Bergamot Polyphenol Fraction Exerts Effects on Bone Biology by Activating ERK 1/2 and Wnt/β-Catenin Pathway and Regulating Bone Biomarkers in Bone Cell Cultures
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
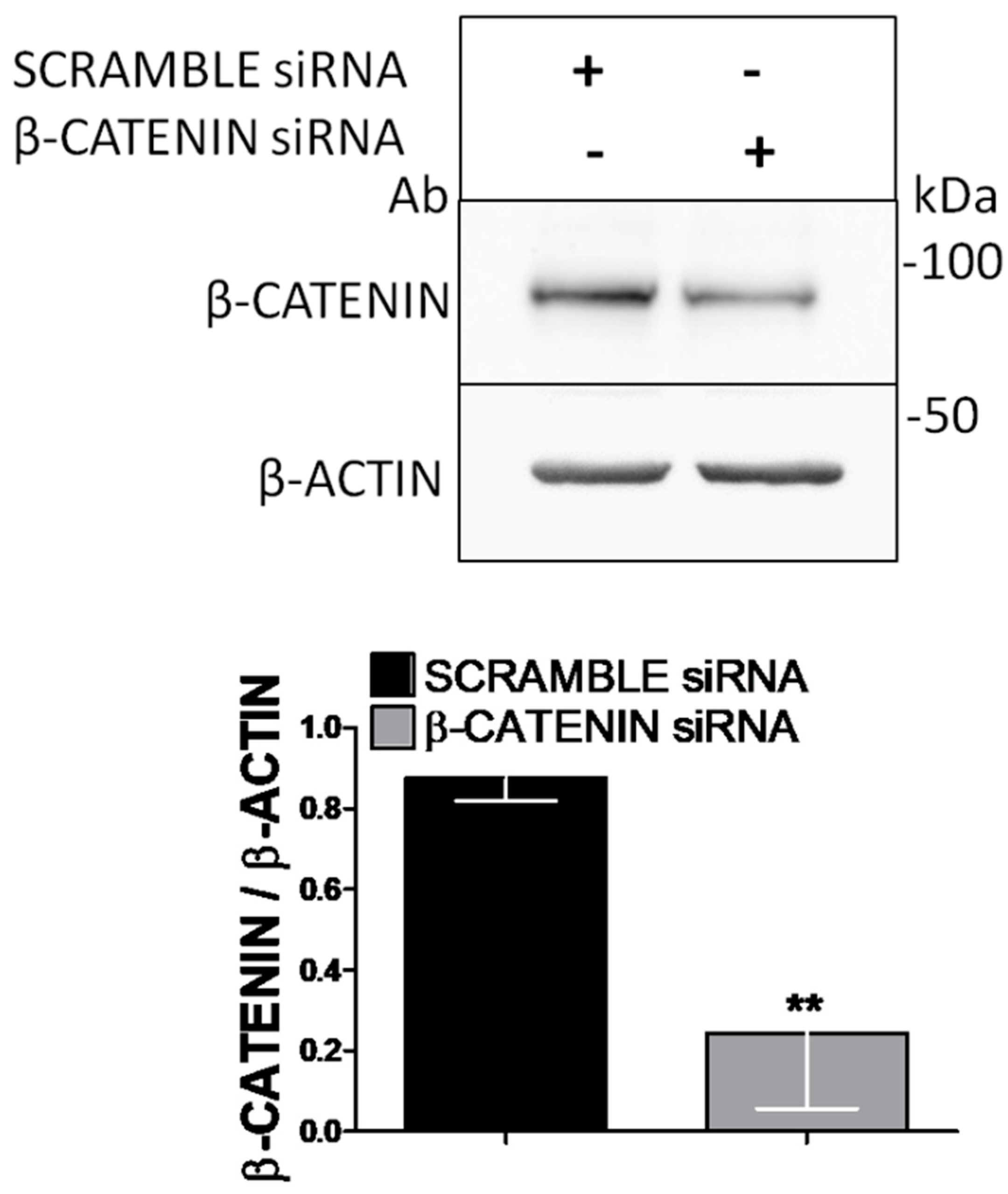
2.2. Western Blotting and β-Catenin Knockdown
2.3. Real Time-PCR
2.4. ALP Activity
2.5. Cell Viability Assay
2.6. Cell Proliferation
2.7. Statistical Analysis
3. Results
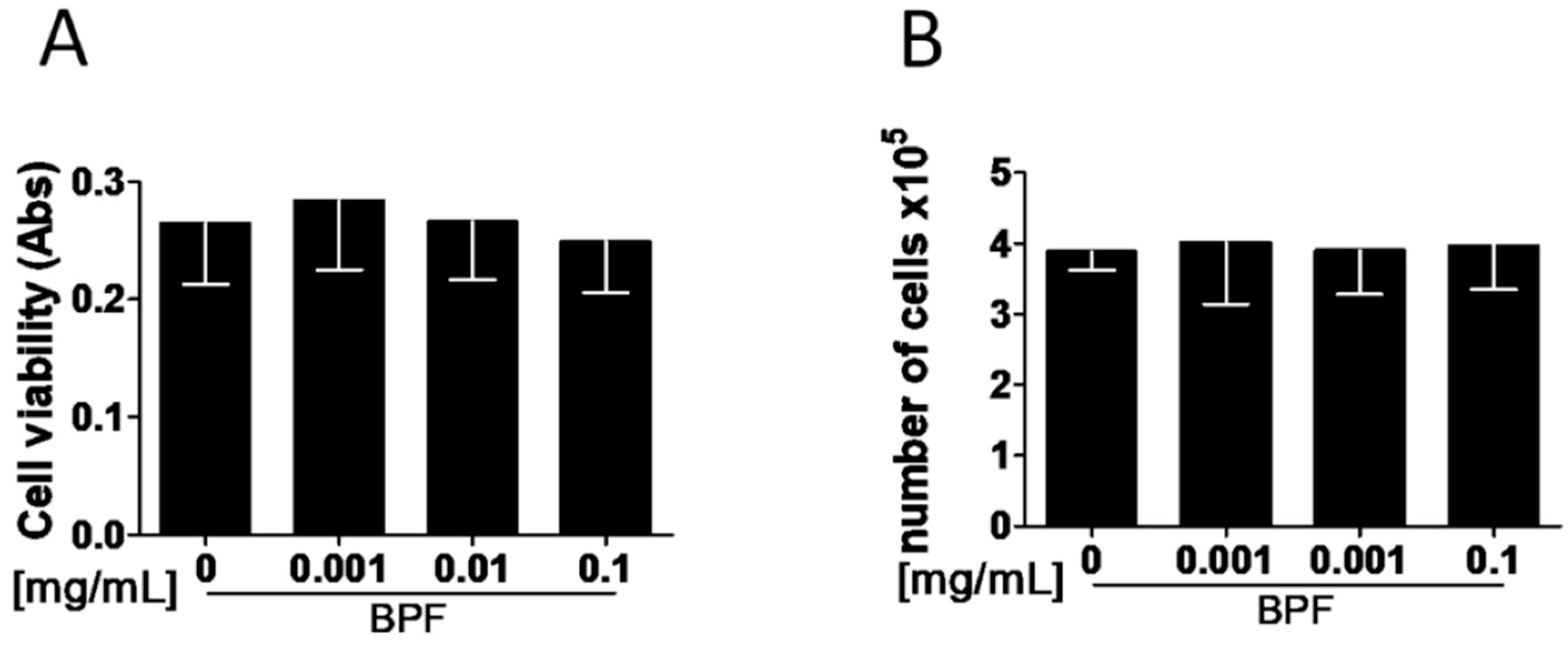
3.1. BPF Does Not Act on Osteoblast Viability and Proliferation In Vitro
3.2. BPF Induces Higher pERK1/2 Levels in Saos-2
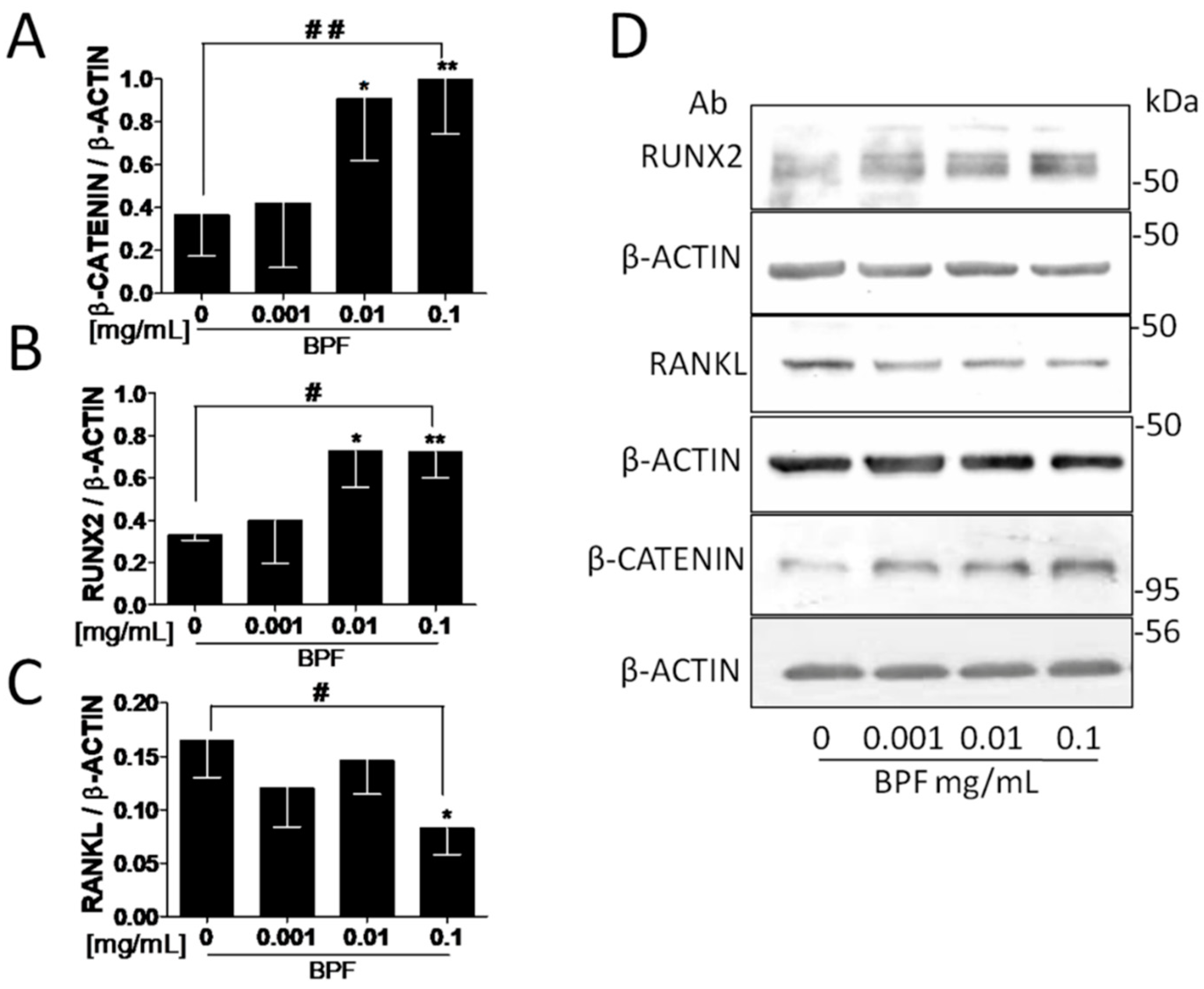
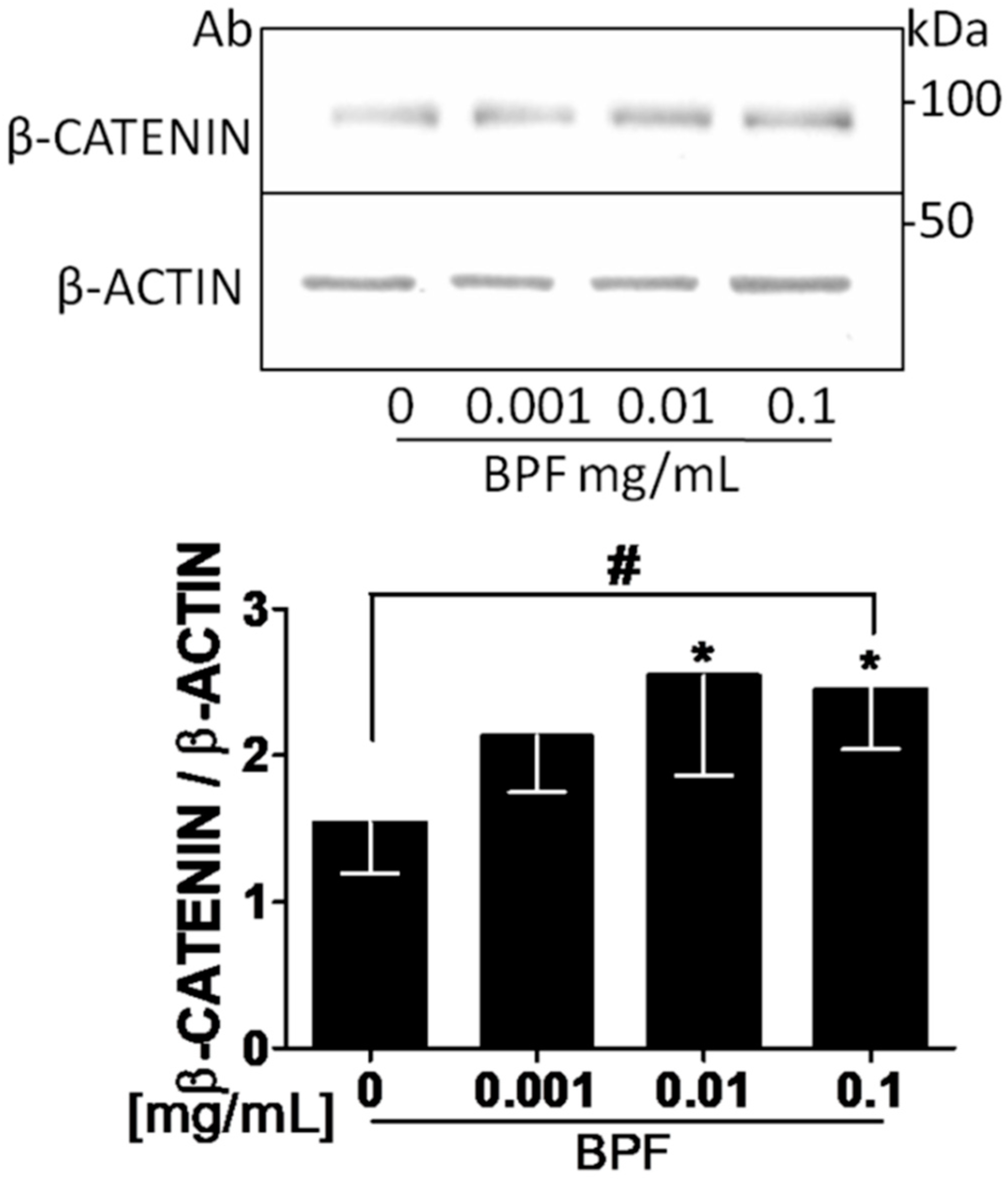
3.3. BPF Increases β-Catenin, RUNX2 and Intracellular COL1A Proteins and Decreases Both the RANKL and Extracellular COL1A Protein Levels in Saos-2
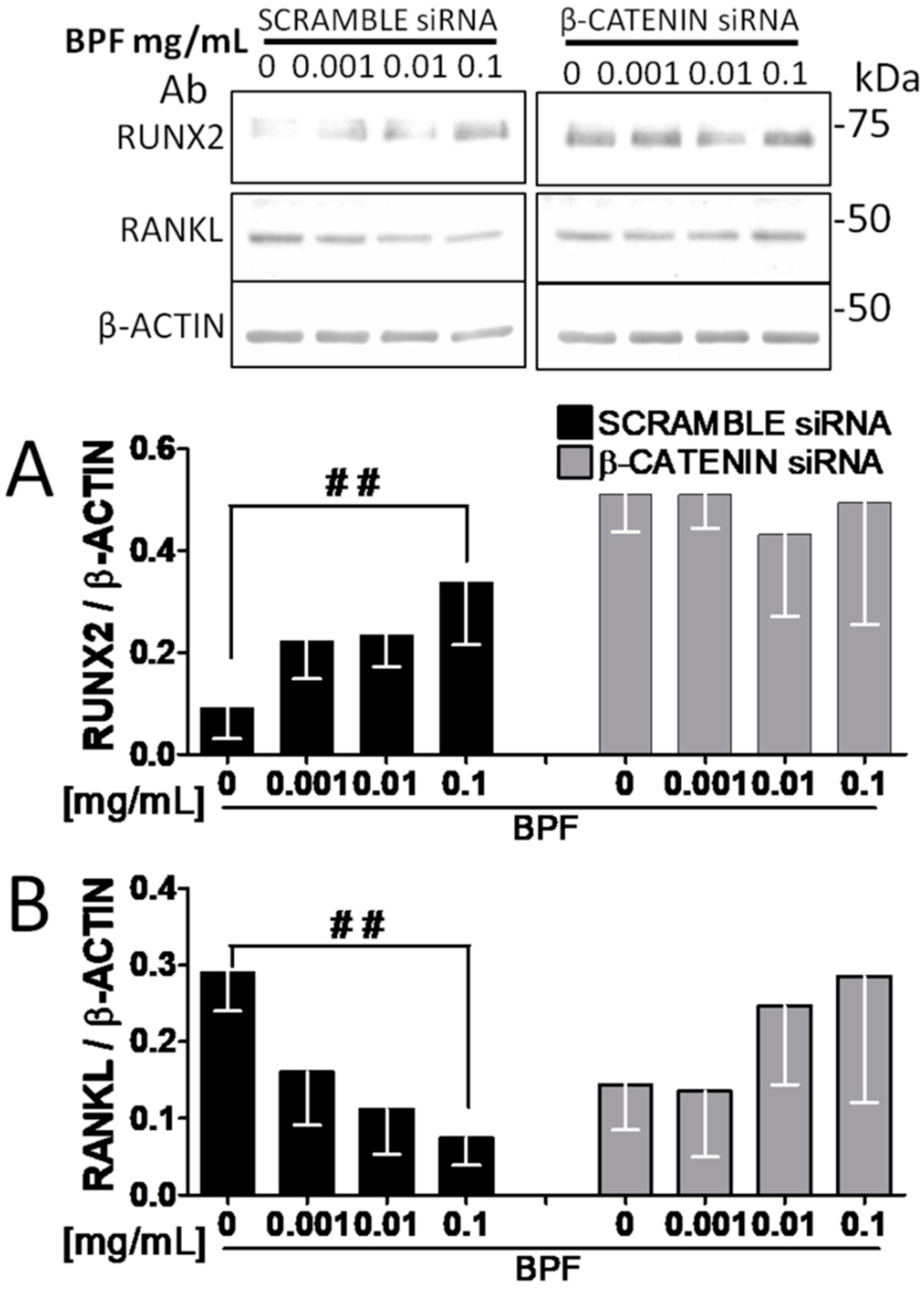
3.4. RNA Interference Shows that RUNX2 and RANKL Expression Is Mediated by β-Catenin
3.5. BPF Increases β-Catenin Protein Levels in MG63.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| BPF | Bergamot polyphenol fraction |
| ERK | Extracellular Signal-regulated Kinase |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| ONJ | osteonecrosis of the jaw |
| RANKL | receptor activator of nuclear factor-κB ligand |
| RUNX2 | Runt-related transcription factor 2 |
| siRNA | small interfering RNA |
| COL1 | type I collagen |
References
- Cummings, S.R.; Black, D.M.; Thompson, D.E.; Applegate, W.B.; Barrett-Connor, E.; Musliner, T.A.; Palermo, L.; Prineas, R.; Rubin, S.M.; Scott, J.C.; et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: Results from the Fracture Intervention Trial. JAMA 1998, 280, 2077–2082. [Google Scholar] [CrossRef] [PubMed]
- McClung, M.R.; Geusens, P.; Miller, P.D.; Zippel, H.; Bensen, W.G.; Roux, C.; Adami, S.; Fogelman, I.; Diamond, T.; Eastell, R.; et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N. Engl. J. Med. 2001, 344, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.T.; Watts, N.B.; Genant, H.K.; McKeever, C.D.; Hangartner, T.; Keller, M.; Chesnut, C.H., III; Brown, J.; Eriksen, E.F.; Hoseyni, M.S.; et al. Effects of risedronate treatment on vertebral and non vertebral fractures in women with postmenopausal osteoporosis: A randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. JAMA 1999, 282, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A.; Seeley, D.G.; Ensrud, K.; Ettinger, B.; Black, D.; Cummings, S.R. Estrogen replacement therapy and fractures in older women. Study of Osteoporotic Fractures Research Group. Ann. Intern. Med. 1995, 122, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Delmas, P.D.; Eastell, R.; Reid, I.R.; Boonen, S.; Cauley, J.A.; Cosman, F.; Lakatos, P.; Leung, P.C.; Man, Z.; et al. Once-year zoledronic acid for treatment of postmenopausal osteoporosis. N. Engl. J. Med. 2007, 356, 1809–1822. [Google Scholar] [CrossRef] [PubMed]
- Neer, R.M.; Arnaud, C.D.; Zanchetta, J.R.; Prince, R.; Gaich, G.A.; Reginster, J.Y.; Hodsman, A.B.; Eriksen, E.F.; Ish-Shalom, S.; Genant, H.K.; et al. Effect of parathyroid hormone (1–34) on fractures bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 2001, 344, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; San Martin, J.; McClung, M.R.; Siris, E.S.; Eastell, R.; Reid, I.R.; Delmas, P.; Zoog, H.B.; Austin, M.; Wang, A.; et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 2009, 361, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Delmas, P.D.; Ensrud, K.E.; Adachi, J.D.; Harper, K.D.; Sarkar, S.; Gennari, C.; Reginster, J.Y.; Pols, H.A.; Recker, R.R.; Harris, S.T.; et al. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: Four-year results from a randomized clinical trial. J. Clin. Endocrinol. Metab. 2002, 87, 3609–3617. [Google Scholar] [CrossRef] [PubMed]
- Schilcher, J.; Michaëlsson, K.; Aspenberg, P. Bisphosphonate use and a typical fractures of the femoral shaft. N. Engl. J. Med. 2011, 364, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Lowe, C.E.; Depew, W.T.; Vanner, S.J.; Paterson, W.G.; Meddings, J.B. Upper gastrointestinal toxicity of alendronate. Am. J. Gastroenterol. 2000, 95, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hamadeh, I.S.; Song, S.; Katz, J.; Moreb, J.S.; Langaee, T.Y.; Lesko, L.J.; Gong, Y. Osteonecrosis of the Jaw in the United States Food and Drug Administration’s Adverse Event Reporting System (FAERS). J. Bone Miner. Res. 2016, 31, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Morrison, A.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.R.; Ruggiero, S.L.; Taguchi, A.; Tetradis, S.; et al. Diagnosis management of osteonecrosis of the jaw: A systematic review international consensus. J. Bone Miner. Res. 2015, 30, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Risks benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar] [PubMed]
- Hulley, S.; Grady, D.; Bush, T.; Furberg, C.; Herrington, D.; Riggs, B.; Vittinghoff, E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998, 280, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Jolette, J.; Wilker, C.E.; Smith, S.Y.; Doyle, N.; Hardisty, J.F.; Metcalfe, A.J.; Marriot, T.B.; Fox, J.; Wells, D.S. Defining a non carcinogenic dose of recombinant human parathyroid hormone 1–84 in a 2-year study in Fischer 344 rats. Toxicol. Pathol. 2006, 34, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Musette, P.; Brandi, M.L.; Cacoub, P.; Kaufman, J.M.; Rizzoli, R.; Reginster, J.Y. Treatment of osteoporosis: Recognizing and managing cutaneous adverse reactions and drug-induced hypersensitivity. Osteoporos. Int. 2010, 21, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Schousboe, J.T. Adherence with medications used to treat osteoporosis: Behavioral insights. Curr. Osteoporos. Rep. 2013, 11, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; von Bergen, V.; Chyu, M.C.; Jenkins, M.R.; Mo, H.; Chen, C.H.; Kwun, I.S. Fruits and dietary phytochemicals in bone protection. Nutr. Res. 2012, 32, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Lerner, U.H.; Ohlssonm, C. The WNT system: Background its role in bone. J. Intern. Med. 2015, 277, 630–649. [Google Scholar] [CrossRef] [PubMed]
- Gliozzi, M.; Walker, R.; Muscoli, S.; Vitale, C.; Gratteri, S.; Carresi, C.; Musolino, V.; Russo, V.; Janda, E.; Ragusa, S.; et al. Bergamot polyphenolic fraction enhances rosuvastatin-induced effect on LDL-cholesterol, LOX-1 expression and protein kinase B phosphorylation in patients with hyperlipidemia. Int. J. Cardiol. 2013, 170, 140–145. [Google Scholar] [CrossRef] [PubMed]
- McQuillan, D.J.; Richardson, M.D.; Bateman, J.F. Matrix deposition by a calcifying human osteogenic sarcoma cell line (SAOS-2). Bone 1995, 16, 415–426. [Google Scholar] [CrossRef]
- Qiu, R.; Cao, W.T.; Tian, H.Y.; He, J.; Chen, G.D.; Chen, Y.M. Greater Intake of Fruit and Vegetables Is Associated with Greater Bone Mineral Density and Lower Osteoporosis Risk in Middle-Aged and Elderly Adults. PLoS ONE 2017, 12, e0168906. [Google Scholar] [CrossRef] [PubMed]
- Byberg, L.; Bellavia, A.; Orsini, N.; Wolk, A.; Michaëlsson, K. Fruit and vegetable intake and risk of hip fracture: A cohort study of Swedish men and women. J. Bone Miner. Res. 2015, 30, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Zalloua, P.A.; Hsu, Y.H.; Terwedow, H.; Zang, T.; Wu, D.; Tang, G.; Li, Z.; Hong, X.; Azar, S.T.; Wang, B.; et al. Impact of seafood and fruit consumption on bone mineral density. Maturitas 2007, 56, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chiba, H.; Uehara, M.; Wu, J.; Wang, X.; Masuyama, R.; Suzuki, K.; Kanazawa, K.; Ishimi, Y. Hesperidin, a citrus flavonoid, inhibits bone loss and decreases serum and hepatic lipids in ovariectomized mice. J. Nutr. 2003, 133, 1892–1897. [Google Scholar] [CrossRef] [PubMed]
- Deyhim, F.; Garica, K.; Lopez, E.; Gonzalez, J.; Ino, S.; Garcia, M.; Patil, B.S. Citrus juice modulates bone strength in male senescent rat model of osteoporosis. Nutrition 2006, 22, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Horcajada-Molteni, M.N.; Crespy, V.; Coxam, V.; Davicco, M.J.; Rémésy, C.; Barlet, J.P. Rutin inhibits ovariectomy-induced osteopenia in rats. J. Bone Miner. Res. 2000, 15, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Mollace, V.; Sacco, I.; Janda, E.; Malara, C.; Ventrice, D.; Colica, C.; Visalli, V.; Muscoli, S.; Ragusa, S.; Muscoli, C.; et al. Hypolipemic and hypoglycaemic activity of bergamot polyphenols: From animal models to human studies. Fitoterapia 2011, 82, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Carresi, C.; Musolino, V.; Gliozzi, M.; Maiuolo, J.; Mollace, R.; Nucera, S.; Maretta, A.; Sergi, D.; Muscoli, S.; Gratteri, S.; et al. Anti-oxidanteffect of bergamot polyphenolic fraction counteract doxorubicin-induced cardiomyopathy: Role of autophagy and c-kit(pos) CD45(neg) CD31(neg) cardiac stem cell activation. J. Mol. Cell. Cardiol. 2018, 119, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Xiao, G.; Jiang, D.; Franceschi, R.T. Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J. Cell Biol. 2007, 176, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Hamidouche, Z.; Fromigué, O.; Ringe, J.; Häupl, T.; Vaudin, P.; Pagès, J.C.; Srouji, S.; Livne, E.; Marie, P.J. Priming integrin alpha5 promotes human mesenchymal stromal cell osteoblast differentiation and osteogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 18587–18591. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.H.; Shin, C.S.; Stains, J.P.; Cheng, S.L.; Sheikh, S.; Mbalaviele, G.; Szejnfeld, V.L.; Civitelli, R. Targeted expression of a dominant-negative N-cadherin in vivo delays peak bone mass and increases adipogenesis. J. Cell Sci. 2004, 117, 2853–2864. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.K.; Jaiswal, N.; Bruder, S.P.; Mbalaviele, G.; Marshak, D.R.; Pittenger, M.F. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J. Biol. Chem. 2000, 275, 9645–9652. [Google Scholar] [CrossRef] [PubMed]
- Cortizo, A.M.; Lettieri, M.G.; Barrio, D.A.; Mercer, N.; Etcheverry, S.B.; McCarthy, A.D. Advanced glycation end-products (AGEs) induce concerted changes in the osteoblastic expression of their receptor RAGE and in the activation of extracellular signal-regulated kinases (ERK). Mol. Cell. Biochem. 2003, 250, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.L.; Liang, H.L.; Hung, C.H.; Kuo, P.L. Syringetin, a flavonoid derivative in grape and wine, induces human osteoblast differentiation through bone morphogenetic protein-2/extracellularsignal-regulatedkinase1/2 pathway. Mol. Nutr. Food Res. 2009, 53, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ma, W.; Wang, F.; Dong, J.; Wang, D.; Sun, B.; Wang, B. Stimulation of Wnt/β-Catenin Signaling to Improve Bone Development by Naringin via Interacting with AMPK and Akt. Cell. Physiol. Biochem. 2015, 36, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hsu, Y.H.; Mo, C.; Abreu, E.; Kiel, D.P.; Bonewald, L.F.; Brotto, M.; Karasik, D. METTL21C is a potential pleiotropic gene for osteoporosis sarcopenia acting through the modulation of the NF-κB signaling pathway. J. Bone Miner. Res. 2014, 29, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.K.; Jeon, O.; Krebs, M.D.; Schapira, D.; Alsberg, E. Sustained localized presentation of RNA interfering molecules from in situ forming hydrogels to guide stem cell osteogenic differentiation. Biomaterials 2014, 35, 6278–6286. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.; Xiu, L.; Li, L. Sphingosine 1-phosphate receptors negatively regulate collagen type I/III expression in human bone marrow-derived mesenchymal stem cell. J. Cell. Biochem. 2014, 115, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Poundarik, A.A.; Diab, T.; Sroga, G.E.; Ural, A.; Boskey, A.L.; Gundberg, C.M.; Vashishth, D. Dilatational band formation in bone. Proc. Natl. Acad. Sci. USA 2012, 109, 19178–19183. [Google Scholar] [CrossRef] [PubMed]
- Akinci, B.; Bayraktar, F.; Saklamaz, A.; Demir, T.; Yener, S.; Comlekci, A.; Ozcan, M.A.; Kebapcilar, L.; Yuksel, F.; Yesil, S. Low transforming growth factor-beta1 serum levels in idiopathic male osteoporosis. J. Endocrinol. Investig. 2007, 30, 350–355. [Google Scholar] [CrossRef]
- Qi, W.N.; Scully, S.P. Extracellular collagen regulates expression of transforming growth factor-beta1 gene. J. Orthop. Res. 2000, 18, 928–932. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.Y.; Lerner, M.; Stoecker, B.J.; Boldrin, E.; Brackett, D.J.; Lucas, E.A.; Smith, B.J. Dried plum polyphenols inhibit osteoclastogenesis by downregulating NFATc1 and inflammatory mediators. Calcif. Tissue Int. 2008, 82, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Patel, J.D.; Mumper, R.J. Characterization of blackberry extract and its antiproliferative and anti-inflammatory properties. J. Med. Food. 2007, 10, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Akagawa, M.; Shigemitsu, T.; Suyama, K. Production of hydrogen peroxide by polyphenols polyphenol-rich beverages under quasi-physiological conditions. Biosci. Biotechnol. Biochem. 2003, 67, 2632–2640. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid Composition of Citrus Juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Wang, Y.; Ma, Y.; Su, W.; Bai, Y.; Zhao, P. A rapid LC/MS/MS quantitation assay for naringin its two metabolites in rat’s plasma. J. Pharm. Biomed. Anal. 2006, 40, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Mohseny, A.B.; Karperien, M.; Hogendoorn, P.C.; Zhou, G.; Cleton-Jansen, A.M. Inactive Wnt/beta-catenin pathway in conventional high-grade osteosarcoma. J. Pathol. 2010, 220, 24–33. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward | Reverse |
|---|---|---|
| RUNX2 | 5′-TTACTTACACCCCGCCAGTC-3′ | 5′-TATGGAGTGCTGCTGGTCTG-3′ |
| RANKL | 5′-AGAGCGCAGATGGATCCTAA-3′ | 5′-TTCCTTTTGCACAGCTCCTT-3′ |
| COL1A | 5′-CCCCAGCCCACAAAGAGTCTA-3′ | 5′-CTGTACGCAGGTGATTGGTG-3′ |
| Osteoprotegerin | 5′-TGCAGTACGTCAAGCAGGAG-3′ | 5′-GTGTCTTGGTCGCCATTTTT-3′ |
| β-actin | 5′-GACTGTGACGAGTTGGCTGA-3′ | 5′-CTGGAGAGGAGCAGAACTGG-3′ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pujia, A.; Russo, C.; Maurotti, S.; Pujia, R.; Mollace, V.; Romeo, S.; Montalcini, T. Bergamot Polyphenol Fraction Exerts Effects on Bone Biology by Activating ERK 1/2 and Wnt/β-Catenin Pathway and Regulating Bone Biomarkers in Bone Cell Cultures. Nutrients 2018, 10, 1305. https://doi.org/10.3390/nu10091305
Pujia A, Russo C, Maurotti S, Pujia R, Mollace V, Romeo S, Montalcini T. Bergamot Polyphenol Fraction Exerts Effects on Bone Biology by Activating ERK 1/2 and Wnt/β-Catenin Pathway and Regulating Bone Biomarkers in Bone Cell Cultures. Nutrients. 2018; 10(9):1305. https://doi.org/10.3390/nu10091305
Chicago/Turabian StylePujia, Arturo, Cristina Russo, Samantha Maurotti, Roberta Pujia, Vincenzo Mollace, Stefano Romeo, and Tiziana Montalcini. 2018. "Bergamot Polyphenol Fraction Exerts Effects on Bone Biology by Activating ERK 1/2 and Wnt/β-Catenin Pathway and Regulating Bone Biomarkers in Bone Cell Cultures" Nutrients 10, no. 9: 1305. https://doi.org/10.3390/nu10091305
APA StylePujia, A., Russo, C., Maurotti, S., Pujia, R., Mollace, V., Romeo, S., & Montalcini, T. (2018). Bergamot Polyphenol Fraction Exerts Effects on Bone Biology by Activating ERK 1/2 and Wnt/β-Catenin Pathway and Regulating Bone Biomarkers in Bone Cell Cultures. Nutrients, 10(9), 1305. https://doi.org/10.3390/nu10091305






