrasound Measurement of the Brachial Artery Flow-Mediated Dilation Without ECG Gating. Ultrasound Med. Biol. 2008, 34, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Cosaro, E.; Bonafini, S.; Montagnana, M.; Danese, E.; Trettene, M.S.; Minuz, P.; Delva, P.; Fava, C. Effects of magnesium supplements on blood pressure, endothelial function and metabolic parameters in healthy young men with a family history of metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Giannarelli, C.; Bianchini, E.; Bruno, R.M.; Magagna, A.; Landini, L.; Faita, F.; Gemignani, V.; Penno, G.; Taddei, S.; Ghiadoni, L. Local carotid stiffness and intima-media thickness assessment by a novel ultrasound-based system in essential hypertension. Atherosclerosis 2012, 223, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Faita, F.; Gemignani, V.; Bianchini, E.; Giannarelli, C.; Demi, M. Real-Time Measurement System for the Evaluation of the Intima Media Thickness with a New Edge Detector. Available online: https://ieeexplore.ieee.org/abstract/document/4461851/ (accessed on 16 July 2018).
- Senn, S.S.; Senn, S. Cross-Over Trials In Clinical Research; John Wiley & Sons: Chichester, UK, 2002. [Google Scholar]
- Rudolph, T.K.; Ruempler, K.; Schwedhelm, E.; Tan-Andresen, J.; Riederer, U.; Böger, R.H.; Maas, R. Acute effects of various fast-food meals on vascular function and cardiovascular disease risk markers: The Hamburg Burger Trial. Am. J. Clin. Nutr. 2007, 86, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.P.; Liu, L.; Gao, M.; Zhou, Q.C.; Li, Y.L.; Xia, B. Impairment of endothelial function after a high-fat meal in patients with coronary artery disease. Coron. Artery Dis. 2001, 12, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Draijer, R.; Schalkwijk, C.; Desideri, G.; D’Angeli, A.; Francavilla, S.; Mulder, T.; Ferri, C. Black Tea Increases Circulating Endothelial Progenitor Cells and Improves Flow Mediated Dilatation Counteracting Deleterious Effects from a Fat Load in Hypertensive Patients: A Randomized Controlled Study. Nutrients 2016, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Ghiadoni, L.; Faita, F.; Salvetti, M.; Cordiano, C.; Biggi, A.; Puato, M.; Di Monaco, A.; De Siati, L.; Volpe, M.; Ambrosio, G.; et al. Assessment of flow-mediated dilation reproducibility: A nationwide multicenter study. J. Hypertens. 2012, 30, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Gemignani, V.; Faita, F.; Ghiadoni, L.; Poggianti, E.; Demi, M. A system for real-time measurement of the brachial artery diameter in B-mode ultrasound images. IEEE Trans. Med. Imag. 2007, 26, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, D.A.; Goulding, M.G.; Nguyen, A.; Malaver, T.; Walker, C.F.; George, T.W.; Methven, L.; Lovegrove, J.A. Acute Ingestion of Beetroot Bread Increases Endothelium-Independent Vasodilation and Lowers Diastolic Blood Pressure in Healthy Men: A Randomized Controlled Trial. J. Nutr. 2013, 143, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Hmelak Gorenjak, A.; Cencič, A. Nitrate in vegetables and their impact on human health. A review. Acta Aliment. 2013, 42, 158–172. [Google Scholar] [CrossRef]
- Bahra, M.; Kapil, V.; Pearl, V.; Ghosh, S.; Ahluwalia, A. Inorganic nitrate ingestion improves vascular compliance but does not alter flow-mediated dilatation in healthy volunteers. Nitric Oxide 2012, 26, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Upritchard, J.E.; Sutherland, W.H.; Mann, J.I. Effect of supplementation with tomato juice, vitamin E, and vitamin C on LDL oxidation and products of inflammatory activity in type 2 diabetes. Diabetes Care 2000, 23, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Paik, J.K.; Kim, O.Y.; Park, H.W.; Lee, J.H.; Jang, Y.; Lee, J.H. Effects of lycopene supplementation on oxidative stress and markers of endothelial function in healthy men. Atherosclerosis 2011, 215, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Burton-Freeman, B.; Talbot, J.; Park, E.; Krishnankutty, S.; Edirisinghe, I. Protective activity of processed tomato products on postprandial oxidation and inflammation: A clinical trial in healthy weight men and women. Mol. Nutr. Food Res. 2012, 56, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, C.; Trettene, M.; Degan, M.; Delva, P.; Molesini, B.; Minuz, P.; Pandolfini, T. Anti-angiogenic effects of two cystine-knot miniproteins from tomato fruit. Br. J. Pharmacol. 2011, 162, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Xaplanteris, P.; Vlachopoulos, C.; Pietri, P.; Terentes-Printzios, D.; Kardara, D.; Alexopoulos, N.; Aznaouridis, K.; Miliou, A.; Stefanadis, C. Tomato paste supplementation improves endothelial dynamics and reduces plasma total oxidative status in healthy subjects. Nutr. Res. 2012, 32, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Stangl, V.; Kuhn, C.; Hentschel, S.; Jochmann, N.; Jacob, C.; Böhm, V.; Fröhlich, K.; Müller, L.; Gericke, C.; Lorenz, M. Lack of effects of tomato products on endothelial function in human subjects: Results of a randomised, placebo-controlled cross-over study. Br. J. Nutr. 2011, 105, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Paran, E.; Novack, V.; Engelhard, Y.N.; Hazan-Halevy, I. The effects of natural antioxidants from tomato extract in treated but uncontrolled hypertensive patients. Cardiovasc. Drugs Ther. 2009, 23, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Thies, F.; Masson, L.F.; Rudd, A.; Vaughan, N.; Tsang, C.; Brittenden, J.; Simpson, W.G.; Duthie, S.; Horgan, G.W.; Duthie, G. Effect of a tomato-rich diet on markers of cardiovascular disease risk in moderately overweight, disease-free, middle-aged adults: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1013–1022. [Google Scholar] [PubMed]
- Ried, K.; Frank, O.R.; Stocks, N.P. Dark chocolate or tomato extract for prehypertension: A randomised controlled trial. BMC Complement. Altern. Med. 2009, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Lithander, F.E.; Herlihy, L.K.; Walsh, D.M.; Burke, E.; Crowley, V.; Mahmud, A. Postprandial effect of dietary fat quantity and quality on arterial stiffness and wave reflection: A randomised controlled trial. Nutr. J. 2013, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E. Postprandial peaks as a risk factor for cardiovascular disease: Epidemiological perspectives. Int. J. Clin. Pract. 2002, 129, 5–11. [Google Scholar]
- Peter, R.; Okoseime, O.E.; Rees, A.; Owens, D.R. Postprandial glucose—A potential therapeutic target to reduce cardiovascular mortality. Curr. Vasc. Pharmacol. 2009, 7, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Tabara, Y.; Okada, Y.; Uetani, E.; Nagai, T.; Igase, M.; Kido, T.; Ochi, N.; Ohara, M.; Takita, R.; Kohara, K.; et al. Postprandial hypotension as a risk marker for asymptomatic lacunar infarction. J. Hypertens. 2014, 32, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Zanasi, A.; Tincani, E.; Evandri, V.; Giovanardi, P.; Bertolotti, M.; Rioli, G. Meal-induced blood pressure variation and cardiovascular mortality in ambulatory hypertensive elderly patients. J. Hypertens. 2012, 30, 2125–2132. [Google Scholar] [CrossRef] [PubMed]
- Uetani, E.; Tabara, Y.; Igase, M.; Guo, H.; Kido, T.; Ochi, N.; Takita, R.; Kohara, K.; Miki, T. Postprandial hypertension, an overlooked risk marker for arteriosclerosis. Atherosclerosis 2012, 224, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G. Short-and long-term blood pressure variability: Present and future. Hypertension 2012, 60, 512–517. [Google Scholar] [CrossRef] [PubMed]
| Variable at Baseline | LFD & TP | LFD-Only | Difference LFD & TP vs. LFD-Only | p-Value LFD & TP vs. LFD-Only |
|---|---|---|---|---|
| SBP (mmHg) | 120.4 ± 7.1 | 122.2 ± 13.2 | −1.8 ± 8.9 | 0.39 |
| DBP (mmHg) | 73.3 ± 6.1 | 72.2 ± 7.2 | 1.1 ± 5.4 | 0.37 |
| HR (bpm) | 64.0 ± 9.5 | 64.1 ± 13.1 | −0.1 ± 10.6 | 0.98 |
| Brachial artery diameter (mm) | 4.0 ± 0.4 | 4.0 ± 0.3 | −0.1 ± 0.3 | 0.53 |
| FMD (%) | 3.5 ± 1.6 | 4.4 ± 3.3 | −0.9 ± 3.4 | 0.27 |
| NMD (%) | 2.6 ± 1.9 | 3.4 ± 2.6 | −0.9 ± 2.6 | 0.31 |
| Carotid DC (KPa −1 10 −3) | 36.4 ± 5.3 | 35.3 ± 10.3 | 1.0 ± 9.4 | 0.49 |
| SI (m/s) | 6.6 ± 0.6 | 6.3 ± 0.5 | 0.3 ± 0.5 | 0.025 |
| RI (%) | 71.6 ± 11.2 | 68.0 ± 14.0 | 3.6 ± 13.4 | 0.25 |
| Total cholesterol (mg/dL) | 154.5 ± 27.2 | 151.1 ± 26.8 | 3.4 ± 15.0 | 0.34 |
| HDL-cholesterol (mmol/L) | 52.4 ± 15.3 | 52.0 ± 13.5 | 0.4 ± 6.2 | 0.77 |
| LDL-cholesterol (mmol/L) | 87.4 ± 24.5 | 84.2 ± 25.8 | 3.3 ± 12.4 | 0.51 |
| Triglycerides (mmol/L) | 78.2 ± 28.0 | 71.5 ± 30.3 | 6.7 ± 22.3 | 0.21 |
| Glucose (mmol/L) | 86.2 ± 6.3 | 85.8 ± 4.5 | 0.5 ± 7.2 | 0.78 |
| Variable at Baseline | Difference LFD & TP + FM (2 h) vs. Baseline | p-Value LFD & TP + FM (2 h) vs. Baseline | Difference LFD-Only + FM (2 h) vs. Baseline | p-Value LFD-Only + FM (2 h) vs. Baseline | Difference LFD & TP + FM (3.5 h) vs. Baseline | p-Value LFD & TP + FM (3.5 h) vs. Baseline | Difference LFD-Only + FM (3.5 h) vs. Baseline | p-Value LFD-Only + FM (3.5 h) vs. Baseline |
|---|---|---|---|---|---|---|---|---|
| SBP (mmHg) | 1.5 ± 6.9 | 0.35 | 0.2 ± 8.2 | 0.93 | 0.8 ± 7.8 | 0.67 | −1.2 ± 9.0 | 0.56 |
| DBP (mmHg) | −2.6 ± 4.3 | 0.017 | −0.3 ± 6.3 | 0.82 | −0.3 ± 6.4 | 0.82 | −1.3 ± 5.1 | 0.30 |
| HR (bpm) | 4.1 ± 4.8 | 0.001 | 4.5 ± 6.9 | 0.011 | 2.4 ± 6.2 | 0.11 | 0.3 ± 9.9 | 0.90 |
| Brachial artery diameter (mm) | 0.2 ± 0.2 | 0.004 | 0.1 ± 0.3 | 0.26 | 0.1 ± 0.6 | 0.62 | 0.1 ± 0.3 | 0.28 |
| FMD (%) | 0.7 ± 3.0 | 0.32 | −1.0 ± 3.5 | 0.23 | 0.6 ± 3.0 | 0.41 | 0.1 ± 5.0 | 0.95 |
| NMD (%) | 1.4 ± 3.1 | 0.16 | 0.6 ± 1.7 | 0.36 | n.p. | n.p. | n.p. | n.p. |
| Carotid DC (KPa −1 10 −3) | −0.2 ± 5.7 | 0.91 | 3.4 ± 10.5 | 0.17 | 0.7 ± 4.5 | 0.50 | 2.2 ± 8.7 | 0.29 |
| SI (m/s) | −0.2 ± 0.4 | 0.046 | 0.2 ± 0.6 | 0.17 | −0.2 ± 0.5 | 0.09 | 0.4 ± 0.6 | 0.023 |
| RI (%) | −11 ± 7.4 | <0.001 | −6.6 ± 7.2 | 0.006 | −5.7 ± 9.5 | 0.019 | 1.3 ± 9.8 | 0.56 |
| Total cholesterol (mg/dL) | −3.3 ± 10.7 | 0.19 | −2.8 ± 7.3 | 0.11 | −2.0 ± 8.4 | 0.32 | −3.3 ± 10.2 | 0.17 |
| HDL-cholesterol (mmol/L) | −3.4 ± 4.1 | 0.002 | −3.7 ± 3.3 | <0.001 | −4.3 ± 3.2 | <0.001 | −4.8 ± 3.8 | <0.001 |
| LDL-cholesterol (mmol/L) | −13.0 ± 10.0 | <0.001 | −10.9 ± 8.5 | <0.001 | −10.2 ± 8.9 | <0.001 | −10.1 ± 12.4 | 0.002 |
| Triglycerides (mmol/L) | 72.9 ± 40.8 | <0.001 | 69.4 ± 34.3 | <0.001 | 65.9 ± 45.0 | <0.001 | 66.8 ± 44.6 | <0.001 |
| Glucose (mmol/L) | 6.6 ± 20.4 | 0.18 | 9.9 ± 12.8 | 0.003 | 2.3 ± 8.8 | 0.28 | 5.2 ± 5.4 | <0.001 |
| Variable at Baseline | Difference between Δ-LFD & TP + FM vs. Δ-LFD-Only +FM (2 h) | p-Value Δ-LFD & TP + FM vs. Δ-LFD-Only + FM (2 h) | Difference between Δ-LFD & TP + FM vs. Δ-LFD-Only + FM (3.5 h) | p-Value Δ-LFD & TP + FM vs. Δ-LFD-Only + FM (3.5 h) |
|---|---|---|---|---|
| SBP (mmHg) | 1.4 ± 11.6 | 0.61 | 0.6 ± 12.4 | 0.83 |
| DBP (mmHg) | −2.29 ± 7.2 | 0.18 | 0.9 ± 7.0 | 0.58 |
| HR (bpm) | −0.3 ± 7.0 | 0.83 | 2.1 ± 9.3 | 0.34 |
| Brachial artery diameter (mm) | 0.1 ± 0.4 | 0.23 | 0.0 ± 0.6 | 0.90 |
| FMD (%) | 1.7 ± 4.1 | 0.085 | 0.5 ± 5.7 | 0.69 |
| NMD (%) | 1.6 ± 3.0 | 0.19 | n.p. | n.p. |
| Carotid DC (KPa −1 10 −3) | −3.6 ± 11.8 | 0.20 | −1.5 ± 7.6 | 0.41 |
| SI (m/s) | −0.4 ± 0.8 | 0.048 | −0.6 ± 0.9 | 0.022 |
| RI (%) | −4.5 ± 13.3 | 0.16 | −4.3 ± 15.4 | 0.23 |
| Total cholesterol (mg/dL) | −0.1 ± 14.7 | 0.99 | 1.3 ± 14.9 | 0.70 |
| HDL-cholesterol (mmol/L) | 0.3 ± 5.4 | 0.80 | 0.5 ± 5.7 | 0.72 |
| LDL-cholesterol (mmol/L) | −2.1 ± 11.8 | 0.46 | −0.1 ± 9.5 | 0.97 |
| Triglycerides (mmol/L) | 3.5 ± 41.4 | 0.72 | −0.8 ± 28.3 | 0.90 |
| Glucose (mmol/L) | −3.4 ± 22.9 | 0.53 | −2.9 ± 10.6 | 0.24 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalbeni, A.; Treggiari, D.; Tagetti, A.; Bevilaqua, M.; Bonafini, S.; Montagnana, M.; Scaturro, G.; Minuz, P.; Fava, C. Positive Effects of Tomato Paste on Vascular Function After a Fat Meal in Male Healthy Subjects. Nutrients 2018, 10, 1310. https://doi.org/10.3390/nu10091310
Dalbeni A, Treggiari D, Tagetti A, Bevilaqua M, Bonafini S, Montagnana M, Scaturro G, Minuz P, Fava C. Positive Effects of Tomato Paste on Vascular Function After a Fat Meal in Male Healthy Subjects. Nutrients. 2018; 10(9):1310. https://doi.org/10.3390/nu10091310
Chicago/Turabian StyleDalbeni, Andrea, Davide Treggiari, Angela Tagetti, Michele Bevilaqua, Sara Bonafini, Martina Montagnana, Giuliana Scaturro, Pietro Minuz, and Cristiano Fava. 2018. "Positive Effects of Tomato Paste on Vascular Function After a Fat Meal in Male Healthy Subjects" Nutrients 10, no. 9: 1310. https://doi.org/10.3390/nu10091310
APA StyleDalbeni, A., Treggiari, D., Tagetti, A., Bevilaqua, M., Bonafini, S., Montagnana, M., Scaturro, G., Minuz, P., & Fava, C. (2018). Positive Effects of Tomato Paste on Vascular Function After a Fat Meal in Male Healthy Subjects. Nutrients, 10(9), 1310. https://doi.org/10.3390/nu10091310







