Dietary Flavonoids, Copper Intake, and Risk of Metabolic Syndrome in Chinese Adults
Abstract
1. Introduction
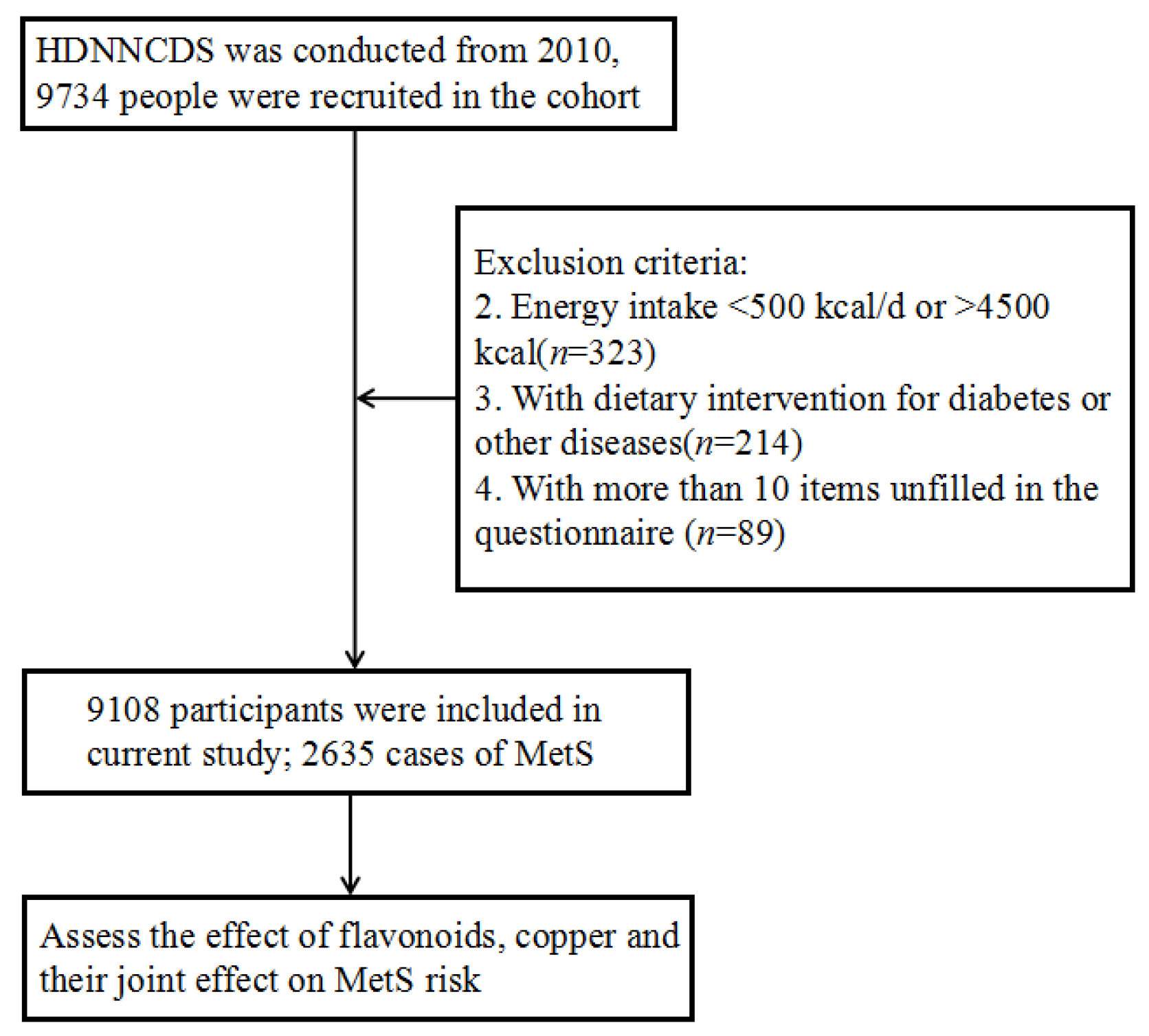
2. Materials and Methods
2.1. Study Population
2.2. Questionnaire Data Collection
2.3. Dietary Flavonoid Assessment
2.4. Anthropometric Measurement and Biochemical Assessment
2.5. Study Outcome Definition
2.6. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Dietary Nutrient and Flavonoid Intakes
3.3. Relationship between Flavonoid and Cu Intake, and MetS Risk
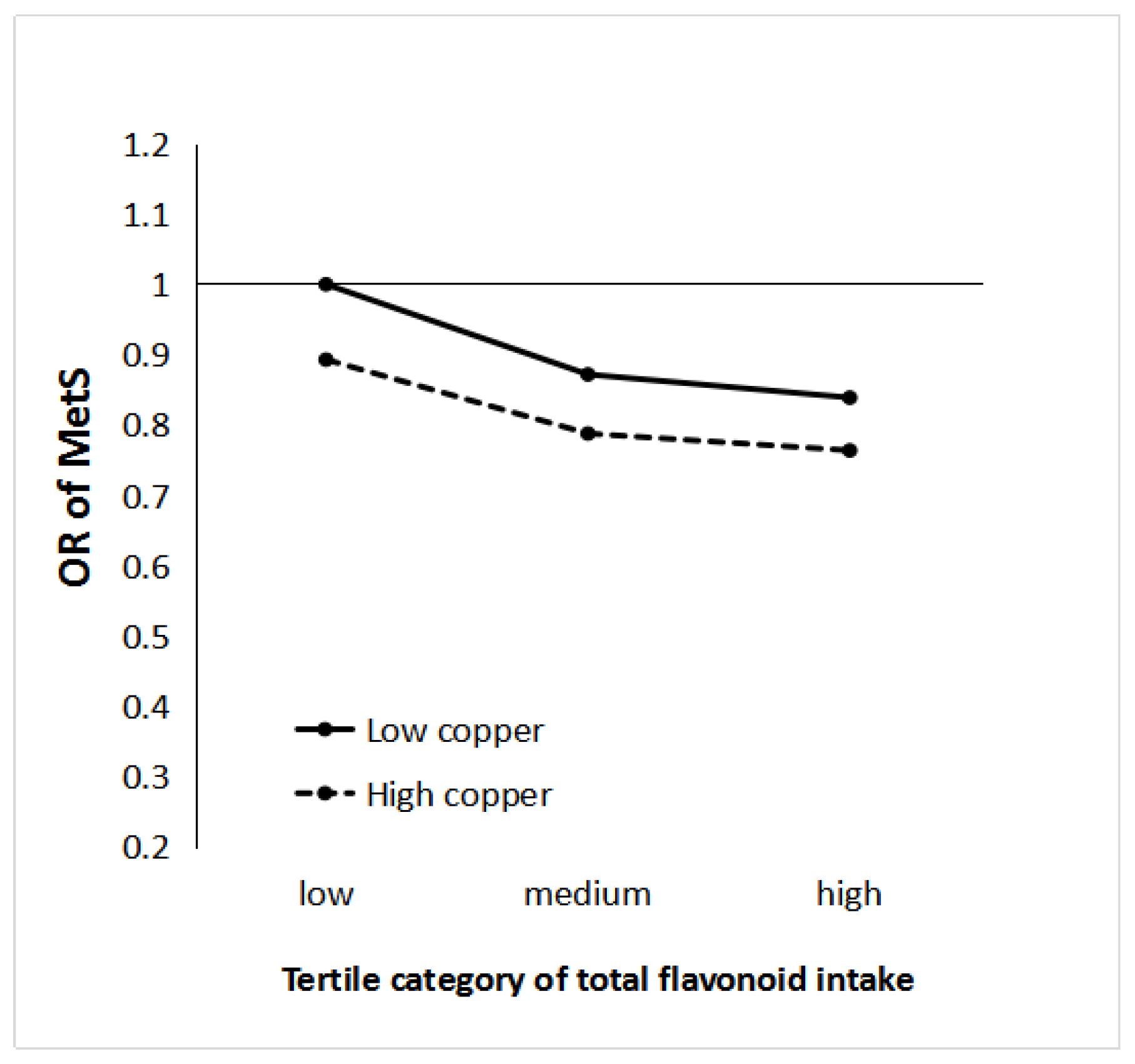
3.4. Joint Analyses of Flavonoid and Cu Intake on MetS Risk
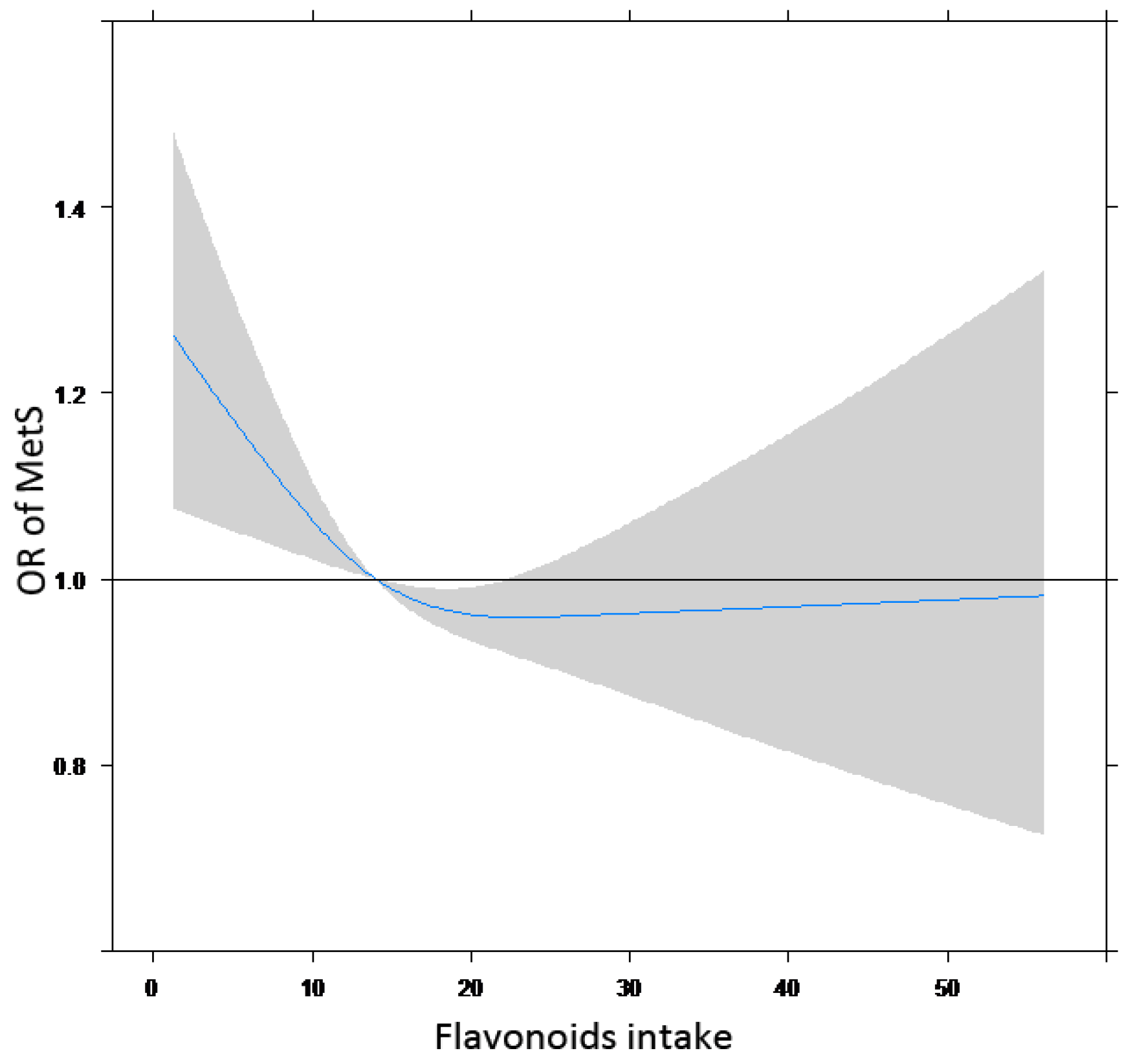
3.5. Suggestions for Flavonoid Intake for the Prevention of MetS
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bonora, E.; Kiechl, S.; Willeit, J.; Oberhollenzer, F.; Egger, G.; Bonadonna, R.C.; Muggeo, M.; Bruneck Study. Metabolic syndrome: Epidemiology and more extensive phenotypic description. Cross-sectional data from the Bruneck Study. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Sahyoun, N.R.; Jacques, P.F.; Zhang, X.L.; Juan, W.; McKeown, N.M. Whole-grain intake is inversely associated with the metabolic syndrome and mortality in older adults. Am. J. Clin. Nutr. 2006, 83, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Kim, S.M.; Lee, J.S.; Oh, H.J.; Han, J.H.; Song, Y.; Joung, H.; Park, H.S. Dietary patterns and the metabolic syndrome in Korean adolescents: 2001 Korean National Health and Nutrition Survey. Diabetes Care 2007, 30, 1904–1905. [Google Scholar] [CrossRef] [PubMed]
- Esmaillzadeh, A.; Kimiagar, M.; Mehrabi, Y.; Azadbakht, L.; Hu, F.B.; Willett, W.C. Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome. Am. J. Clin. Nutr. 2007, 85, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Lutsey, P.L.; Steffen, L.M.; Stevens, J. Dietary intake and the development of the metabolic syndrome: The atherosclerosis risk in communities study. Circulation 2008, 117, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Kandaswami, C.; Middleton, E., Jr. Free radical scavenging and antioxidant activity of plant flavonoids. Adv. Exp. Med. Biol. 1994, 366, 35–76. [Google Scholar]
- Chen, G.; Li, X.; Saleri, F.; Guo, M. Analysis of flavonoids in rhamnus davurica and its antiproliferative activities. Molecules 2016, 21, 1275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cheng, X.R.; da Silva, I.S.; Hung, V.W.; Veloso, A.J.; Angnes, L.; Kerman, K. Electroanalysis of the interaction between (–)-epigallocatechin-3-gallate (EGCG) and amyloid-β in the presence of copper. Metallomics 2013, 5, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Scheiber, I.; Dringen, R.; Mercer, J.F. Copper: Effects of deficiency and overload. Met. Ions Life Sci. 2013, 13, 359–387. [Google Scholar] [PubMed]
- Obeid, O.; Elfakhani, M.; Hlais, S.; Iskandar, M.; Batal, M.; Mouneimne, Y.; Adra, N.; Hwalla, N. Plasma copper, zinc, and selenium levels and correlates with metabolic syndrome components of lebanese adults. Biol. Trace Elem. Res. 2008, 123, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.K.; Bae, Y.J. Relationship between dietary magnesium, manganese, and copper and metabolic syndrome risk in Korean adults: The Korea National Health and Nutrition Examination Survey (2007–2008). Biol. Trace Elem. Res. 2013, 156, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Temerk, Y.M.; Ibrahim, M.S.; Kotb, M.; Schuhmann, W. Interaction of antitumor flavonoids with dsDNA in the absence and presence of Cu (II). Anal. Bioanal. Chem. 2013, 405, 3839–3846. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, E.; Janjua, N.K.; Hameed, S. β-Cyclodextrin assisted solubilization of Cu and Cr complexes of flavonoids in aqueous medium: A DNA-interaction study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 128, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Wang, B.; Zhu, L. DNA binding and oxidative DNA damage induced by a quercetin copper (II) complex: Potential mechanism of its antitumor properties. J. Biol. Inorg. Chem. 2009, 14, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Na, L.; Wu, X.; Feng, R.; Li, J.; Han, T.; Lin, L.; Lan, L.; Yang, C.; Li, Y.; Sun, C. The Harbin cohort study on diet, nutrition and chronic non-communicable diseases: Study design and baseline characteristics. PLoS ONE 2015, 10, e0122598. [Google Scholar] [CrossRef] [PubMed]
- He, Y.N.; Zhai, F.Y.; Yang, X.G.; Ge, K.Y. The Chinese diet balance index revised. Acta Nutr. Sin. 2009, 316, 532–536. [Google Scholar]
- Cao, J.; Chen, W.; Zhang, Y.; Zhang, Y.Q.; Zhao, X.J. Content of selected flavonoids in 100 edible vegetables and fruits. Food Sci. Technol. Res. 2010, 16, 395–402. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J.; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome-a new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Grosso, G.; Stepaniak, U.; Micek, A.; Stefler, D.; Bobak, M.; Pająk, A. Dietary polyphenols are inversely associated with metabolic syndrome in Polish adults of the HAPIEE study. Eur. J. Nutr. 2017, 56, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Sohrab, G.; Hosseinpour-Niazi, S.; Hejazi, J.; Yuzbashian, E.; Mirmiran, P.; Azizi, F. Dietary polyphenols and metabolic syndrome among Iranian adults. Int. J. Food Sci. Nutr. 2013, 64, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Rizza, S.; Muniyappa, R.; Iantorno, M.; Kim, J.A.; Chen, H.; Pullikotil, P.; Senese, N.; Tesauro, M.; Lauro, D.; Cardillo, C.; et al. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J. Clin. Endocrinol. Metab. 2011, 96, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Schewe, T.; Steffen, Y.; Sies, H. How do dietary flavanols improve vascular function? A position paper. Arch. Biochem. Biophys. 2008, 476, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, M.; Keen, C.L.; Lönnerdal, B.; Hurley, L.S.; Schneeman, B.O. Copper deficiency-induced hypercholesterolemia: Effects on HDL subfractions and hepatic lipoprotein receptor activity in the rat. J. Nutr. 1986, 116, 1735–1746. [Google Scholar] [CrossRef] [PubMed]
- Salonen, J.T.; Salonen, R.; Korpela, H.; Suntionen, S.; Tuomilehto, J. Serum copper and the risk of acute myocardial infarction: A prospective population study in men in Estern Finland. Am. J. Epidemiol. 1991, 134, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Sitasawad, S.; Deshpande, M.; Katdare, M.; Tirth, S.; Parab, P. Beneficial effect of supplementation with copper sulfate on STZ-diabetic mice (IDDM). Diabetes Res. Clin. Pract. 2001, 52, 77–84. [Google Scholar] [CrossRef]
- Suarez-Ortegón, M.F.; Ordoñez-Betancourth, J.E.; Aguilar-de Plata, C. Dietary zinc intake is inversely associated to metabolic syndrome in male but not in female urban adolescents. Am. J. Hum. Biol. 2013, 25, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Florêncio, M.H.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Chun, O.K.; Chung, S.J.; Song, W.O. Estimated dietary flavonoid intake and major food sources of U.S. adults. J. Nutr. 2007, 137, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Andres-Lacueva, C.; Lamuela-Raventós, R.M.; Berenguer, T.; Jakszyn, P.; Barricarte, A.; Ardanaz, E.; Amiano, P.; Dorronsoro, M.; Larrañaga, N.; et al. Estimation of dietary sources and flavonoid intake in a Spanish adult population (EPIC-Spain). J. Am. Diet. Assoc. 2010, 110, 390–398. [Google Scholar] [CrossRef] [PubMed]
| Variable | Control (n = 6473) | MetS (n = 2635) | p-Value |
|---|---|---|---|
| Sociodemographics | |||
| Age (years) | 49.33 ± 10.34 | 54.17 ± 9.34 | 0.000 |
| Male, no. (%) | 2266 (35.0) | 978 (37.1) | 0.057 |
| Drinking, no. (%) | 2140 (34.1) | 889 (34.5) | 0.747 |
| Smoking, no. (%) | 0.000 | ||
| Yes | 1064 (16.6) | 500 (19.1) | |
| No | 5174 (80.7) | 1988 (76.0) | |
| Quit | 175 (2.7) | 127 (4.9) | |
| Physical activity, no. (%) | 0.047 | ||
| Light | 5250 (82.9) | 2189 (85.0) | |
| Middle | 973 (15.4) | 352 (13.7) | |
| Heavy | 110 (1.7) | 35 (1.4) | |
| Body Composition and Clinical Parameters | |||
| BMI (kg/m2) | 24.12 ± 3.24 | 27.13 ± 3.26 | 0.000 |
| Total Cholesterol (mmol/L) | 5.1 ± 1.01 | 5.31 ± 1.08 | 0.000 |
| LDL-c (mmol/L) | 1.31 ± 0.33 | 1.13 ± 0.29 | 0.000 |
| Uric Acid | 306.38 ± 84.71 | 335.49 ± 89.42 | 0.000 |
| Metabolic Parameters | |||
| Waist Circumference (cm) | 82.97 ± 9.67 | 92.98 ± 8.07 | 0.000 |
| SBP (mmHg) | 130.93 ± 16.83 | 144.83 ± 19.15 | 0.000 |
| DBP (mmHg) | 79.64 ± 8 | 85.62 ± 9.68 | 0.000 |
| FPG (mmol/L) | 4.68 ± 1.32 | 5.8 ± 2.24 | 0.000 |
| Triglycerides (mmol/L) | 1.59 ± 1.6 | 2.31 ± 2.17 | 0.000 |
| HDL-c (mmol/L) | 2.98 ± 0.84 | 3.18 ± 0.9 | 0.000 |
| Variable | Control (n = 6473) | MetS (n = 2635) | p-Value |
|---|---|---|---|
| Nutrient Intake | |||
| Energy (kcal/day) | 2297.17 ± 731.26 | 2328.96 ± 754.7 | 0.049 |
| Carbohydrate | |||
| Intake (g/day) | 355.97 ± 135.28 | 360.85 ± 137.48 | 0.120 |
| Intake (g/kcal) | 152.95 ± 21.57 | 153.2 ± 21.66 | 0.607 |
| Fat | |||
| Intake (g/day) | 72.52 ± 23.89 | 73.34 ± 25.2 | 0.145 |
| Intake (g/kcal) | 32.67 ± 8.22 | 32.51 ± 8.11 | 0.394 |
| Protein | |||
| Intake (g/day) | 70.2 ± 29.53 | 71.33 ± 31.11 | 0.101 |
| Intake (g/kcal) | 30.22 ± 6.22 | 30.28 ± 6.6 | 0.673 |
| Fiber | |||
| Intake (g/day) | 13.66 ± 6.58 | 13.76 ± 6.61 | 0.536 |
| Intake (g/kcal) | 6.05 ± 2.41 | 6.05 ± 2.5 | 0.975 |
| Copper | |||
| Intake (ug/day) | 2433.62 ± 904.96 | 2454 ± 927.69 | 0.328 |
| Intake (ug/kcal) | 1053.54 ± 181.91 | 1044.85 ± 193.73 | 0.048 |
| Flavonoid intake | |||
| Total flavonoid | |||
| Intake (mg/day) | 34.68 ± 20.88 | 34.12 ± 20.81 | 0.241 |
| Intake (mg/kcal) | 15.43 ± 8.19 | 15.04 ± 8.31 | 0.042 |
| Kaempferol | |||
| Intake (mg/day) | 11.34 ± 6.81 | 11.4 ± 6.9 | 0.687 |
| Intake (mg/kcal) | 5.05 ± 2.7 | 5.05 ± 2.82 | 0.981 |
| Luteolin | |||
| Intake (mg/day) | 9.08 ± 5.66 | 8.85 ± 5.54 | 0.081 |
| Intake (mg/kcal) | 4.03 ± 2.18 | 3.89 ± 2.12 | 0.004 |
| Isorhamnetin | |||
| Intake (mg/day) | 8.32 ± 5.11 | 8.25 ± 5.07 | 0.534 |
| intake (mg/kcal) | 3.71 ± 2.02 | 3.65 ± 2.04 | 0.221 |
| Quercetin | |||
| Intake (mg/day) | 3.58 ± 2.21 | 3.52 ± 2.17 | 0.236 |
| Intake (mg/kcal) | 1.59 ± 0.86 | 1.55 ± 0.85 | 0.044 |
| Apigenin | |||
| Intake (mg/day) | 2.36 ± 1.54 | 2.42 ± 1.6 | 0.086 |
| intake (mg/kcal) | 1.05 ± 0.64 | 1.08 ± 0.69 | 0.099 |
| Q1 | Q2 | Q3 | Q4 | Ptrend | ||
|---|---|---|---|---|---|---|
| Flavonoid | Case/control | 712/1594 | 662/1607 | 645/1622 | 616/1650 | |
| Mode1 | 1.00 | 0.87 (0.76–0.99) | 0.79 (0.69–0.90) | 0.72 (0.63–0.82) | 0.000 | |
| Mode2 | 1.00 | 0.98 (0.84–1.13) | 0.83 (0.72–0.97) | 0.77 (0.66–0.90) | 0.002 | |
| Copper | Case/control | 678/1568 | 681/1602 | 633/1657 | 628/1661 | |
| Mode1 | 1.00 | 0.94 (0.83–1.07) | 0.83 (0.73–0.95) | 0.79 (0.69–0.90) | 0.001 | |
| Mode2 | 1.00 | 0.95 (0.82–1.11) | 0.85 (0.74–0.99) | 0.81 (0.70–0.94) | 0.020 |
| Q1 | Q2 | Q3 | Pinteraction | ||
|---|---|---|---|---|---|
| Flavonoid | Case/control | 932/2105 | 858/2174 | 845/2194 | 0.000 |
| Model 1 | 1.00 | 0.81 (0.73–0.91) | 0.75 (0.67–0.85) | ||
| Model 2 | 1.00 | 0.85 (0.75–0.97) | 0.80 (0.70–0.91) | ||
| Copper | Case/control | 1374/3180 | 1261/3293 | ||
| Model 1 | 1.00 | 0.83 (0.76–0.91) | |||
| Model 2 | 1.00 | 0.85 (0.77–0.95) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, R.; Jia, Y.; Liu, J.; Jin, S.; Han, T.; Na, L. Dietary Flavonoids, Copper Intake, and Risk of Metabolic Syndrome in Chinese Adults. Nutrients 2018, 10, 991. https://doi.org/10.3390/nu10080991
Qu R, Jia Y, Liu J, Jin S, Han T, Na L. Dietary Flavonoids, Copper Intake, and Risk of Metabolic Syndrome in Chinese Adults. Nutrients. 2018; 10(8):991. https://doi.org/10.3390/nu10080991
Chicago/Turabian StyleQu, Rongge, Yubing Jia, Junyi Liu, Shanshan Jin, Tianshu Han, and Lixin Na. 2018. "Dietary Flavonoids, Copper Intake, and Risk of Metabolic Syndrome in Chinese Adults" Nutrients 10, no. 8: 991. https://doi.org/10.3390/nu10080991
APA StyleQu, R., Jia, Y., Liu, J., Jin, S., Han, T., & Na, L. (2018). Dietary Flavonoids, Copper Intake, and Risk of Metabolic Syndrome in Chinese Adults. Nutrients, 10(8), 991. https://doi.org/10.3390/nu10080991




