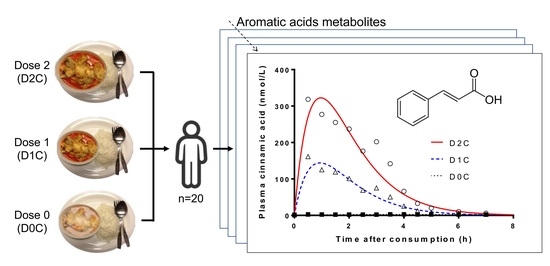
Dose-Dependent Increase in Unconjugated Cinnamic Acid Concentration in Plasma Following Acute Consumption of Polyphenol Rich Curry in the Polyspice Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Total Polyphenol Content (TPC) of Test Meals
2.3. Blood Sample Collection
2.4. Plasma Phenolic/Aromatic Acids Analyses
2.5. UHPLC-MS/MS Analysis
2.6. Statistical Analyses
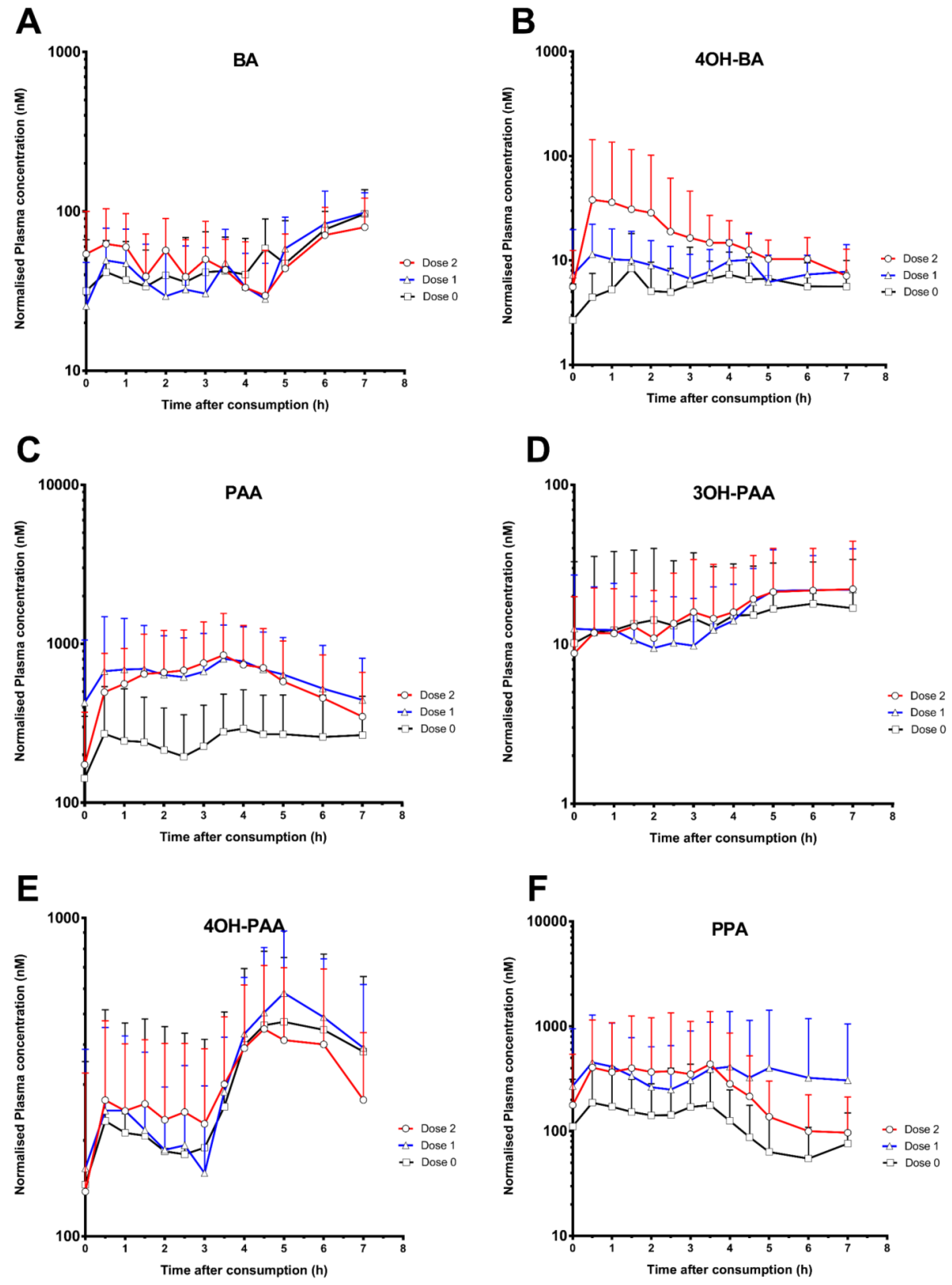
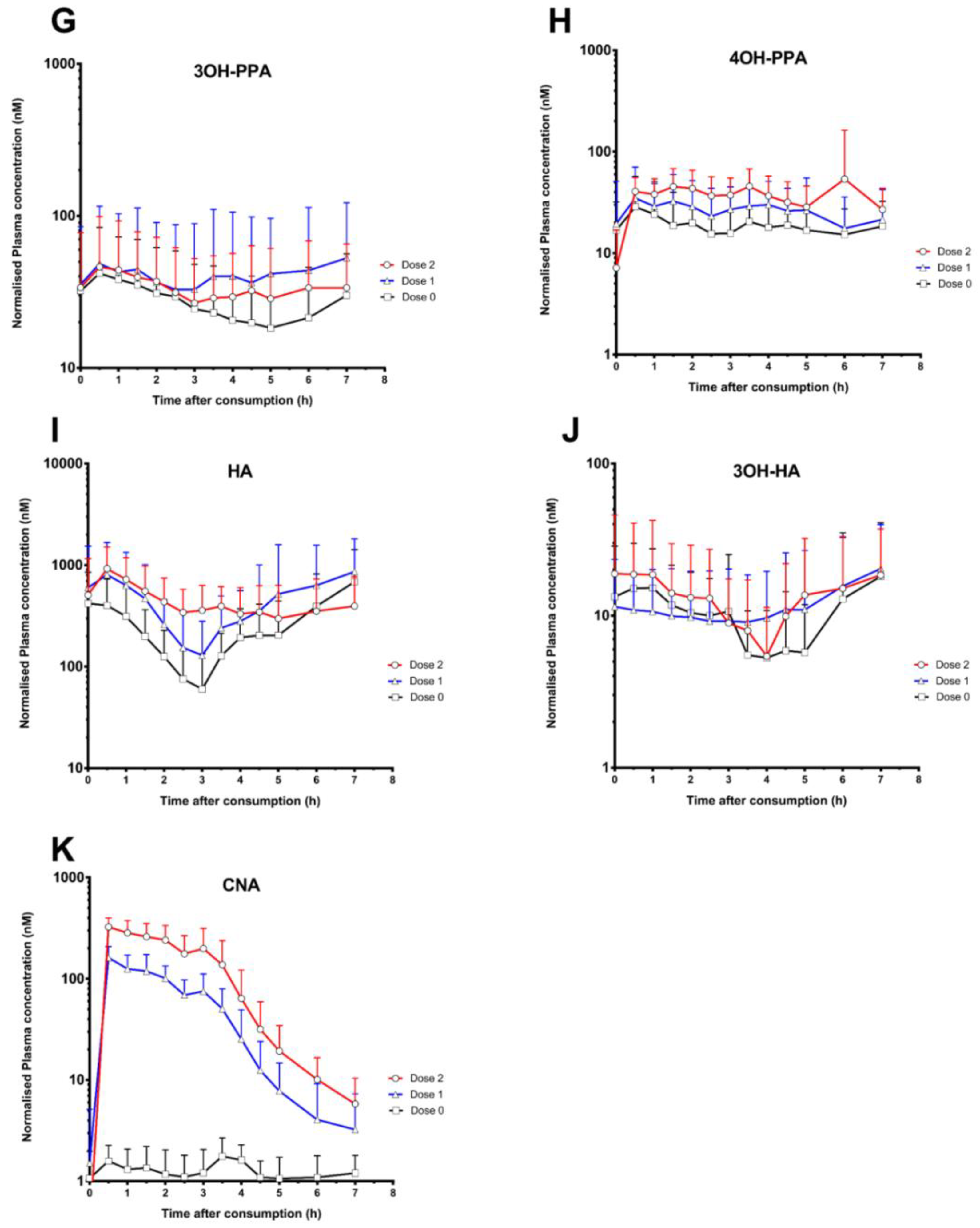
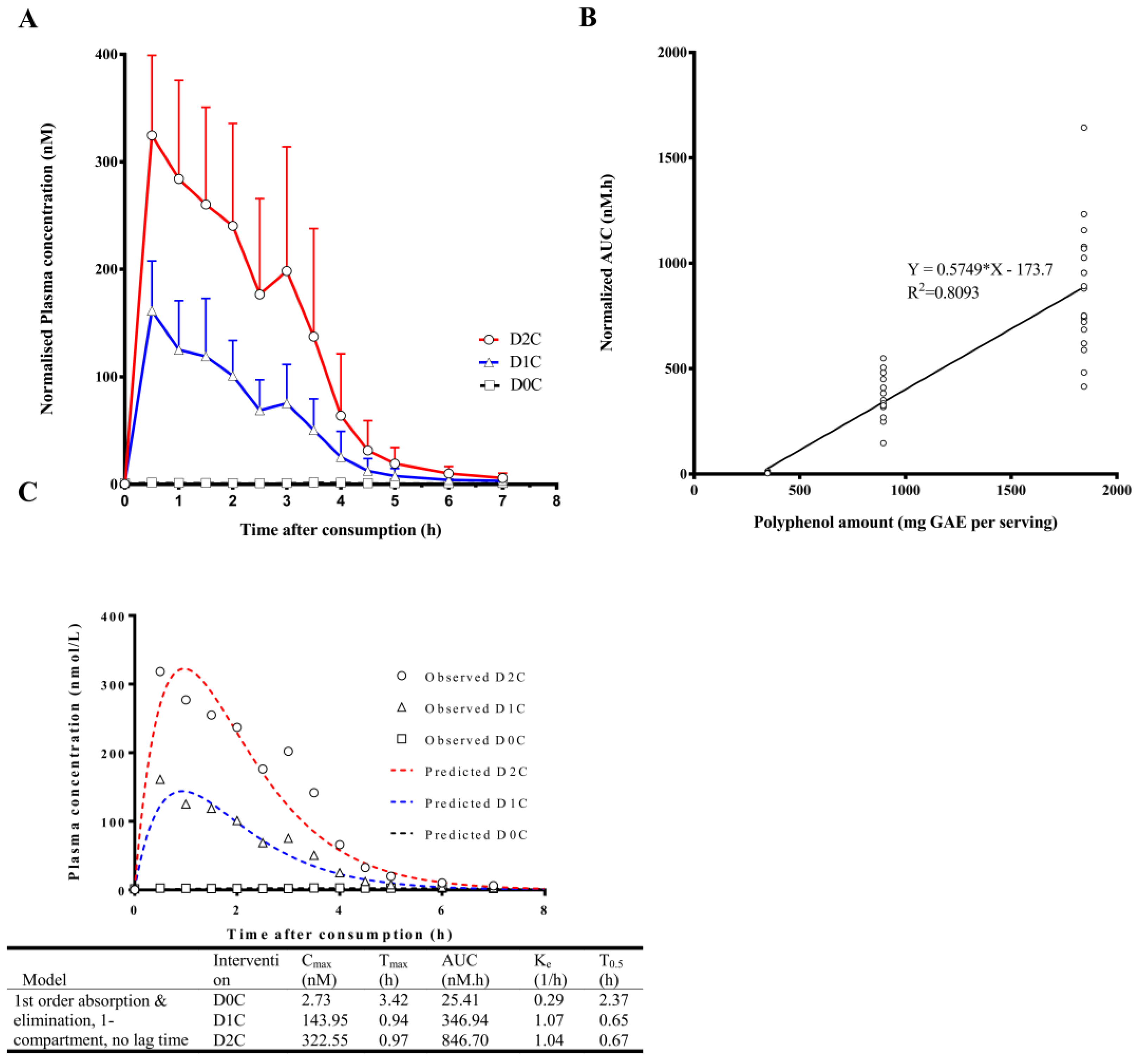
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tapsell, L.C.; Hemphill, I.; Cobiac, L.; Patch, C.S.; Sullivan, D.R.; Fenech, M.; Roodenrys, S.; Keogh, J.B.; Clifton, P.M.; Williams, P.G.; et al. Health benefits of herbs and spices: The past, the present, the future. Med. J. Aust. 2006, 185, S4–S24. [Google Scholar] [PubMed]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remón, A.; M’Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Van Duynhoven, J.; van Velzen, E.J.J.; Westerhuis, J.A.; Foltz, M.; Jacobs, D.M.; Smilde, A.K. Nutrikinetics: Concept, technologies, applications, perspectives. Trends Food Sci. Technol. 2012, 26, 4–13. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; van Velzen, E.J.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Dore, J.; et al. Metabolic fate of polyphenols in the human superorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 4531–4538. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Wang, P.; Abgaryan, N.; Vicinanza, R.; de Oliveira, D.M.; Zhang, Y.; Lee, R.P.; Carpenter, C.L.; Aronson, W.J.; Heber, D. Phenolic acid concentrations in plasma and urine from men consuming green or black tea and potential chemopreventive properties for colon cancer. Mol. Nutr. Food Res. 2013, 57, 483–493. [Google Scholar] [CrossRef] [PubMed]
- De Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.; Mullen, W.; Mullan, A.; Lean, M.E.J.; Roberts, S.A.; Crozier, A. Bioavailability of multiple components following acute ingestion of a polyphenol-rich juice drink. Mol. Nutr. Food Res. 2010, 54, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.; van der Hooft, J.; Crozier, A. Human studies on the absorption, distribution, metabolism, and excretion of tea polyphenols. Am. J. Clin. Nutr. 2013, 98. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Rothwell, J.A.; Scalbert, A.; Knaze, V.; Romieu, I.; Slimani, N.; Fagherazzi, G.; Perquier, F.; Touillaud, M.; Molina-Montes, E.; et al. Dietary intakes and food sources of phenolic acids in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br. J. Nutr. 2013, 110. [Google Scholar] [CrossRef] [PubMed]
- Lafay, S.; Gil-Izquierdo, A. Bioavailability of phenolic acids. Phytochem. Rev. 2008, 7, 301–311. [Google Scholar] [CrossRef]
- Srinivasan, K. Role of spices beyond food flavoring: Nutraceuticals with multiple health effects. Food Rev. Int. 2005, 21, 167–188. [Google Scholar] [CrossRef]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant Activity of Spices and Their Impact on Human Health: A Review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Haldar, S.; Chia, S.C.; Lee, S.H.; Lim, J.; Leow, M.K.-S.; Chan, E.C.Y.; Henry, C.J. Polyphenol-rich curry made with mixed spices and vegetables benefits glucose homeostasis in Chinese males (Polyspice Study): A dose–response randomized controlled crossover trial. Eur. J. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Haldar, S.; Chia, S.C.; Henry, C.J. Polyphenol-rich curry made with mixed spices and vegetables increases postprandial plasma GLP-1 concentration in a dose-dependent manner. Eur. J. Clin. Nutr. 2017, 1. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S. Cinnamic acid and its derivatives: Mechanisms for prevention and management of diabetes and its complications. Nutrients 2017, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.L.; Clifford, M.N.; Morgan, L.M. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: Glycemic effects of chlorogenic acid and caffeine. Am. J. Clin. Nutr. 2003, 78, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Asp. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef] [PubMed]
- Thakare, R.; Chhonker, Y.S.; Gautam, N.; Alamoudi, J.A.; Alnouti, Y. Quantitative analysis of endogenous compounds. J. Pharm. Biomed. Anal. 2016, 128, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, L.; Scazzina, F.; Leonardi, R.; Dall’Aglio, E.; Newell, M.; Dall’Asta, M.; Melegari, C.; Ray, S.; Brighenti, F.; Del Rio, D. Bioavailability and metabolism of phenolic compounds from wholegrain wheat and aleurone-rich wheat bread. Mol. Nutr. Food Res. 2016, 60, 2343–2354. [Google Scholar] [CrossRef] [PubMed]
- Renouf, M.; Marmet, C.; Giuffrida, F.; Lepage, M.; Barron, D.; Beaumont, M.; Williamson, G.; Dionisi, F. Dose-response plasma appearance of coffee chlorogenic and phenolic acids in adults. Mol. Nutr. Food Res. 2014, 58, 301–309. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Chen, C.Y.O.; Zampariello, C.A.; Blumberg, J.B. Flavonoids and phenolic acids from cranberry juice are bioavailable and bioactive in healthy older adults. Food Chem. 2015, 168, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, K.A.; Caldwell, J. The formation of β-glucuronidase resistant glucuronides by the intramolecular rearrangement of glucuronic acid conjugates at mild alkaline pH. Biochem. Pharmacol. 1982, 31, 953–957. [Google Scholar] [CrossRef]
- Faed, E.M. Properties of acyl glucuronides: Implications for studies of the pharmacokinetics and metabolism of acidic drugs. Drug Metab. Rev. 1984, 15, 1213–1249. [Google Scholar] [CrossRef] [PubMed]
- Langguth, H.S.; Benet, L.Z. Acyl Glucuronides Revisited: Is the Glucuronidation Proces a Toxification as Well as a Detoxification Mechanism? Drug Metab. Rev. 1992, 24, 5–47. [Google Scholar] [CrossRef] [PubMed]
- Piazzon, A.; Vrhovsek, U.; Masuero, D.; Mattivi, F.; Mandoj, F.; Nardini, M. Antioxidant activity of phenolic acids and their metabolites: Synthesis and antioxidant properties of the sulfate derivatives of ferulic and caffeic acids and of the acyl glucuronide of ferulic acid. J. Agric. Food Chem. 2012, 60, 12312–12323. [Google Scholar] [CrossRef] [PubMed]
- Cren-Olivé, C.; Teissier, E.; Duriez, P.; Rolando, C. Effect of catechin O-methylated metabolites and analogues on human LDL oxidation. Free Radic. Biol. Med. 2003, 34, 850–855. [Google Scholar] [CrossRef]
- Cerdá, B.; Tomás-Barberán, F.A.; Espín, J.C. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: Identification of biomarkers and individual variability. J. Agric. Food Chem. 2005, 53, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, R.P.; Boeres, A.; Massacessi, L.; Istas, G.; Ventura, M.R.; Nunes Dos Santos, C.; Heiss, C.; Rodriguez-Mateos, A. Identification and quantification of novel cranberry-derived plasma and urinary (poly)phenols. Arch. Biochem. Biophys. 2016, 599, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Yip, L.Y.; Aw, C.C.; Lee, S.H.; Hong, Y.S.; Ku, H.C.; Xu, H.; Mei, J.; Chan, X.; Jing, E.; Cheong, Y.; et al. The Liver-Gut Microbiota Axis Modulates Hepatotoxicity of Tacrine in the Rat. Hepatology 2017, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Van Duynhoven, J.; van Der Hooft, J.J.J.; van Dorsten, F.A.; Peters, S.; Foltz, M.; Gomez-Roldan, V.; Vervoort, J.; De Vos, R.C.H.; Jacobs, D.M. Rapid and sustained systemic circulation of conjugated gut microbial catabolites after single-dose black tea extract consumption. J. Proteome Res. 2014, 13, 2668–2678. [Google Scholar] [CrossRef] [PubMed]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13 C-tracer study 1–3. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Pallister, T.; Jackson, M.A.; Martin, T.C.; Zierer, J.; Jennings, A.; Mohney, R.P.; MacGregor, A.; Steves, C.J.; Cassidy, A.; Spector, T.D.; et al. Hippurate as a metabolomic marker of gut microbiome diversity: Modulation by diet and relationship to metabolic syndrome. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Clifford, M.N. Colonic metabolites of berry polyphenols: The missing link to biological activity? Br. J. Nutr. 2010, 104, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, B.L.; Ruthven, C.R.J.; Sandler, M. Gut flora and the origin of some urinary aromatic phenolic compounds. Biochem. Pharmacol. 1994, 47, 2294–2297. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Rinaldi Alvarenga, J.F.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Clifford, M.N. Dietary hydroxybenzoic acid derivatives-nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1024–1032. [Google Scholar] [CrossRef]
- Hoskins, J.A. The occurrence, metabolism and toxicity of cinnamic acid and related compounds. J. Appl. Toxicol. 1984, 4, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Liu, H.; Liu, C.; Wang, L.; Ma, R.; Chen, B.; Li, L.; Niu, J.; Fu, M.; Zhang, D.; et al. Cinnamaldehyde in diabetes: A review of pharmacology, pharmacokinetics and safety. Pharmacol. Res. 2017, 122, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, Y.; Ma, W. Pharmacokinetics and bioavailability of cinnamic acid after oral administration of Ramulus Cinnamomi in rats. Eur. J. Drug Metab. Pharmacokinet. 2009, 34, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, M.; Ju, W.; Liu, S.; Xu, M.; Chu, J.; Wu, T. Liquid chromatograph/tandem mass spectrometry assay for the simultaneous determination of chlorogenic acid and cinnamic acid in plasma and its application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2010, 51, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Zhao, Y.; Zhang, Q.; Wang, P.; Guan, J.; Rong, R.; Yu, Z. Simultaneous determination of cinnamaldehyde, cinnamic acid, and 2-methoxy cinnamic acid in rat whole blood after oral administration of volatile oil of Cinnamoni Ramulus by UHPLC-MS/MS: An application for a pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 1001, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Garrait, G.; Jarrige, J.F.; Blanquet, S.; Beyssac, E.; Cardot, J.M.; Alric, M. Gastrointestinal absorption and urinary excretion of trans-cinnamic and p-coumaric acids in rats. J. Agric. Food Chem. 2006, 54, 2944–2950. [Google Scholar] [CrossRef] [PubMed]
- Platel, K.; Srinivasan, K. Digestive stimulant action of spices: A myth or reality? Indian J. Med. Res. 2004, 119, 167–179. [Google Scholar] [PubMed]
- Achaintre, D.; Buleté, A.; Cren-Olivé, C.; Li, L.; Rinaldi, S.; Scalbert, A. Differential Isotope Labeling of 38 Dietary Polyphenols and Their Quantification in Urine by Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry. Anal. Chem. 2016, 88, 2637–2644. [Google Scholar] [CrossRef] [PubMed]
| Nutrikinetic Parameters | Cmax (nM) | Tmax (h) | AUC0–7 h (nM.h) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Polyphenols Intake | D0C | D1C | D2C | D0C | D1C | D2C | D0C | D1C | D2C |
| Benzoic acids | |||||||||
| Benzoic acid (BA) | 114.3 ± 36.7 | 113.3 ± 33.6 | 109.0 ± 34.6 | 5.3 ± 2.2 | 5.7 ± 2.0 | 3.8 ± 2.8 | 347.9 ± 86.8 | 347.0 ± 107.2 | 354.7 ± 122.8 |
| 3-Hydoxybenzoic acid (3OH-BA) | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 4-Hydroxybenzoic acid (4OH-BA) | 13.7 ± 9.9 | 18.3 ± 12.4 | 41.5 ± 101.2 | 3.1 ± 1.7 | 3.4 ± 2.1 | 3.5 ± 1.5 | 36.0 ± 23.3 | 54.2 ± 34.6 | 114.1 ± 207.0 |
| Phenylacetic acids | |||||||||
| Phenylacetic acid (PAA) | 433.3 ± 286.9 | 1019.1 ± 825.9 | 1001.4 ± 666.2 | 3.4 ± 2.4 | 3.6 ± 1.4 | 3.2 ± 1.3 | 1742.3 ± 1243.4 | 4437.2 ± 2974.3 | 4132.1 ± 2947.1 |
| 3-Hydroxyphenylacetic acid (3OH-PAA) | 28.1 ± 26.2 | 27.2 ± 15.8 | 29.1 ± 20.5 | 4.9 ± 2.0 | 4.7 ± 2.6 | 5.7 ± 1.7 | 101.0 ± 112.2 | 105.7 ± 70.0 | 113.3 ± 90.4 |
| 4-Hydroxyphenylacetic acid (4OH-PAA) | 565.9 ± 322.3 | 667.3 ± 304.9 | 633.4 ± 285.1 | 4.7 ± 2.0 | 4.4 ± 2.0 | 3.8 ± 1.8 | 2070.7 ± 1453.7 | 2378.0 ± 1065.8 | 2166.7 ± 1067.5 |
| Phenylpropanoic acids | |||||||||
| 3-Phenylpropanoate acid (PPA) | 278.7 ± 322.4 | 730.7 ± 1224.0 | 554.4 ± 984.6 | 2.5 ± 1.8 | 2.8 ± 2.3 | 3.0 ±2.2 | 831.1 ± 783.9 | 2390.8 ± 4371.0 | 1859.8 ± 3426.7 |
| 3-(3-Hydroxyphenyl)-propanoic acid (3OH- PPA) | 58.9 ± 39.1 | 83.3 ± 79.5 | 72.5 ± 48.6 | 3.6 ± 2.8 | 4.0± 2.8 | 3.5 ± 2.9 | 182.2 ± 126.3 | 283.0 ± 388.2 | 226.3 ± 176.6 |
| 3-(4-Hydroxyphenyl)-propanoic acid (4OH-PPA) | 31.4 ± 25.0 | 48.6 ± 36.6 | 81.7 ± 102.2 | 3.1 ± 2.7 | 2.6 ± 1.7 | 3.3 ± 2.0 | 126.3 ± 115.1 | 178.0 ± 120.3 | 260.6 ± 131.7 |
| Hippuric acids | |||||||||
| Hippuric acid (HA) | 1286.2 ± 706.5 | 1369.2 ± 1167.5 | 1075.8 ± 615.7 | 2.4 ± 3.3 | 3.4 ± 3.2 | 0.5 ± 0.3 | 1832.7 ± 1421.5 | 3224.5 ± 3095.2 | 3063.1 ± 1795.5 |
| 3-Hydroxyhippuric acid (3OH-HA) | 26.3 ± 22.5 | 27.4 ± 19.1 | 31.3 ± 26.5 | 3.3 ± 3.1 | 4.8 ± 2.9 | 3.8 ± 3.2 | 70.7 ± 61.6 | 80.1 ± 68.3 | 88.4 ± 85.4 |
| 4-Hydroxyhippuric acid (4OH-HA) | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Cinnamic acids | |||||||||
| Cinnamic acid (CNA) * | 2.4 ± 0.7 | 164.4 ± 47.7 | 342.0 ± 83.0 | 2.8 ± 1.9 | 0.7 ± 0.4 | 1.4 ± 1.2 | 8.4 ± 3.4 | 376.1 ± 104.7 | 875.7 ± 291.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haldar, S.; Lee, S.H.; Tan, J.J.; Chia, S.C.; Henry, C.J.; Chan, E.C.Y. Dose-Dependent Increase in Unconjugated Cinnamic Acid Concentration in Plasma Following Acute Consumption of Polyphenol Rich Curry in the Polyspice Study. Nutrients 2018, 10, 934. https://doi.org/10.3390/nu10070934
Haldar S, Lee SH, Tan JJ, Chia SC, Henry CJ, Chan ECY. Dose-Dependent Increase in Unconjugated Cinnamic Acid Concentration in Plasma Following Acute Consumption of Polyphenol Rich Curry in the Polyspice Study. Nutrients. 2018; 10(7):934. https://doi.org/10.3390/nu10070934
Chicago/Turabian StyleHaldar, Sumanto, Sze Han Lee, Jun Jie Tan, Siok Ching Chia, Christiani Jeyakumar Henry, and Eric Chun Yong Chan. 2018. "Dose-Dependent Increase in Unconjugated Cinnamic Acid Concentration in Plasma Following Acute Consumption of Polyphenol Rich Curry in the Polyspice Study" Nutrients 10, no. 7: 934. https://doi.org/10.3390/nu10070934
APA StyleHaldar, S., Lee, S. H., Tan, J. J., Chia, S. C., Henry, C. J., & Chan, E. C. Y. (2018). Dose-Dependent Increase in Unconjugated Cinnamic Acid Concentration in Plasma Following Acute Consumption of Polyphenol Rich Curry in the Polyspice Study. Nutrients, 10(7), 934. https://doi.org/10.3390/nu10070934