Acceptability and Utilization of Three Nutritional Supplements during Pregnancy: Findings from a Longitudinal, Mixed-Methods Study in Niger
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Population
2.2. Study Intervention
2.3. Study Design and Data Collection
2.4. Data Analysis
3. Results
3.1. Typical Utilization
Since she was given this supplement, she takes it easily … and swallows it with water. She does not have any fear of taking it.(Interviewer field notes, IFA group, Phase 1, ID#52)
Early in the morning after I take my breakfast I will open the capsule of the supplement and take two supplements, and likewise in the evening … We have heard the layer that covers the supplement does not dissolve in the body; that is why I remove it before I take the supplement … We are removing the capsule then pouring the solid medicine into water for consuming.(Pregnant woman, MMN group, Phase 2, ID#47)
Since the time that (the health assistant) gave me the supplement, I spent two weeks vomiting whenever I used to take it. Later, I complained to him and he told me that I should be taking it with fura and put sugar inside it, or I should be taking it just like that and I will become used to it. That is how I have done and now I am enjoying it.(Pregnant woman, MQ-LNS group, Phase 1, ID#86)
3.2. Perceived Benefits
It promotes and protects my health, protects my growing fetus, increases my appetite, makes me strong, limits the risk of developing minor infections and diseases, enhances safe delivery, and prevents shortage of blood in the body.(Pregnant woman, IFA group, Phase 1, ID#68)
Before, I couldn’t do any work; other people had to do it for me. But since I started consuming this supplement, by God’s grace, I can cook, sweep, bathe my children, and do all the minor housework without getting tired. This supplement is giving me strength and energy; it also builds up my body and the baby in the womb.(Pregnant woman, MMN group, Phase 1, ID#88)
When a woman is using the supplement, it increases the volume of blood in her body, so that when she delivers she will not need any blood transfusion because she has enough blood, even after delivery.(Pregnant woman, IFA group, Phase 1, ID#62)
3.3. Facilitating Factors
We don’t know the actual benefits (the supplement) will provide to us, but we are totally sure it will do something good in our body, because whatever comes from the hand of a doctor must be essential to life.(Pregnant woman, MMN group, Phase 2, ID#45)
Yes, the supplement is easy to consume. Also, it makes a delivery easier for the pregnant woman that consumes it. For instance, my neighbor gave birth to a baby girl two weeks ago. The baby looks very big and healthy. The mother also told me that she delivered the baby in a very short period of time and she thinks it is the supplement that made it easier for her to deliver. This is one of the things that encourages me to consume the supplement every day.(Pregnant woman, IFA group, Phase 1, ID#93)
3.4. Acceptability-Related Barriers
I don’t have any problem consuming the supplement, but after taking the supplement, I feel some choky thing inside my throat and I am feeling as if I want to vomit out what I took in ... After some time, it goes away and I get back to my normal state of being healthy and enjoying my day.(Pregnant woman, IFA group, Phase 1, ID#31)
Sometimes we wake up in the morning without having breakfast, so we must look for what to eat, and the drug is to be taken after eating food, so if time passes without getting something to eat, we must skip that instance (of taking the supplement).(Pregnant woman, MMN group, Phase 1, ID#47)
3.5. Utilization-Related Barriers
At first, there are some people in this community that tried so hard to see that pregnant women didn’t use the supplement. They went on spreading rumors that the supplement will cause nothing but harm to the people of this community; they also tried to scare us off the supplement by telling us all sort of lies that the doctors use to remove some liters of blood from the body, and they also said that the doctors use to cause miscarriage for the women. They also said that it is a male doctor that does the diagnosis [including vaginal exam].(Pregnant woman, MMN group, Phase 1, ID#116)
It is because the supplement makes the a baby to grow big inside the womb, and when the baby is too big, the mother will have a tear during delivery or may not be able to deliver the baby herself until she undergoes a Cesarean section.(Pregnant woman, IFA group, Phase 1, ID#112)
I used to forget about the supplement … I don’t have any specific reason … But I don’t want to have excessive blood in my body, that is why I decided to reduce my consumption of the nutritional supplement.(Pregnant woman, IFA group, Phase 1, ID#25)
Other women are saying about the supplement that it causes severe bleeding during childbirth, which leads some women to not consume the supplement. They may put it aside or discard it in the trash.(Pregnant woman, IFA group, Phase 1, ID#68)
If the supplement is going to be given to me for the whole of the year, I wouldn’t mind consuming it; I even hide it from the children so that they won’t take it, because of its sweetness. If I eat it in front of them, they will be the one to consume half of the supplement, because they would say I must give it to them too. I also don’t store it where the children will see it.(Pregnant woman, MQ-LNS group, Phase 1, ID#4)
Some just take it as a business, especially the LNS, thinking that it is a nutritional supplement for malnourished children, and they sell it 25 CFA or 50 CFA [US $0.04 to $0.09]. But now they have stopped that; since we are asking them to bring the used empty sachets, we get fewer cases of this.(Health assistant, MQ-LNS group, Phase 1, ID#109)
You will see that, especially for new programs, people will be saying different things about that program for some time. In the village, people were saying that the supplement will result in the death of the baby. If they were right, I believe from the day Epicentre started this program, thousands of children would have died by now. So these are just rumors, because since I started taking the supplement I have become healthy and I have seen many other successfully delivering a healthy baby. I have not ever listened to them, because in my mind I have no doubt about the program. That is why I am taking the supplement and even taking my children for immunization, despite all different kinds of rumors I am hearing from people.(Pregnant woman, MMN group, Phase 1, ID#88)
3.6. Family, Community, and Health Staff Perceptions
I don’t know how they (my daughters-in-law) take the supplement. I just advise them to take it, and not to quit. Every pregnant woman should take her supplements and go for antenatal care, this is indeed a great improvement to our nation; we really appreciate the help and we enjoy how the care is given to us. The mother and the baby will remain healthy. The healthcare and the medication are also free. I really appreciate it.(Mother-in-law, MMN group, Phase 1, ID#14)
My impression on the supplement is neutral. (My wife) went to the hospital and collected the supplement; I will not tell her to stop consuming the supplement … If there is anything wrong with the supplement, the doctors would not bring it here and ask pregnant women to consume it. But I do not know any additional information about the supplement … She could have told me she doesn’t like it, but from what I understand, my wife enjoys consuming this nutritional supplement.(Husband, IFA group, Phase 1, ID#64)
My first child did not experience this program, but my second child did. My first son had many problems when his mother gave birth to him, but my second son was born very healthy, so I came to realize that it is the supplement that his mother consumed during pregnancy that prevented those diseases from reaching him.(Husband, MQ-LNS group, Phase 1, ID#19)
There is nobody that can stop me from taking the supplements. Even my in-laws cannot, only my husband can stop me ... and my husband likes the supplement, he even encourages me to be taking the supplement on time ... He is the one that guides me every day. I am his second wife, but even if he didn’t sleep in my room [that night], in the morning he will come to my room and ask me whether I took the supplement or not.(Pregnant woman, MMN group, Phase 1, ID#89)
Whenever I want to take (the supplement) I have to hide so that my mother-in-law will not see me, because she doesn’t like the supplement ... She believes the rumors in town that it makes a woman not to be able to deliver the baby herself due to the big size of the baby; she has to undergo an operation. But we don’t believe this; it is not the supplement, this happens by God’s will.(Pregnant woman, MQ-LNS group, Phase 1, ID#105)
The women taking IFA complain of an irritating smell, so we tell her to put it in food and take it when going to bed (to avoid morning sickness); we tell them to do such because they will not feel it and if they go to sleep nothing will happen. For those who cannot take MMN because of its size, we tell them to take off the capsule and put the powder in fura or cool porridge – not the hot one, because the hot one makes the MMN less active – and we explain to them that it is not a problem. The LNS should be mixed with water for those that cannot endure to take it the way it is, or mixed in a porridge.(Midwife, Phase 1, ID#81)
The challenge we had from the initial point of the service was that some husbands were not happy because we are men going to their wives, but everything became normal after they understood what the service is. Some husbands even come to us themselves to inform us that their wives are pregnant so that we can involve them in the service, because they are happy with the project. Truly, we don’t have a problem.(Health assistant, MMN group, Phase 1, ID#134)
I recommend (the women) to take the supplement, because it is very essential to their lives, especially during pregnancy. Here in the village, our foods mostly do not contain vitamins, it is only millet porridge. If they can be taking the supplement as well, it will help in building their bodies, including the fetus.(Health assistant, IFA group, Phase 1, ID#104)
3.7. Change over the Course of Pregnancy (Longitudinal Analysis)
Before, when I started consuming the supplement during early pregnancy, I don’t like it, but as time goes on I started to like it, and now I don’t want to stop consuming the supplement ... The supplement I used to consume before is not sweet like the one I am consuming now that the pregnancy has become matured.(Pregnant woman, MQ-LNS group, Phase 2, ID#85)
What scares me about the supplement is that some women told me that consuming the Plumpy’Nut (MQ-LNS supplement) makes the baby to be big inside the womb to the extent that she cannot deliver the baby by herself that she has to undergo Cesarean section. This is what scares the pregnant women about consuming the Plumpy’Nut because no woman would want to undergo that Cesarean section.(Pregnant woman, MQ-LNS group, Phase 1, ID#59)
When you have a healthy pregnancy … I feel strength and much movement of the fetus. I hear people saying that the baby will be of moderate weight with good complexion. And this is my wish as well … Big or small (baby), it is all from God, and they will all pass through the same passage. You may see a mother who gave birth to a big baby without suffering, while another suffers from delivering a small baby … Most of the women who take this supplement, even if I did not ask them, I know they will have a healthy, mature, and moderate weight baby.(Pregnant woman, MQ-LNS group, Phase 2, ID#59)
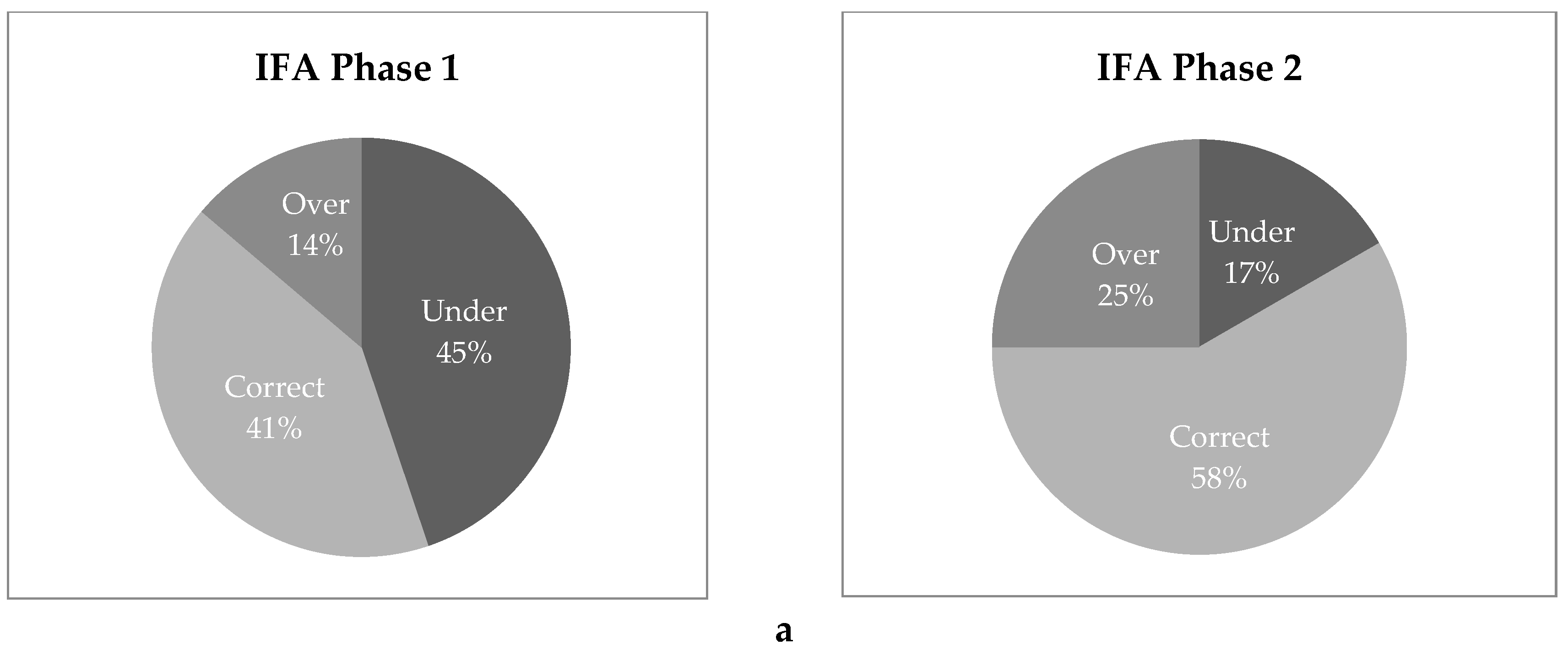
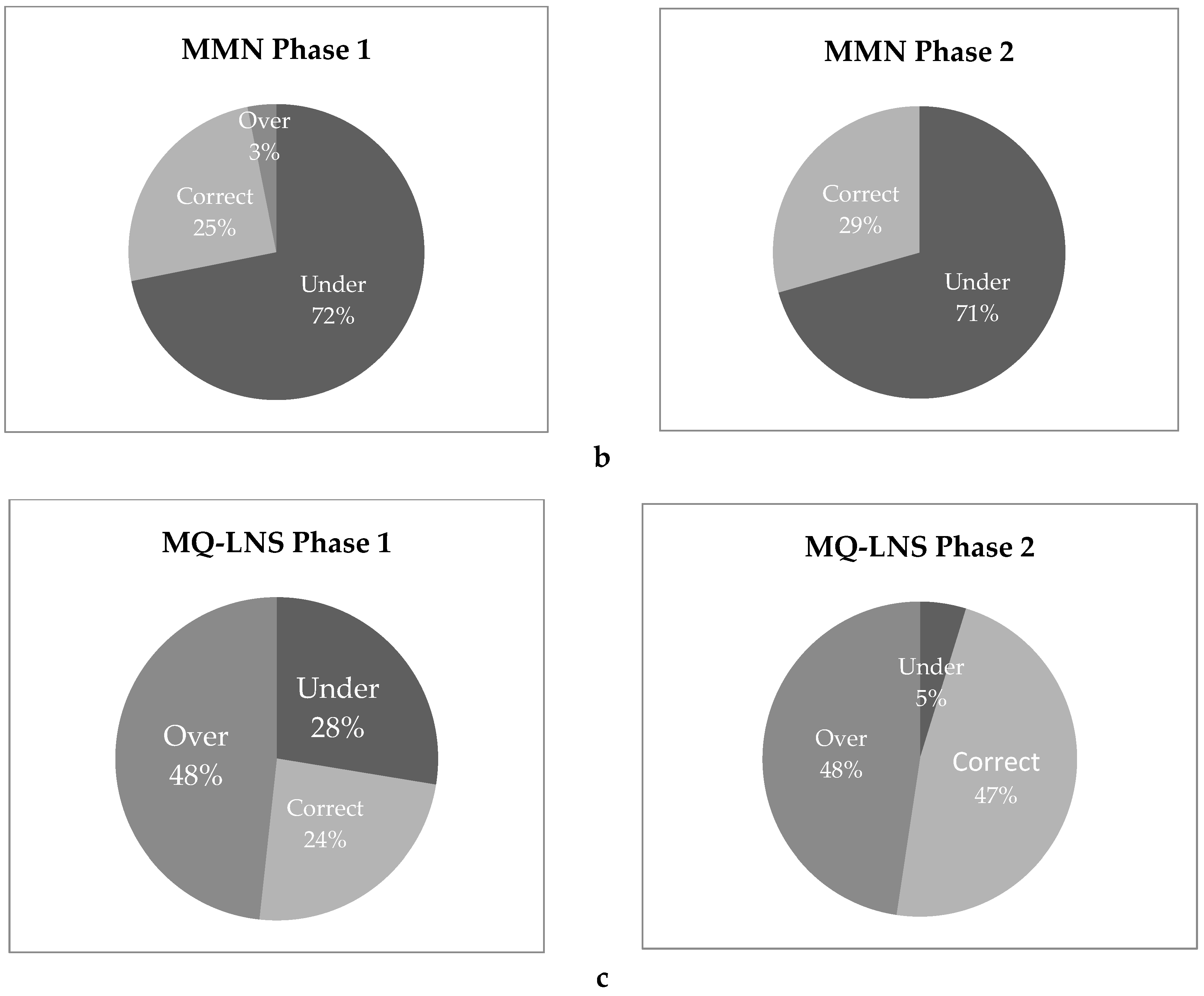
3.8. Household Spot Checks
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhutta, Z.A.; Das, J.K.; Rizvi, A.; Gaffey, M.F.; Walker, N.; Horton, S.; Webb, P.; Lartey, A.; Black, R.E.; The Lancet Nutrition Interventions Review Group; the Maternal and Child Nutrition Study Group. Evidence-based interventions for improvement of maternal and child nutrition: What can be done and at what cost? Lancet 2013, 382, 452–477. [Google Scholar] [CrossRef]
- Miller, S.; Abalos, E.; Chamillard, M.; Ciapponi, A.; Colaci, D.; Comandé, D.; Diaz, V.; Geller, S.; Hanson, C.; Langer, A.; et al. Beyond too little, too late and too much, too soon: A pathway towards evidence-based, respectful maternity care worldwide. Lancet 2016, 388, 2176–2192. [Google Scholar] [CrossRef]
- Christian, P.; Srihari, S.B.; Thorne-Lyman, A.; Khatry, S.K.; LeClerq, S.C.; Shrestha, S.R. Eating Down in Pregnancy: Exploring Food-Related Beliefs and Practices of Pregnancy in Rural Nepal. Ecol. Food Nutr. 2006, 45, 253–278. [Google Scholar] [CrossRef]
- Harding, K.L.; Matias, S.L.; Mridha, M.K.; Vosti, S.A.; Hussain, S.; Dewey, K.G.; Stewart, C.P. Eating down or simply eating less? The diet and health implications of these practices during pregnancy and postpartum in rural Bangladesh. Public Health Nutr. 2017, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ojofeitimi, E.O.; Elegbe, I.; Babafemi, J. Diet restriction by pregnant women in Nigeria. Int. J. Gynaecol. Obstet. 1982, 20, 99–103. [Google Scholar] [CrossRef]
- Hutter, I. Reduction of food intake during pregnancy in rural south India. Trop. Med. Int. Health 1996, 1, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; Mullany, L.C.; Hurley, K.M.; Katz, J.; Black, R.E. Nutrition and maternal, neonatal, and child health. Semin. Perinatol. 2015, 39, 361–372. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Institut National de la Statistique (INS). ICF International Enquête Démographique et de Santé et à Indicateurs Multiples du Niger 2012; Institut National de la Statistique: Niamey, Niger, 2013. [Google Scholar]
- Haider, B.A.; Bhutta, Z.A. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2015, 11, CD004905. [Google Scholar] [CrossRef]
- Ghanekar, J.; Kanani, S.; Patel, S. Toward better compliance with iron-folic acid supplements: Understanding the behavior of poor urban pregnant women through ethnographic decision models in Vadodara, India. Food Nutr. Bull. 2002, 23, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Lacerte, P.; Pradipasen, M.; Temcharoen, P.; Imamee, N.; Vorapongsathorn, T. Determinants of adherence to iron/folate supplementation during pregnancy in two provinces in Cambodia. Asia. Pac. J. Public Health 2011, 23, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Lutsey, P.L.; Dawe, D.; Villate, E.; Valencia, S.; Lopez, O. Iron supplementation compliance among pregnant women in Bicol, Philippines. Public Health Nutr. 2008, 11, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Seck, B.C.; Jackson, R.T. Determinants of compliance with iron supplementation among pregnant women in Senegal. Public Health Nutr. 2008, 11, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Zavaleta, N.; Caulfield, L.E.; Figueroa, A.; Chen, P. Patterns of compliance with prenatal iron supplementation among Peruvian women. Matern. Child Nutr. 2014, 10, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Harding, K.L.; Matias, S.L.; Mridha, M.K.; Moniruzzaman, M.; Vosti, S.A.; Hussain, S.; Dewey, K.G.; Stewart, C.P. Adherence to recommendations on lipid-based nutrient supplement and iron and folic acid tablet consumption among pregnant and lactating women participating in a community health programme in northwest Bangladesh. Matern. Child Nutr. 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Nisar, Y.B.; Alam, A.; Aurangzeb, B.; Dibley, M.J. Perceptions of antenatal iron-folic acid supplements in urban and rural Pakistan: A qualitative study. BMC Pregnancy Childbirth 2014, 14, 344. [Google Scholar] [CrossRef] [PubMed]
- Aguayo, V.M.; Koné, D.; Bamba, S.I.; Diallo, B.; Sidibé, Y.; Traoré, D.; Signé, P.; Baker, S.K. Acceptability of multiple micronutrient supplements by pregnant and lactating women in Mali. Public Health Nutr. 2005, 8, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.V.; Asrilla, Z.; Kadha, J.K.; Sebayang, S.; Apriatni, M.; Sulastri, A.; Sunarsih, E.; Shankar, A.H.; SUMMIT Study Group. Programmatic effects of a large-scale multiple-micronutrient supplementation trial in Indonesia: Using community facilitators as intermediaries for behavior change. Food Nutr. Bull. 2009, 30, S207–S214. [Google Scholar] [CrossRef] [PubMed]
- Young, S.L.; Blanco, I.; Hernandez-Cordero, S.; Pelto, G.H.; Neufeld, L.M. Organoleptic properties, ease of use, and perceived health effects are determinants of acceptability of micronutrient supplements among poor Mexican women. J. Nutr. 2010, 140, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, B.; Christian, P.; LeClerq, S.C.; Khatry, S.K. Determinants of compliance to antenatal micronutrient supplementation and women’s perceptions of supplement use in rural Nepal. Public Health Nutr. 2010, 13, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Klevor, M.K.; Adu-Afarwuah, S.; Ashorn, P.; Arimond, M.; Dewey, K.G.; Lartey, A.; Maleta, K.; Phiri, N.; Pyykkö, J.; Zeilani, M.; et al. A mixed method study exploring adherence to and acceptability of small quantity lipid-based nutrient supplements (SQ-LNS) among pregnant and lactating women in Ghana and Malawi. BMC Pregnancy Childbirth 2016, 16, 253. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Natamba, B.; Luwedde, F.; Nyafwono, D.; Okia, B.; Osterbauer, B.; Natureeba, P.; Johnson, L.; Michel, C.; Zheng, A.; et al. “I Have Remained Strong Because of That Food”: Acceptability and Use of Lipid-Based Nutrient Supplements Among Pregnant HIV-Infected Ugandan Women Receiving Combination Antiretroviral Therapy. AIDS Behav. 2015, 19, 1535–1547. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Isanaka, S.; Guindo, O.; Langendorf, C.; Matar Seck, A.; Plikaytis, B.D.; Sayinzoga-Makombe, N.; McNeal, M.M.; Meyer, N.; Adehossi, E.; Djibo, A.; et al. Efficacy of a Low-Cost, Heat-Stable Oral Rotavirus Vaccine in Niger. N. Engl. J. Med. 2017, 376, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Palinkas, L.A.; Horwitz, S.M.; Green, C.A.; Wisdom, J.P.; Duan, N.; Hoagwood, K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm. Policy Ment. Health 2015, 42, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Fusch, P.; Ness, L. Are We There Yet? Data Saturation in Qualitative Research. Qual. Rep. 2015, 20, 1408–1416. [Google Scholar]
- Arimond, M.; Zeilani, M.; Jungjohann, S.; Brown, K.H.; Ashorn, P.; Allen, L.H.; Dewey, K.G. Considerations in developing lipid-based nutrient supplements for prevention of undernutrition: Experience from the International Lipid-Based Nutrient Supplements (iLiNS) Project. Matern. Child Nutr. 2015, 11 (Suppl. 4), 31–61. [Google Scholar] [CrossRef]
- Dedoose, Version 7.5.27; Web Application for Managing, Analyzing, and Presenting Qualitative and Mixed Method Research Data; SocioCultural Research Consultants, LLC: Los Angeles, CA, USA, 2017.
- Charmaz, K. Constructing Grounded Theory: A Practical Guide Through Qualitative Analysis; Sage Publications Ltd.: London, UK, 2006; ISBN 978-0-7619-7352-2. [Google Scholar]
- Saldana, J. The Coding Manual for Qualitative Researchers; Sage Publications Ltd.: London, UK, 2015; ISBN 978-1-4739-4359-9. [Google Scholar]
- Mack, N.; Woodsong, C. Qualitative Research Methods: A Data Collector’s Field Guide; Family Health International: Research Triangle Park, NC, USA, 2005; ISBN 978-0-939704-98-9. [Google Scholar]
- Yin, R.K. Qualitative Research from Start to Finish, 2nd ed.; The Guilford Press: New York, NY, USA, 2016; ISBN 978-1-4625-2134-0. [Google Scholar]
- Kodish, S.R.; Aburto, N.J.; Nseluke Hambayi, M.; Dibari, F.; Gittelsohn, J. Patterns and determinants of small-quantity LNS utilization in rural Malawi and Mozambique: Considerations for interventions with specialized nutritious foods. Matern. Child Nutr. 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.P.; Okronipa, H.; Adu-Afarwuah, S.; Arimond, M.; Kumordzie, S.; Oaks, B.M.; Ocansey, M.E.; Young, R.R.; Vosti, S.A.; Dewey, K.G. Ghanaian parents’ perceptions of pre and postnatal nutrient supplements and their effects. Matern. Child Nutr. 2018, e12608. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.J. Social desirability bias and the validity of indirect questioning. J. Consum. Res. 1993, 20, 303–315. [Google Scholar] [CrossRef]
| Phase 1 Individual Interviews | Phase 1 Focus Groups | Phase 1 Spot Checks | Phase 2 Individual Interviews | Phase 2 Focus Groups | Phase 2 Spot Checks | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IFA | MMN | LNS | IFA | MMN | LNS | IFA | MMN | LNS | IFA | MMN | LNS | IFA | MMN | LNS | IFA | MMN | LNS | |
| Pregnant women (<20 weeks gestation) | 14 | 14 | 12 | 2 | 3 | 7 | 9 | 8 | 10 | |||||||||
| Pregnant women (≥20 weeks gestation) | 14 | 16 | 14 | 1 | 2 | 2 | 20 | 24 | 19 | 12 | 13 | 14 | 2 | 3 | 7 | 12 | 17 | 21 |
| Husbands | 7 | 6 | 5 | |||||||||||||||
| In-laws | 2 | 4 | 3 | |||||||||||||||
| Health assistants | 4 | 2 | 3 | |||||||||||||||
| Study midwives | 1 | 1 | 1 | |||||||||||||||
| Typical Consumption | ||||||
| Consume only after eating food | Consume with water | Consume with other food | Open capsule before consumption | Overconsume when hungry | ||
| IFA | ✔ | ✔✔ | ✔ | |||
| MMN | ✔✔✔ | ✔ | ✔✔ | ✔✔ | ||
| MQ-LNS | ✔ | ✔ | ||||
| Perceived Benefits | ||||||
| Increases appetite | Increases strength | Increases blood volume | Improved health of mother and baby | Promotes safe delivery of a healthy child | ||
| IFA | ✔✔✔ | ✔✔ | ✔✔✔ | ✔✔✔ | ✔ | |
| MMN | ✔✔✔ | ✔✔✔ | ✔✔ | ✔✔✔ | ✔ | |
| MQ-LNS | ✔✔✔ | ✔✔✔ | ✔✔ | ✔✔ | ✔ | |
| Side Effects | ||||||
| Bad odor | Too large for consumption | “Excess blood” in the body | Bad taste | |||
| IFA | ✔ | ✔ | ✔✔ | |||
| MMN | ✔✔ | ✔✔ | ||||
| MQ-LNS | ✔ | |||||
| Facilitating Factors | ||||||
| Trust in medical system | Positive perception of parent study | Saw healthy babies delivered in community | ||||
| IFA | ✔✔ | ✔✔ | ✔ | |||
| MMN | ✔✔ | ✔✔ | ✔ | |||
| MQ-LNS | ✔✔ | ✔✔ | ✔ | |||
| Barriers to Consumption | ||||||
| Rumors about parent study | Fear of delivering large baby | Fear of Bleeding/Complications during delivery | Limited access to food to consume supplement | Overconsume when hungry | Share with children or sell for money | |
| IFA | ✔✔✔ | ✔✔✔ | ✔✔ | ✔ | ||
| MMN | ✔✔✔ | ✔✔ | ✔ | ✔✔ | ||
| MQ-LNS | ✔✔✔ | ✔✔ | ✔ | ✔✔ | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clermont, A.; Kodish, S.R.; Matar Seck, A.; Salifou, A.; Rosen, J.; Grais, R.F.; Isanaka, S. Acceptability and Utilization of Three Nutritional Supplements during Pregnancy: Findings from a Longitudinal, Mixed-Methods Study in Niger. Nutrients 2018, 10, 1073. https://doi.org/10.3390/nu10081073
Clermont A, Kodish SR, Matar Seck A, Salifou A, Rosen J, Grais RF, Isanaka S. Acceptability and Utilization of Three Nutritional Supplements during Pregnancy: Findings from a Longitudinal, Mixed-Methods Study in Niger. Nutrients. 2018; 10(8):1073. https://doi.org/10.3390/nu10081073
Chicago/Turabian StyleClermont, Adrienne, Stephen R. Kodish, Amadou Matar Seck, Aichatou Salifou, Joseph Rosen, Rebecca F. Grais, and Sheila Isanaka. 2018. "Acceptability and Utilization of Three Nutritional Supplements during Pregnancy: Findings from a Longitudinal, Mixed-Methods Study in Niger" Nutrients 10, no. 8: 1073. https://doi.org/10.3390/nu10081073
APA StyleClermont, A., Kodish, S. R., Matar Seck, A., Salifou, A., Rosen, J., Grais, R. F., & Isanaka, S. (2018). Acceptability and Utilization of Three Nutritional Supplements during Pregnancy: Findings from a Longitudinal, Mixed-Methods Study in Niger. Nutrients, 10(8), 1073. https://doi.org/10.3390/nu10081073





