Vitamin D Regulates Maternal T-Helper Cytokine Production in Infertile Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Vitamin D Prospective Intervention Study
2.3. Analysis of T-helper Cells
2.4. Analysis of Vitamin D
2.5. Cytokine Analysis in Primary Human Endometrial Cell Culture
2.6. Immunohistochemical Staining for Vitamin D Receptor
2.7. Statistical Analysis
3. Results
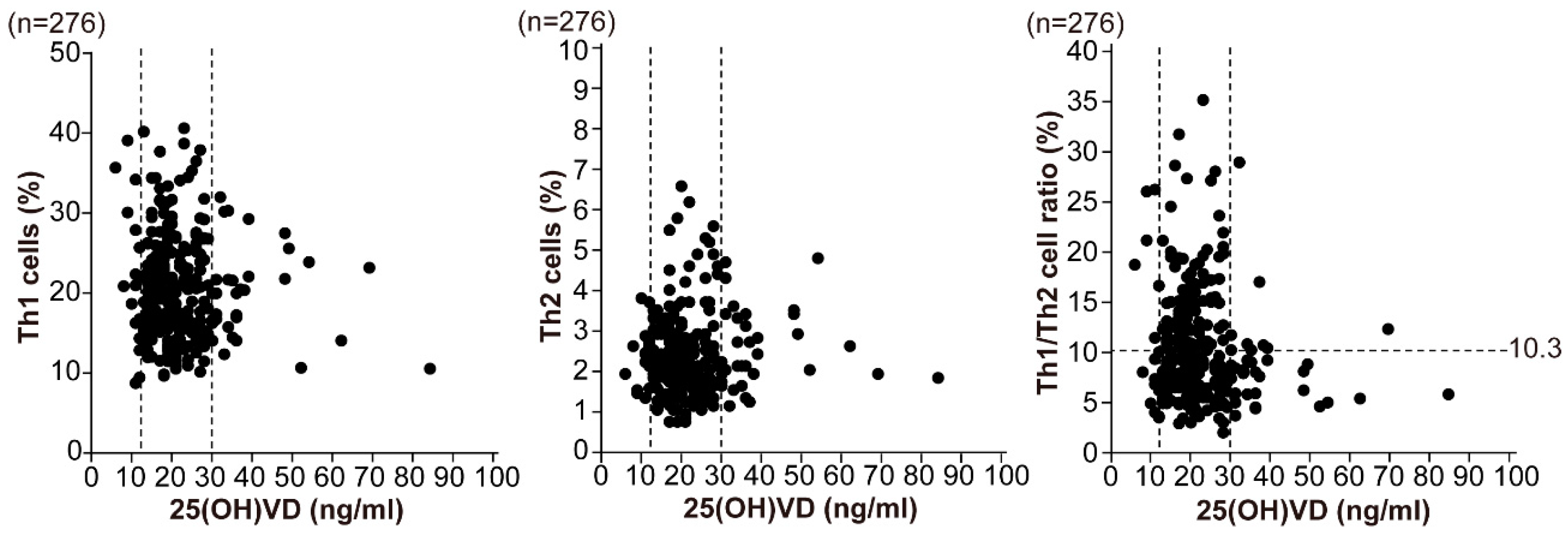
3.1. Status of Helper T-Cell Immunity and Vitamin D in Infertile Women
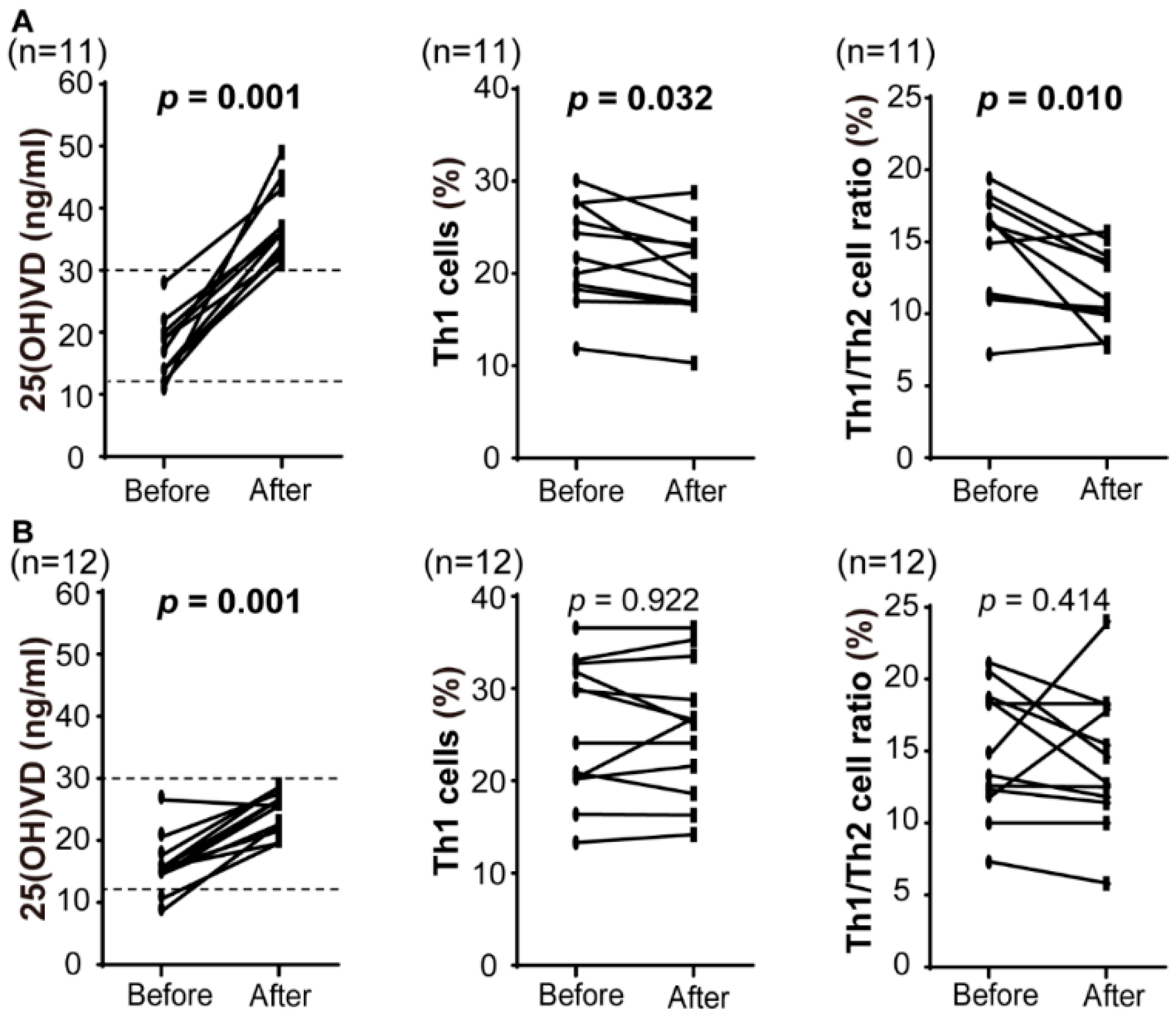
3.2. Immunological Impact of Preconception Vitamin D Supplementation
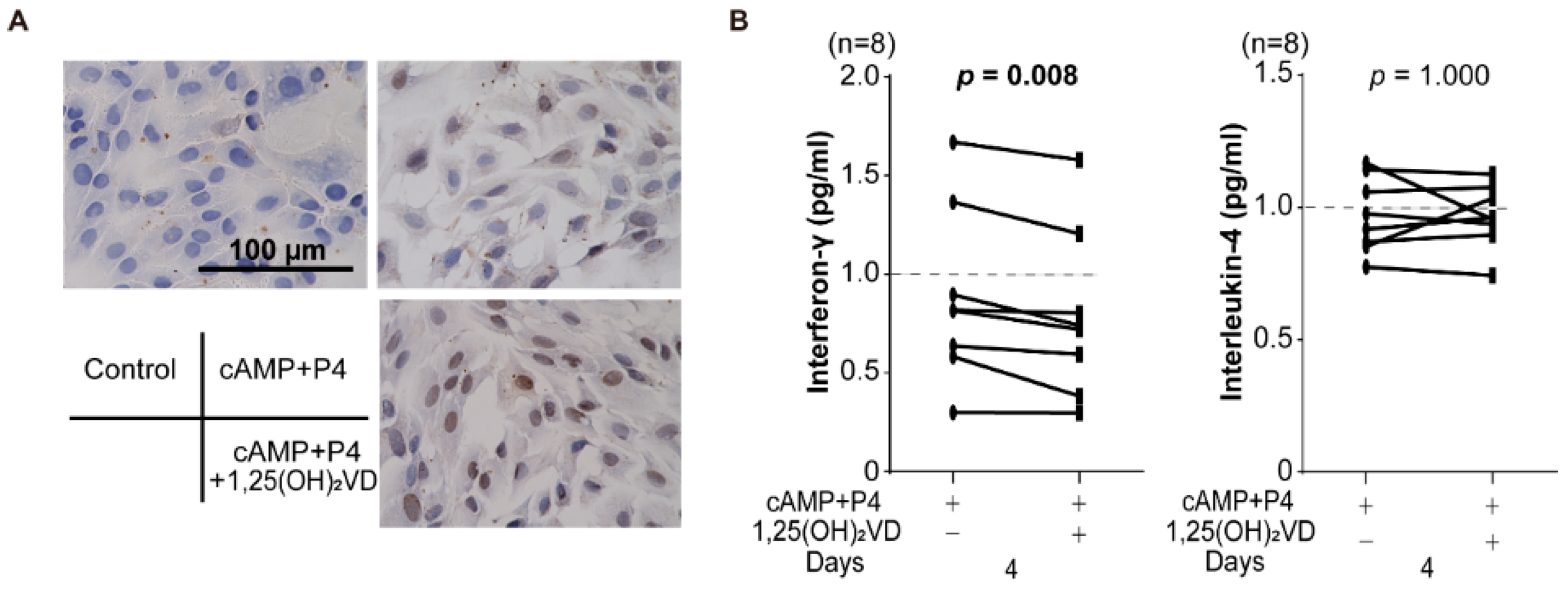
3.3. Effect of Vitamin D on Decidualized Endometrial Stromal Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and the immune system: New perspectives on an old theme. Endocrinol. Metab. Clin. N. Am. 2010, 39, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Bizzaro, G.; Shoenfeld, Y. Vitamin D and autoimmune thyroid diseases: Facts and unresolved questions. Immunol. Res. 2015, 61, 46–52. [Google Scholar] [CrossRef] [PubMed]
- El-Fakhri, N.; McDevitt, H.; Shaikh, M.G.; Halsey, C.; Ahmed, S.F. Vitamin D and its effects on glucose homeostasis, cardiovascular function and immune function. Horm. Res. Paediatr. 2014, 81, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Terrier, B.; Derian, N.; Schoindre, Y.; Chaara, W.; Geri, G.; Zahr, N.; Mariampillai, K.; Rosenzwajg, M.; Carpentier, W.; Musset, L.; et al. Restoration of regulatory and effector T cell balance and B cell homeostasis in systemic lupus erythematosus patients through vitamin D supplementation. Arthritis Res. Ther. 2012, 14, R221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.X.; Pan, G.T.; Guo, J.F.; Li, B.Y.; Qin, L.Q.; Zhang, Z.L. Vitamin D deficiency increases the risk of gestational diabetes mellitus: A meta-analysis of observational studies. Nutrients 2015, 7, 8366–8375. [Google Scholar] [CrossRef] [PubMed]
- Mirzakhani, H.; Litonjua, A.A.; McElrath, T.F.; O’Connor, G.; Lee-Parritz, A.; Iverson, R.; Macones, G.; Strunk, R.C.; Bacharier, L.B.; Zeiger, R.; et al. Early pregnancy vitamin D status and risk of preeclampsia. J. Clin. Investig. 2016, 126, 4702–4715. [Google Scholar] [CrossRef] [PubMed]
- Ota, K.; Dambaeva, S.; Han, A.R.; Beaman, K.; Gilman-Sachs, A.; Kwak-Kim, J. Vitamin D deficiency may be a risk factor for recurrent pregnancy losses by increasing cellular immunity and autoimmunity. Hum. Reprod. 2014, 29, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Rudick, B.J.; Ingles, S.A.; Chung, K.; Stanczyk, F.Z.; Paulson, R.J.; Bendikson, K.A. Influence of vitamin D levels on in vitro fertilization outcomes in donor-recipient cycles. Fertil. Steril. 2014, 101, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Raghupathy, R.; Makhseed, M.; Azizieh, F.; Omu, A.; Gupta, M.; Farhat, R. Cytokine production by maternal lymphocytes during normal human pregnancy and in unexplained recurrent spontaneous abortion. Hum. Reprod. 2000, 15, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Gilman-Sachs, A.; Thaker, P.; Beaman, K.D.; Beer, A.E.; Kwak-Kim, J. Expression of intracellular Th1 and Th2 cytokines in women with recurrent spontaneous abortion, implantation failures after IVF/ET or normal pregnancy. Am. J. Reprod. Immunol. 2002, 48, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Aluvihare, V.R.; Kallikourdis, M.; Betz, A.G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 2004, 5, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Sakai, M.; Miyazaki, S.; Higuma, S.; Shiozaki, A.; Saito, S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol. Hum. Reprod. 2004, 10, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Crome, S.Q.; Wang, A.Y.; Levings, M.K. Translational mini-review series on Th17 cells: Function and regulation of human T helper 17 cells in health and disease. Clin. Exp. Immunol. 2010, 159, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Hao, C.F.; Yi, L.; Yin, G.J.; Bao, S.H.; Qiu, L.H.; Lin, Q.D. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J. Reprod. Immunol. 2010, 84, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, J.Y.; Hur, S.E.; Kim, C.J.; Na, B.J.; Lee, M.; Gilman-Sachs, A.; Kwak-Kim, J. An imbalance in interleukin-17-producing T and Foxp3(+) regulatory T cells in women with idiopathic recurrent pregnancy loss. Hum. Reprod. 2011, 26, 2964–2971. [Google Scholar] [CrossRef] [PubMed]
- Agic, A.; Xu, H.; Altgassen, C.; Noack, F.; Wolfler, M.M.; Diedrich, K.; Friedrich, M.; Taylor, R.N.; Hornung, D. Relative expression of 1,25-dihydroxyvitamin D3 receptor, vitamin D 1 alpha-hydroxylase, vitamin D 24-hydroxylase, and vitamin D 25-hydroxylase in endometriosis and gynecologic cancers. Reprod. Sci. 2007, 14, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Perez-Fernandez, R.; Alonso, M.; Segura, C.; Munoz, I.; Garcia-Caballero, T.; Diguez, C. Vitamin D receptor gene expression in human pituitary gland. Life Sci. 1997, 60, 35–42. [Google Scholar] [CrossRef]
- Evans, K.N.; Bulmer, J.N.; Kilby, M.D.; Hewison, M. Vitamin D and placental-decidual function. J. Soc. Gynecol. Investig. 2004, 11, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Vigano, P.; Lattuada, D.; Mangioni, S.; Ermellino, L.; Vignali, M.; Caporizzo, E.; Panina-Bordignon, P.; Besozzi, M.; Di Blasio, A.M. Cycling and early pregnant endometrium as a site of regulated expression of the vitamin D system. J. Mol. Endocrinol. 2006, 36, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.N.; Nguyen, L.; Chan, J.; Innes, B.A.; Bulmer, J.N.; Kilby, M.D.; Hewison, M. Effects of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on cytokine production by human decidual cells. Biol. Reprod. 2006, 75, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Kinuta, K.; Tanaka, H.; Moriwake, T.; Aya, K.; Kato, S.; Seino, Y. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology 2000, 141, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Guillot, X.; Semerano, L.; Saidenberg-Kermanac’h, N.; Falgarone, G.; Boissier, M.C. Vitamin D and inflammation. Jt. Bone Spine 2010, 77, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, A.; Barrat, F.J.; Crain, C.; Heath, V.L.; Savelkoul, H.F.; O’Garra, A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J. Immunol. 2001, 167, 4974–4980. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Snyder, L.; Lin, Y.D.; Yang, L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, R.; Kuroda, K.; Ikemoto, Y.; Ochiai, A.; Matsumoto, A.; Kumakiri, J.; Kitade, M.; Itakura, A.; Muter, J.; Brosens, J.J.; et al. Reprogramming of the retinoic acid pathway in decidualizing human endometrial stromal cells. PLoS ONE 2017, 12, e0173035. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, M.; Jeddi-Tehrani, M.; Salek-Moghaddam, A.; Rajaei, S.; Mohammadzadeh, A.; Sheikhhasani, S.; Kazemi-Sefat, G.E.; Zarnani, A.H. Effects of 1,25(OH)2 vitamin D3 on cytokine production by endometrial cells of women with recurrent spontaneous abortion. Fertil. Steril. 2011, 96, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Salker, M.S.; Zhang, S.; Singh, Y.; Shi, B.; Stournaras, C.; Lang, F. 1alpha,25(OH)2D3 induces actin depolymerization in endometrial carcinoma cells by targeting RAC1 and PAK1. Cell. Physiol. Biochem. 2016, 40, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Kwak-Kim, J.; Ota, K.; Kuroda, K.; Hisano, M.; Sugiyama, R.; Yamaguchi, K. Immunosuppression with tacrolimus improved reproductive outcome of women with repeated implantation failure and elevated peripheral blood TH1/TH2 cell ratios. Am. J. Reprod. Immunol. 2015, 73, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Looker, A.C.; Pfeiffer, C.M.; Lacher, D.A.; Schleicher, R.L.; Picciano, M.F.; Yetley, E.A. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am. J. Clin. Nutr. 2008, 88, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Al-Jaroudi, D.; Al-Banyan, N.; Aljohani, N.J.; Kaddour, O.; Al-Tannir, M. Vitamin D deficiency among subfertile women: Case-control study. Gynecol. Endocrinol. 2016, 32, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Triggianese, P.; Watad, A.; Cedola, F.; Perricone, C.; Amital, H.; Giambini, I.; Perricone, R.; Shoenfeld, Y.; De Carolis, C. Vitamin D deficiency in an Italian cohort of infertile women. Am. J. Reprod. Immunol. 2017, 78. [Google Scholar] [CrossRef] [PubMed]
- Rojansky, N.; Brzezinski, A.; Schenker, J.G. Seasonality in human reproduction: An update. Hum. Reprod. 1992, 7, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Hilger, J.; Friedel, A.; Herr, R.; Rausch, T.; Roos, F.; Wahl, D.A.; Pierroz, D.D.; Weber, P.; Hoffmann, K. A systematic review of vitamin D status in populations worldwide. Br. J. Nutr. 2014, 111, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Kuroda, K.; Sugiyama, R.; Yamaguchi, K. After 12 consecutive miscarriages, a patient received immunosuppressive treatment and delivered an intact baby. Reprod. Med. Biol. 2017, 16, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yin, B.; Lian, R.C.; Zhang, T.; Zhang, H.Z.; Diao, L.H.; Li, Y.Y.; Huang, C.Y.; Liang, D.S.; Zeng, Y. Modulatory effects of vitamin D on peripheral cellular immunity in patients with recurrent miscarriage. Am. J. Reprod. Immunol. 2016, 76, 432–438. [Google Scholar] [CrossRef] [PubMed]
| Deficiency (n = 18) | Insufficiency (n = 223) | Sufficiency (n = 35) | p Value | |
|---|---|---|---|---|
| Age (years) | 35.6 ± 4.6 | 36.1 ± 3.6 | 35.7 ± 3.5 | 0.899 |
| 25(OH)VD (ng/mL) | 10.7 ± 1.7 | 20.2 ± 4.4 | 39.3 ± 12.3 | <0.0001 |
| Th1 cells (%) | 21.6 ± 8.9 | 20.8 ± 6.5 | 19.6 ± 5.6 | 0.748 |
| Th2 cells (%) | 2.3 ± 0.7 | 2.3 ± 1.0 | 2.5 ± 1.0 | 0.621 |
| Th1/Th2 cell ratio | 10.9 ± 7.5 | 10.6 ± 5.6 | 8.6 ± 4.5 | 0.411 |
| n = 23 | Before | After | p Value |
|---|---|---|---|
| 25(OH)VD (ng/mL) | 16.7 ± 4.7 | 31.0 ± 7.8 | <0.0001 |
| 1,25(OH)2VD (pg/mL) | 49.9 ± 8.2 | 65.4 ± 16.8 | 0.019 |
| Th1 cells (%) | 24.0 ± 6.7 | 23.0 ± 6.8 | 0.135 |
| Th2 cells (%) | 1.7 ± 0.6 | 1.9 ± 0.6 | 0.141 |
| Th1/Th2 cell ratio | 14.8 ± 4.0 | 13.1 ± 4.1 | 0.004 |
| Th17 cells (%) | 1.65 ± 0.40 | 1.54 ± 0.63 | 0.315 |
| Treg cells (%) | 5.74 ± 0.98 | 6.38 ± 1.03 | 0.059 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikemoto, Y.; Kuroda, K.; Nakagawa, K.; Ochiai, A.; Ozaki, R.; Murakami, K.; Jinushi, M.; Matsumoto, A.; Sugiyama, R.; Takeda, S. Vitamin D Regulates Maternal T-Helper Cytokine Production in Infertile Women. Nutrients 2018, 10, 902. https://doi.org/10.3390/nu10070902
Ikemoto Y, Kuroda K, Nakagawa K, Ochiai A, Ozaki R, Murakami K, Jinushi M, Matsumoto A, Sugiyama R, Takeda S. Vitamin D Regulates Maternal T-Helper Cytokine Production in Infertile Women. Nutrients. 2018; 10(7):902. https://doi.org/10.3390/nu10070902
Chicago/Turabian StyleIkemoto, Yuko, Keiji Kuroda, Koji Nakagawa, Asako Ochiai, Rie Ozaki, Keisuke Murakami, Makoto Jinushi, Akemi Matsumoto, Rikikazu Sugiyama, and Satoru Takeda. 2018. "Vitamin D Regulates Maternal T-Helper Cytokine Production in Infertile Women" Nutrients 10, no. 7: 902. https://doi.org/10.3390/nu10070902
APA StyleIkemoto, Y., Kuroda, K., Nakagawa, K., Ochiai, A., Ozaki, R., Murakami, K., Jinushi, M., Matsumoto, A., Sugiyama, R., & Takeda, S. (2018). Vitamin D Regulates Maternal T-Helper Cytokine Production in Infertile Women. Nutrients, 10(7), 902. https://doi.org/10.3390/nu10070902






