A Balanced Risk-Benefit Analysis to Determine Human Risks Associated with Pyrrolizidine Alkaloids (PA)—The Case of Herbal Medicinal Products Containing St. John’s Wort Extracts (SJW)
Abstract
1. Introduction
2. Pharmacological Effects of St. John’s Wort (SJW) in the Treatment of Depression: Clinical Studies, Side Effects, and Guidelines
2.1. Definitions
2.2. European and Chinese Studies
2.3. U.S.-American Studies
2.4. Side Effects of SJW
2.5. Guidelines and Cross-Cultural Differences
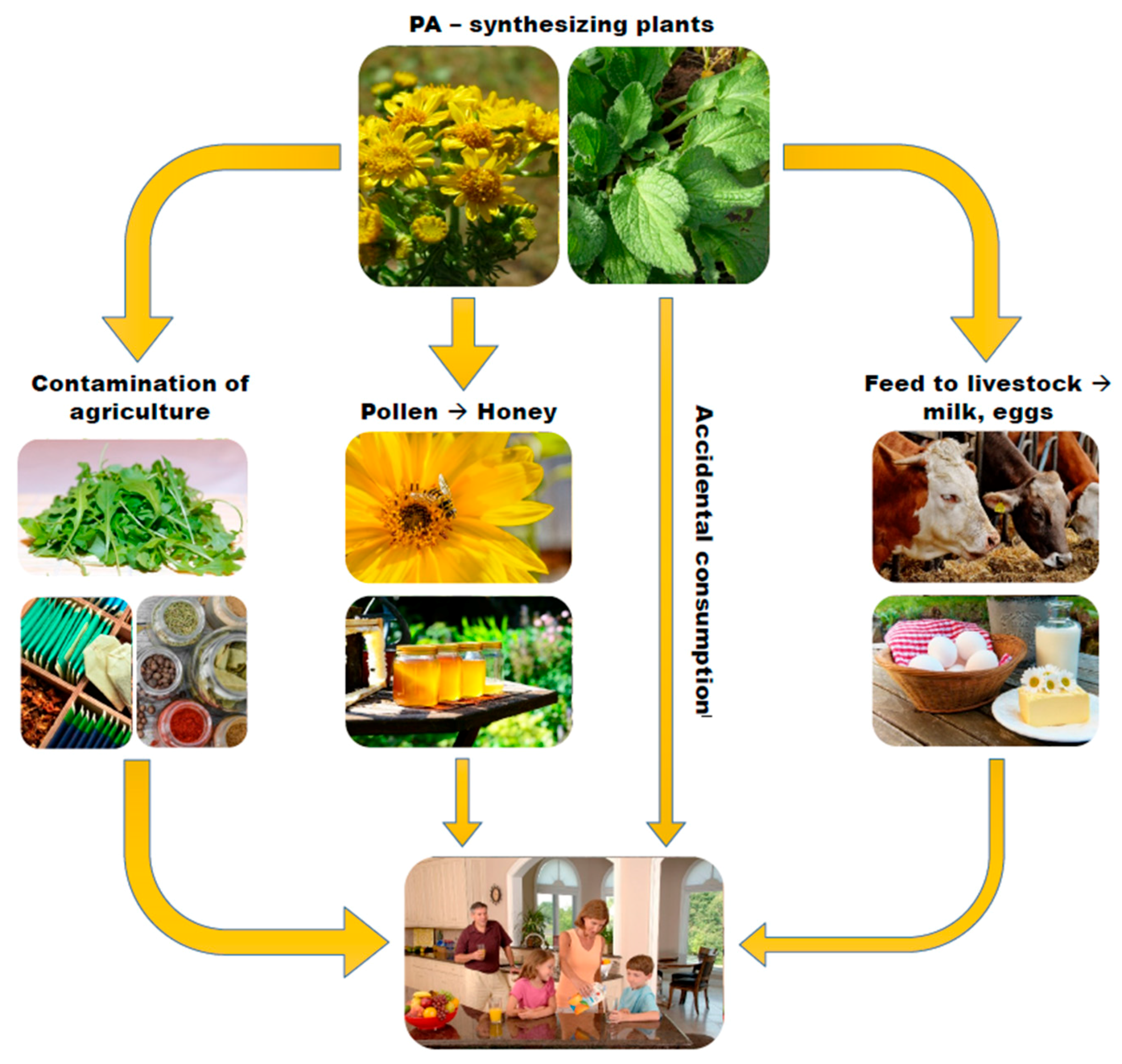
3. SJW Contamination with PA
- Contamination of pollen of plants and resulting bee products such as honey: The PA content of honey has been shown to be directly proportional to the amount of PA pollen in honey, and the transfer of PA from pollen to honey occurs rather quickly [31].
- PA-contamination of feed given to livestock: Such contamination may result in low level PA contamination in the foods produced by the animals such as milk and eggs.
4. PA Toxicity: Detectability, Metabolism, and Serious Health Consequences
5. Balanced Risk-Benefit and Risk Communication
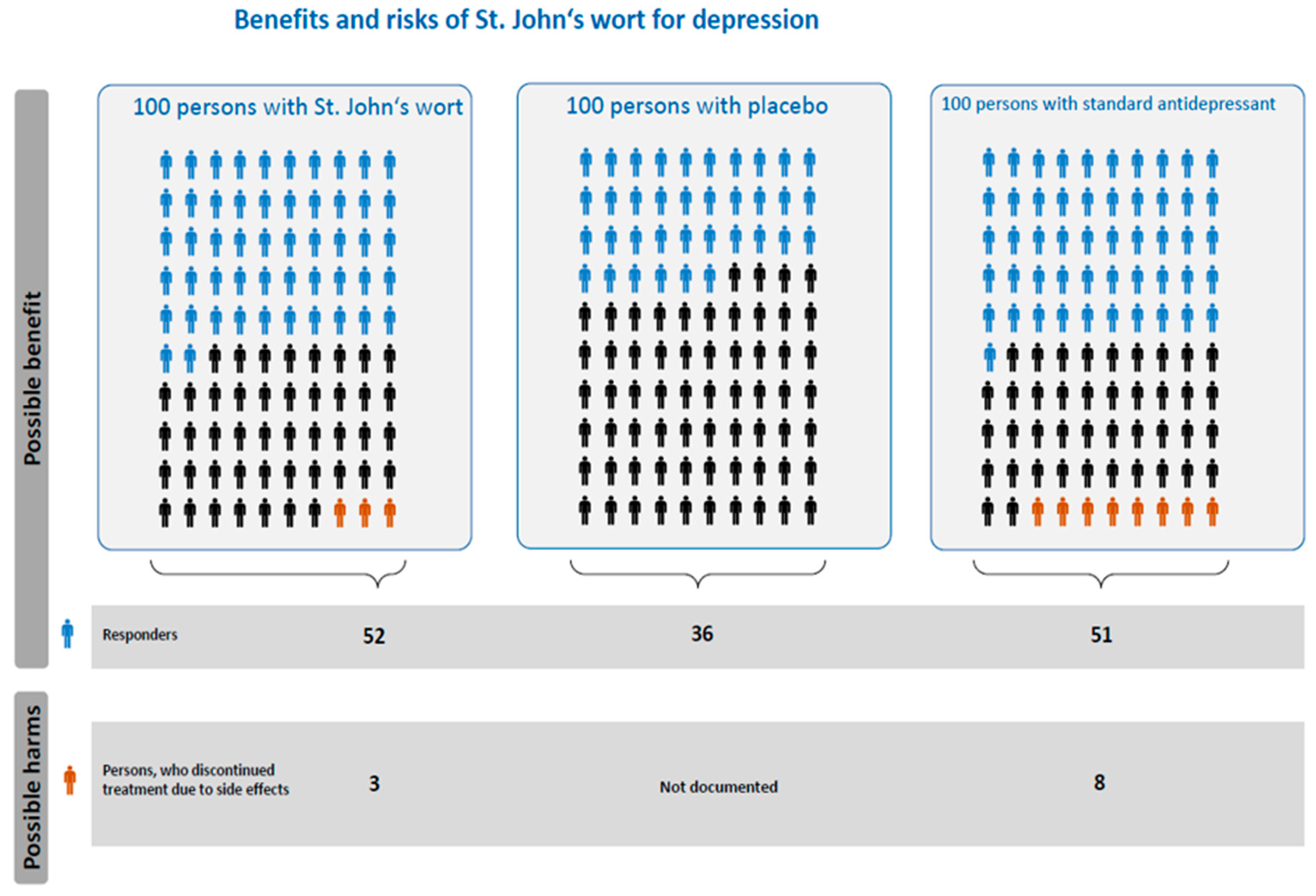
5.1. Rationale for and Methods of Compiling the Risk Ladder
5.2. Methods of Compiling the Icon Array
5.3. Interpretation
6. Points to Consider and Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wiedenfeld, H.; Roeder, E.; Bourauel, T.; Edgar, J.A. Pyrrolizidine Alkaloids: Structure and Toxicity; V&R Unipress: Göttingen, Germany, 2008. [Google Scholar]
- Bundesinstitut für Risikobewertung. Frequently Asked Questions on Pyrrolizidine Alkaloids in Foods. 2016. Available online: http://www.bfr.bund.de/cm/349/frequently-asked-questions-on-pyrrolizidine-alkaloids-in-foods.pdf (accessed on 11 May 2017).
- Merz, K.; Schrenk, D. Interim relative potency factors for the toxicological risk assessment of pyrrolizidine alkaloids in food and herbal medicines. Toxicol. Lett. 2016, 263, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Habs, M.; Binder, K.; Krauss, S.; Müller, K.; Ernst, B.; Valentini, L.; Koller, M. A balanced risk-benefit analysis to determine human risks associated with Pyrrolizidine Alkaloids (PA)-The case of tea and herbal infusions. Nutrients 2017, 9, 717. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Public Statement on the Use of Herbal Medicinal Products Containing Toxic, Unsaturated Pyrrolizidine Alkaloids (PAs). 2014. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Public_statement/2014/12/WC500179559.pdf (accessed on 9 May 2017).
- Mulder, P.P.; Sánchez, P.L.; These, A.; Preiss-Weigert, A.; Castellari, M. Occurrence of Pyrrolizidine Alkaloids in food. EFSA Support. Publ. 2015, 12. [Google Scholar] [CrossRef]
- Hidaka, B.H. Depression as a disease of modernity: Explanations for increasing prevalence. J. Affect. Disord. 2012, 140, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Pöldinger, W. Zur Geschichte des Johanniskrauts. Praxis 2000, 89, 2102–2109. [Google Scholar] [PubMed]
- European Medicines Agency, Committee on Herbal Medicinal Products (HMPC). Community Herbal Monograph on Hypericum perforatium L., Herba (Well-Established Medicinal Use). 2009. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_Community_herbal_monograph/2010/01/WC500059145.pdf (accessed on 9 May 2017).
- National Institute for Health and Care Excellence (NICE). Depression in Adults with a Chronic Physical Health Problem: Recognition and Management. 2009. Available online: https://www.nice.org.uk/guidance/cG91 (accessed on 9 May 2017).
- Linde, K.; Berner, M.M.; Kriston, L. St John’s wort for major depression. Cochrane Libr. 2008, 4. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency, Committee on Herbal Medicinal Product. Community Herbal Monograph on Hypericum perforatium L., Herba (Traditional Use). 2009. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal__Community_herbal_monograph/2010/01/WC500059149.pdf (accessed on 30 March 2018).
- Greeson, J.M.; Sanford, B.; Monti, D.A. St. John’s wort (Hypericum perforatum): A review of the current pharmacological, toxicological, and clinical literature. Psychopharmacology 2001, 153, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Müller, W. Current St. John’s wort research from mode of action to clinical efficacy. Pharmacol. Res. 2003, 47, 101–109. [Google Scholar] [CrossRef]
- Butterweck, V. Mechanism of action of St John’s wort in depression: What is known? CNS Drugs 2003, 17, 539–562. [Google Scholar] [CrossRef] [PubMed]
- Apaydin, E.A.; Maher, A.R.; Shanman, R.; Booth, M.S.; Miles, J.N.; Sorbero, M.E.; Hempel, S. A systematic review of St. John’s wort for major depressive disorder. Syst. Rev. 2016, 5, 148. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zheng, Y. A meta-analysis on the efficacy and safety of St John’s wort extract in depression therapy in comparison with selective serotonin reuptake inhibitors in adults. Neuropsychiatr. Dis. Treat. 2016, 12, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- National Center for Complementary and Integrative Health. St. John’s Wort. 2017. Available online: https://nccih.nih.gov/health/stjohnswort (accessed on 26 November 2017).
- Hypericum Depression Trial Study Group. Effect of Hypericum perforatum (St John’s Wort) in Major Depressive Disorder. JAMA 2002, 287, 1807–1814. [Google Scholar] [CrossRef]
- Rapaport, M.H.; Nierenberg, A.A.; Howland, R.; Dording, C.; Schettler, P.J.; Mischoulon, D. The treatment of minor depression with St. John’s Wort or citalopram: Failure to show benefit over placebo. J. Psychiatr. Res. 2011, 45, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.; Yue, Q.Y.; Bergquist, C.; Gerden, B.; Arlett, P. St John’s wort (Hypericum perforatum): Drug interactions and clinical outcomes. Br. J. Clin. Pharmacol. 2002, 54, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Mannel, M. Drug interactions with St John’s wort: Mechanisms and clinical implications. Drug Saf. 2004, 27, 773–797. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.A. Drug interactions with St. John’s Wort (Hypericum perforatum): A review of the clinical evidence. Int. J. Clin. Pharmacol. Ther. 2004, 42, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Izzo, A.A. Herb-drug interactions with St John’s wort (Hypericum perforatum): An update on clinical observations. AAPS J. 2009, 11, 710–727. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, S.; Bahramsoltani, R.; Rahimi, R.; Abdollahi, M. Clinical risks of St John’s Wort (Hypericum perforatum) co-administration. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1047–1062. [Google Scholar] [CrossRef] [PubMed]
- Brockmöller, J.; Reum, T.; Bauer, S.; Kerb, R.; Hübner, W.D.; Roots, I. Hypericin and pseudohypericin: Pharmacokinetics and effects on photosensitivity in humans. Pharmacopsychiatry 1997, 30, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.E.; da Costa Rocha, I.; Heinrich, M.; Williamson, E.M. Phytopharmacy: An Evidence-Based Guide to Herbal Medicinal Products; Wiley Blackwell: Chichester, UK, 2015. [Google Scholar]
- S3-Leitlinie/Nationale VersorgungsLeitlinie Unipolare Depression-Langfassung. Available online: https://www.leitlinien.de/mdb/downloads/nvl/depression/archiv/depression-1aufl-vers5-lang.pdf (accessed on 9 May 2017).
- Kasper, S.; Caraci, F.; Forti, B.; Drago, F.; Aguglia, E. Efficacy and tolerability of Hypericum extract for the treatment of mild to moderate depression. Eur. Neuropsychopharmacol. 2010, 20, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Barry, M.J.; Kansagara, D. Nonpharmacologic versus pharmacologic treatment of adult patients with major depressive disorder: A clinical practice guideline from the american college of physicians. Ann. Intern. Med. 2016, 164, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Kempf, M.; Wittig, M.; Schönfeld, K.; Cramer, L.; Schreier, P.; Beuerle, T. Pyrrolizidine alkaloids in food: Downstream contamination in the food chain caused by honey and pollen. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2011, 28, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risks for human health related to the presence of pyrrolizidine alkaloids in honey, tea, herbal infusions and food supplements. EFSA J. 2017, 15, 292. [Google Scholar] [CrossRef]
- Roeder, E. Medicinal plants in Europe containing pyrrolizidine alkaloids. Pharmazie 1995, 50, 83–98. [Google Scholar] [PubMed]
- European Food Safety Authority. Dietary exposure assessment to pyrrolizidine alkaloids in the European population. EFSA J. 2016, 14, e04572. [Google Scholar] [CrossRef]
- European Medicines Agency. Public Statement on Contamination of Herbal Medicinal Products/Traditional Herbal Medicinal Products with Pyrrolizidine Alkaloids: Transitional Recommendations for Risk Management and Quality Control. 2016. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Public_statement/2016/06/WC500208195.pdf (accessed on 9 May 2017).
- Gov.uk. Precautionary Recall-Six Batches of St John’s Wort Tablets. 2016. Available online: https://www.gov.uk/government/news/precautionary-recall-six-batches-of-st-johns-wort-tablets (accessed on December 2017).
- Letsyo, E.; Jerz, G.; Winterhalter, P.; Lindigkeit, R.; Beuerle, T. Incidence of pyrrolizidine alkaloids in herbal medicines from German retail markets: Risk assessments and implications to consumers. Phytother. Res. 2017, 31, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
- Lechtenberger, M.; Hensel, A.; Steinhoff, B. Hepatotoxisch und karzinogen: Pyrrolizidinalalkaloide in Arznei- und Lebensmitteln sorgen für Probleme. Deut. Apoth. Ztg. 2017, 157, 32–41. [Google Scholar]
- Code of Practice to Prevent and Reduce Pyrrolizidine Alkaloid Contaminations of Medicinal Products of Plant Origin. 2016. Available online: http://203.187.160.133:9011/ehtpa.eu/c3pr90ntc0td/pdf/cop-revision-20090245.pdf (accessed on 9 May 2017).
- Bundesinstitut für Risikobewertung. Pyrrolizidine Alkaloids (PA). Available online: http://www.bfr.bund.de/en/pyrrolizidine_alkaloids__pa_-192924.html (accessed on 20 March 2018).
- European Food Safety Authority (EFSA) Panel on Contaminants in the Food Chain. Scientific Opinion on Pyrrolizidine alkaloids in food and feed. EFSA J. 2011, 9, 2406. [Google Scholar] [CrossRef]
- Crews, C.; Berthiller, F.; Krska, R. Update on analytical methods for toxic pyrrolizidine alkaloids. Anal. Bioanal. Chem. 2010, 396, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Bundesinstitut für Risikobewertung. Vorläufige Empfehlungen des BfR zur Analytik von Pyrrolizidinalkaloiden (PA) in Kräutertee und Tee Analytspektrum und Probenahmeverfahren). 2016. Available online: http://www.bfr.bund.de/cm/343/vorlaeufige-empfehlungen-des-bfr-zur-analytik-von-pyrrolizidinalkaloiden-pa-in-kraeutertee-und-tee.pdf (accessed on 11 May 2017).
- De Nijs, M.; Elbers, I.J.W.; Mulder, P.P.J. Inter-laboratory comparison study for pyrrolizidine alkaloids in animal feed using spiked and incurred material. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2014, 31, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Bodi, D.; Pydde, Y.; Preiß-Weigert, A. Internationale Laborvergleichsuntersuchung zur Bestimmung von Pyrrolizidinalkaloiden in Kräutertee und Rooibostee; BfR-Wissenschaft: Berlin, Germany, 2016. [Google Scholar]
- Committee on Herbal Medicinal Product. Guideline on Good Agricultural and Collecting Practice (GACP) for Starting Material of Herbal Origin. 2006. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003362.pdf (accessed on 11 May 2017).
- Pawar, R.S. Pyrrolizidine alkaloids. In Bad Bug Book: Foodborne Pathogenic Microorganisms and Natural Toxins; Food and Drug Administration: Silver Spring, MD, USA, 2012; Volume 2, pp. 242–244. [Google Scholar]
- Ruan, J.; Yang, M.; Fu, P.; Ye, Y.; Lin, G. Metabolic activation of pyrrolizidine alkaloids: Insights into the structural and enzymatic basis. Chem. Res. Toxicol. 2014, 27, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M.; Storer, R.D.; Criswell, K.A.; Doerrer, N.G.; Dellarco, V.L.; Pegg, D.G.; Wojcinski, Z.W.; Malarkey, D.E.; Jacobs, A.C.; Klaunig, J.E.; et al. Hemangiosarcoma in rodents: Mode-of-action evaluation and human relevance. Toxicol. Sci. 2009, 111, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Mei, N.; Xia, Q.; Chen, T.; Chan, P.C.; Fu, P.P. Gene expression profiling as an initial approach for mechanistic studies of toxicity and tumorigenicity of herbal plants and herbal dietary supplements. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2010, 28, 60–87. [Google Scholar] [CrossRef] [PubMed]
- Rasenack, R.; Muller, C.; Kleinschmidt, M.; Rasenack, J.; Wiedenfeld, H. Veno-occlusive disease in a fetus caused by pyrrolizidine alkaloids of food origin. Fetal Diagn. Ther. 2003, 18, 223–225. [Google Scholar] [CrossRef] [PubMed]
- DeLeve, L.D.; McCuskey, R.S.; Wang, X.; Hu, L.; McCuskey, M.K.; Epstein, R.B.; Kanel, G.C. Characterization of a reproducible rat model of hepatic veno-occlusive disease. Hepatology 1999, 29, 1779–1791. [Google Scholar] [CrossRef] [PubMed]
- Kakar, F.; Akbarian, Z.; Leslie, T.; Mustafa, M.L.; Watson, J.; van Egmond, H.P.; Omar, M.F.; Mofleh, J. An outbreak of hepatic veno-occlusive disease in Western Afghanistan associated with exposure to wheat flour contaminated with pyrrolizidine alkaloids. J. Toxicol. 2010, 2010, 313280. [Google Scholar] [CrossRef] [PubMed]
- Wiedenfeld, H. Aufnahmewege von PA Durch Direkte und Indirekte Intoxikation. Available online: https://www.ak-kreuzkraut.de/toxizität-mensch-tier/humangefährdung/aufnahmewege/ (accessed on 11 May 2017).
- Carpenter, D.O.; Bushkin-Bedient, S. Exposure to chemicals and radiation during childhood and risk for cancer later in life. J. Adolesc. Health 2013, 52, S21–S29. [Google Scholar] [CrossRef] [PubMed]
- Wiedenfeld, H. Plants containing pyrrolizidine alkaloids: Toxicity and problems. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2011, 28, 282–292. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer; ebrary, Inc. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Press: Lyon, France, 2002. [Google Scholar]
- International Agency for Research on Cancer. Some naturally occurring substances. In IARC Monographs on the Evaluation of Carcinogenic Risks of Chemicals to Man; International Agency for Research on Cancer: Lyon, France, 1976. [Google Scholar]
- International Agency for Research on Cancer; IARC Working Group on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Some food additives, feed additives and naturally occurring substances. In IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans; IARC: Lyon, France, 1983. [Google Scholar]
- Xia, Q.; Yan, J.; Chou, M.W.; Fu, P.P. Formation of DHP-derived DNA adducts from metabolic activation of the prototype heliotridine-type pyrrolizidine alkaloid, heliotrine. Toxicol. Lett. 2008, 178, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.P.; Yang, Y.; Xia, Q.; Chou, M.W.; Cui, Y.Y.; Lin, G. Pyrrolizidine Alkaloids: Tumorigenic Components in Chinese Herbal Medicines and Dietary Supplements. J. Food Drug Anal. 2002, 10, 198–211. [Google Scholar]
- Habs, H.; Habs, M.; Marquardt, H.; Röder, E.; Schmähl, D.; Wiedenfeld, H. Carcinogenic and mutagenic activity of an alkaloidal extract of Senecio nemorensis ssp. fuchsii. Arzneim. Forsch. 1982, 32, 144–148. [Google Scholar]
- National Toxicology Program. Final Report on Carcinogens Background Document for Riddelliine. 2008. Available online: https://ntp.niehs.nih.gov/ntp/roc/twelfth/2010/finalbds/riddelliine_final_508.pdf (accessed on 9 May 2017).
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Chemical Agents and Related Occupations, Volume 100F. A Review of Human Carcinogens; IARC: Lyon, France, 2012. [Google Scholar]
- Hong, C.B.; Winston, J.M.; Lee, C.C. Hepatic angiosarcoma: Animal model: Angiosarcoma of rats and mice induced by vinyl chloride. Am. J. Pathol. 1980, 101, 737–740. [Google Scholar] [PubMed]
- Falk, H.; Herbert, J.; Crowley, S.; Ishak, K.G.; Thomas, L.B.; Popper, H.; Caldwell, G.G. Epidemiology of hepatic angiosarcoma in the United States: 1964–1974. Environ. Health Perspect. 1981, 41, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Wann, S.; Chang, H.; Lin, S.L.; Wang, J.S.; Guo, H.R. Arsenic, vinyl chloride, viral hepatitis, and hepatic angiosarcoma: A hospital-based study and review of literature in Taiwan. BMC Gastroenterol. 2011, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, X.; Zhang, J.; Hui, Z.Z.; Du, W.J.; Li, R.M.; Ren, X.B. Primary hepatic angiosarcoma and potential treatment options. J. Gastroenterol. Hepatol. 2014, 29, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Zocchetti, C. Angiosarcoma del fegato nell’uomo: Considerazioni epidemiologiche (Liver angiosarcoma in humans: Epidemiologic considerations). Med. Lav. 2001, 92, 39–53. [Google Scholar] [PubMed]
- Chiou, H.Y.; Hsueh, Y.M.; Liaw, K.F.; Horng, S.F.; Chiang, M.H.; Pu, Y.S.; Lin, J.S.; Huang, C.H.; Chen, C.J. Incidence of internal cancers and ingested inorganic arsenic: A seven-year follow-up study in Taiwan. Cancer Res. 1995, 55, 1296–1300. [Google Scholar] [PubMed]
- Falk, H.; Thomas, L.B.; Popper, H.; Ishak, K. Hepatic angiosarcoma associated with androgenic-anabolic steroids. Lancet 1979, 314, 1120–1123. [Google Scholar] [CrossRef]
- Spiegelhalter, D.; Pearson, M.; Short, I. Visualizing uncertainty about the future. Science 2011, 333, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Gigerenzer, G. Why does framing influence judgment? J. Gen. Intern. Med. 2003, 18, 960–961. [Google Scholar] [CrossRef] [PubMed]
- Spiegelhalter, D.J. Understanding uncertainty. Ann. Fam. Med. 2008, 6, 196–197. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Ruan, J.Q.; Chen, J.; Li, N.; Ke, C.Q.; Ye, Y.; Lin, G.; Wang, J.Y. Blood pyrrole-protein adducts as a diagnostic and prognostic index in pyrrolizidine alkaloid-hepatic sinusoidal obstruction syndrome. Drug Des. Dev. Ther. 2015, 25, 4861–4868. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.S.; Pereira, T.N.; Reilly, P.E.; Seawright, A.A. Pyrrolizidine alkaloids in human diet. Mutat. Res. 1999, 443, 53–67. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. Statistics notes: Absence of evidence is not evidence of absence. BMJ 1995, 311, 485. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society Liver Cancer Risk Factors. Available online: https://www.cancer.org/cancer/liver-cancer/causes-risks-prevention/risk-factors.html (accessed on December 2017).
- Tversky, A.; Kahneman, D. Availability: A heuristic for judging frequency and probability. Cogn. Psychol. 1973, 5, 207–232. [Google Scholar] [CrossRef]
- Statistisches Bundesamt. Gesundheit: Todesursachen in Deutschland 2015. 2017. Available online: https://www.destatis.de/DE/Publikationen/Thematisch/Gesundheit/Todesursachen/Todesursachen.html (accessed on 1 April 2017).
- bfu-Swiss Council for Accident Prevention. Status 2017: Statistics on Non-Occupational Accidents and the Level of Safety in Switzerland. 2017. Available online: https://www.bfu.ch/sites/assets/Shop/bfu_2.287.08_STATUS%202017%20%E2%80%93%20Statistics%20on%20non-occupational%20accidents%20and%20the%20level%20of%20safety%20in%20Switzerland.pdf (accessed on 9 May 2017).
- Naghavi, M.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Adetokunboh, O.; Afshin, A.; Agrawal, A.; et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- Galesic, M.; Garcia-Retamero, R.; Gigerenzer, G. Using icon arrays to communicate medical risks: Overcoming low numeracy. Health Psychol. 2009, 28, 210–216. [Google Scholar] [CrossRef] [PubMed]
- McDowell, M.; Rebitschek, F.G.; Gigerenzer, G.; Wegwarth, O. A Simple Tool for Communicating the Benefits and Harms of Health Interventions. MDM Policy Pract. 2016, 1, 1–10. [Google Scholar] [CrossRef]
- Keller, C. Using a familiar risk comparison within a risk ladder to improve risk understanding by low numerates: A study of visual attention. Risk Anal. 2011, 31, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Reeves, A. Working with Risk in Counselling and Psychotherapy; Essential Issues in Counselling and Psychotherapy; SAGE Press: Newcastle upon Tyne, UK, 2015. [Google Scholar]
- Gaynes, B.N.; West, S.L.; Ford, C.A.; Frame, P.; Klein, J.; Lohr, K.N. Screening for suicide risk in adults: A summary of the evidence for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2004, 140, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.W.M.; How, C.H.; Ng, Y.P. Depression in primary care: Assessing suicide risk. Singap. Med. J. 2017, 58, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Nischal, A.; Tripathi, A.; Nischal, A.; Trivedi, J.K. Suicide and antidepressants: What current evidence indicates. Mens Sana Monogr. 2012, 10, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.; et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef]
- Rahimi, R.; Nikfar, S.; Abdollahi, M. Efficacy and tolerability of Hypericum perforatum in major depressive disorder in comparison with selective serotonin reuptake inhibitors: A meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 118–127. [Google Scholar] [CrossRef] [PubMed]
| Death Cases | 2015 * Germany | 2008 * Germany | 2016 ** World | 2014 *** Switzerland | 2014 **** United States |
|---|---|---|---|---|---|
| Absolute number of deaths | 925,200 | 844,439 | 54,700,000 | 63,938 | 2,626,418 |
| Standardized at 1000 cases | |||||
| Cardiovascular disease | 385 | 420 | 323 | 328 | 234 |
| Cancer | 250 | 257 | 163 | 262.3 | 225 |
| Diabetes mellitus | 26 | 26 | 58.3 | 19 | 29 |
| Liver diseases | 16 | 18 | 23 | 14.4 ***** | 15 |
| Suicide | 10.9 | 11.1 | 14.9 | 16 1 | 16 |
| Household accidents | 10 | 8.1 | 23 2 | 10.3 | 6.9 |
| Liver cancer | 8.5 | 8.3 | 12.3 ***** | 9.4 | 9.4 |
| Traffic accidents | 3.8 | 5.5 | 24.5 | 3.6 | 12.8 |
| Liver cancer (unexplained cause) | 1.7 | 0.9 ***** | 3.5 ***** | 1.8 ***** | 2.1 ***** |
| PA-related death 1 | ? | ? | ? | ? | ? |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habs, M.; Binder, K.; Krauss, S.; Müller, K.; Ernst, B.; Valentini, L.; Koller, M. A Balanced Risk-Benefit Analysis to Determine Human Risks Associated with Pyrrolizidine Alkaloids (PA)—The Case of Herbal Medicinal Products Containing St. John’s Wort Extracts (SJW). Nutrients 2018, 10, 804. https://doi.org/10.3390/nu10070804
Habs M, Binder K, Krauss S, Müller K, Ernst B, Valentini L, Koller M. A Balanced Risk-Benefit Analysis to Determine Human Risks Associated with Pyrrolizidine Alkaloids (PA)—The Case of Herbal Medicinal Products Containing St. John’s Wort Extracts (SJW). Nutrients. 2018; 10(7):804. https://doi.org/10.3390/nu10070804
Chicago/Turabian StyleHabs, Michael, Karin Binder, Stefan Krauss, Karolina Müller, Brigitte Ernst, Luzia Valentini, and Michael Koller. 2018. "A Balanced Risk-Benefit Analysis to Determine Human Risks Associated with Pyrrolizidine Alkaloids (PA)—The Case of Herbal Medicinal Products Containing St. John’s Wort Extracts (SJW)" Nutrients 10, no. 7: 804. https://doi.org/10.3390/nu10070804
APA StyleHabs, M., Binder, K., Krauss, S., Müller, K., Ernst, B., Valentini, L., & Koller, M. (2018). A Balanced Risk-Benefit Analysis to Determine Human Risks Associated with Pyrrolizidine Alkaloids (PA)—The Case of Herbal Medicinal Products Containing St. John’s Wort Extracts (SJW). Nutrients, 10(7), 804. https://doi.org/10.3390/nu10070804






