Validation of Different Nutritional Assessment Tools in Predicting Prognosis of Patients with Soft Tissue Spindle-Cell Sarcomas
Abstract
1. Introduction
2. Subjects and Methods
2.1. Patients’ Data
2.2. Geriatric Nutritional Risk Index
2.3. Glasgow Prognostic Score
2.4. Neutrophil–Lymphocyte Ratio
2.5. Platelet–Lymphocyte Ratio
2.6. Controlling Nutritional Score
2.7. Statistical Analysis
2.8. Ethics Approval and Consent to Participate
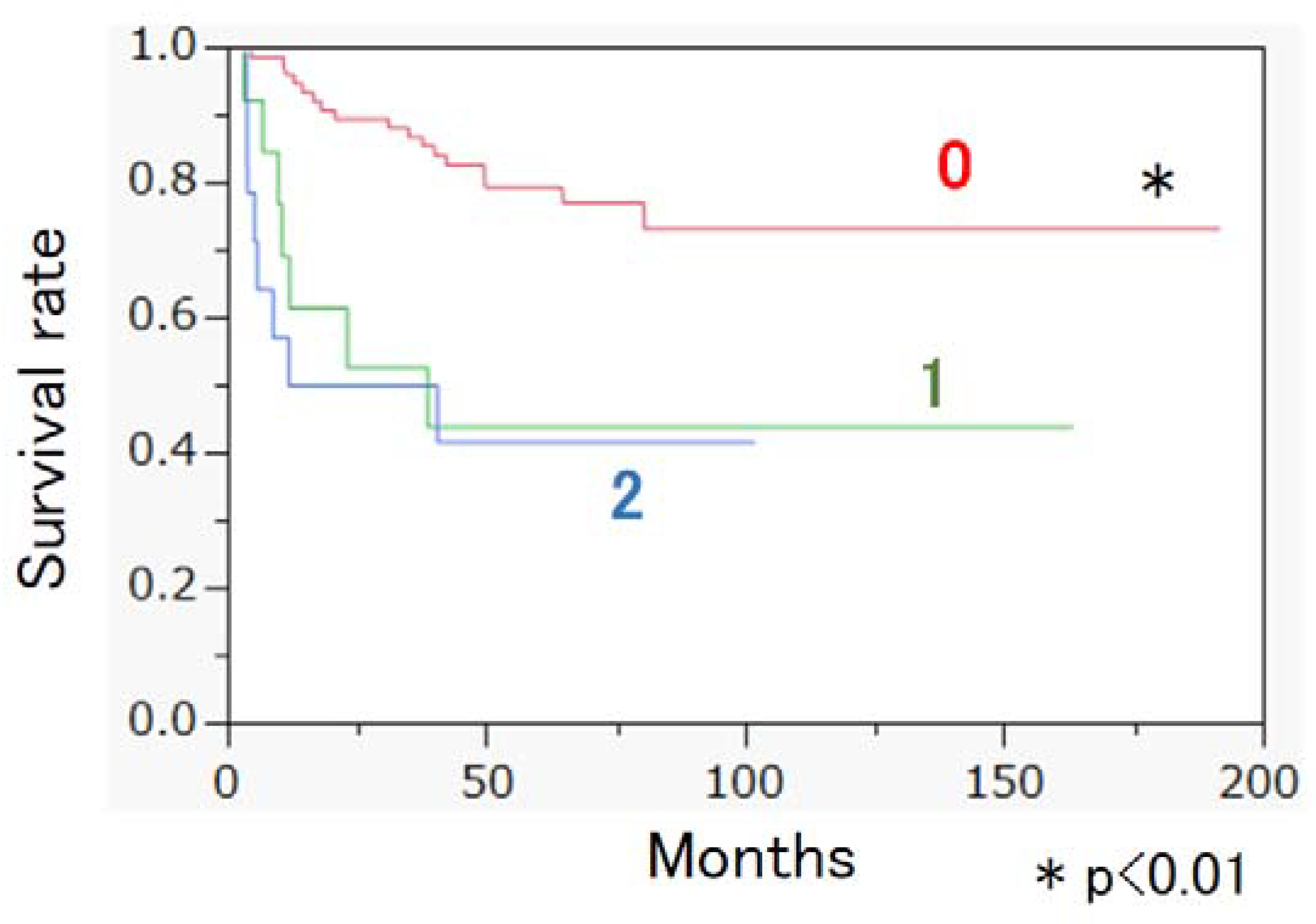
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zambo, I.; Vesely, K. WHO classification of tumours of soft tissue and bone 2013: The main changes compared to the 3rd edition. Ceskoslovenska Patol. 2014, 50, 64–70. [Google Scholar]
- Ogura, K.; Higashi, T.; Kawai, A. Statistics of soft-tissue sarcoma in japan: Report from the bone and soft tissue tumor registry in japan. J. Orthop. Sci. 2017, 22, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Callegaro, D.; Miceli, R.; Mariani, L.; Raut, C.P.; Gronchi, A. Soft tissue sarcoma nomograms and their incorporation into practice. Cancer 2017, 123, 2802–2820. [Google Scholar] [CrossRef] [PubMed]
- Isenring, E.; Bauer, J.; Capra, S. The scored patient-generated subjective global assessment (pg-sga) and its association with quality of life in ambulatory patients receiving radiotherapy. Eur. J. Clin. Nutr. 2003, 57, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Arends, J. The causes and consequences of cancer-associated malnutrition. Eur. J. Oncol. Nurs. 2005, 9 (Suppl. 2), S51–S63. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Jaing, T.H.; Hung, I.J.; Yang, C.P.; Chang, T.Y. High body mass index did not result in poor outcome in Taiwanese children with acute myeloid leukemia: A single-institution experience. Int. J. Hematol. 2015, 102, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Lange, B.J.; Gerbing, R.B.; Feusner, J.; Skolnik, J.; Sacks, N.; Smith, F.O.; Alonzo, T.A. Mortality in overweight and underweight children with acute myeloid leukemia. JAMA 2005, 293, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Doki, N.; Hagino, T.; Miyawaki, S.; Ohtake, S.; Kiyoi, H.; Miyazaki, Y.; Fujita, H.; Usui, N.; Okumura, H.; et al. Underweight status at diagnosis is associated with poorer outcomes in adult patients with acute myeloid leukemia: A retrospective study of jalsg aml 201. Ann. Hematol. 2018, 97, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wu, J.; Ma, M.; Pei, J.; Song, Y.; Zhang, X.; Han, B. Comparison of pg-sga, sga and body-composition measurement in detecting malnutrition among newly diagnosed lung cancer patients in stage iiib/iv and benign conditions. Med. Oncol. 2011, 28, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric nutritional risk index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Tokumoto, H.; Tominaga, H.; Arishima, Y.; Jokoji, G.; Akimoto, M.; Ohtsubo, H.; Taketomi, E.; Sunahara, N.; Nagano, S.; Ishidou, Y.; et al. Association between bone mineral density of femoral neck and geriatric nutritional risk index in rheumatoid arthritis patients treated with biological disease-modifying anti-rheumatic drugs. Nutrients 2018, 10, 234. [Google Scholar] [CrossRef] [PubMed]
- Bo, Y.; Wang, K.; Liu, Y.; You, J.; Cui, H.; Zhu, Y.; Lu, Q.; Yuan, L. The geriatric nutritional risk index predicts survival in elderly esophageal squamous cell carcinoma patients with radiotherapy. PLoS ONE 2016, 11, e0155903. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Furuya, R.; Takita, T.; Maruyama, Y.; Yamaguchi, Y.; Ohkawa, S.; Kumagai, H. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am. J. Clin. Nutr. 2008, 87, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Dohi, T.; Miyauchi, K.; Doi, S.; Naito, R.; Konishi, H.; Tsuboi, S.; Ogita, M.; Kasai, T.; Hassan, A.; et al. Prognostic impact of the geriatric nutritional risk index on long-term outcomes in patients who underwent percutaneous coronary intervention. Am. J. Cardiol. 2017, 119, 1740–1745. [Google Scholar] [CrossRef] [PubMed]
- Mitani, Y.; Oki, Y.; Fujimoto, Y.; Yamaguchi, T.; Iwata, K.; Watanabe, Y.; Takahashi, K.; Yamada, K.; Ishikawa, A. Relationship between functional independence measure and geriatric nutritional risk index in pneumonia patients in long-term nursing care facilities. Geriatr. Gerontol. Int. 2017, 17, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Sargento, L.; Vicente Simoes, A.; Rodrigues, J.; Longo, S.; Lousada, N.; Palma Dos Reis, R. Geriatric nutritional risk index as a nutritional and survival risk assessment tool in stable outpatients with systolic heart failure. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhang, H.; Ye, T.; Ge, S.; Zhuo, R.; Zhu, H. The geriatric nutritional risk index independently predicts mortality in diabetic foot ulcers patients undergoing amputations. J. Diabetes Res. 2017, 2017, 5797194. [Google Scholar] [CrossRef] [PubMed]
- Kawamiya, T.; Suzuki, S.; Ishii, H.; Hirayama, K.; Harada, K.; Shibata, Y.; Tatami, Y.; Harata, S.; Kawashima, K.; Kunimura, A.; et al. Correlations between geriatric nutritional risk index and peripheral artery disease in elderly coronary artery disease patients. Geriatr. Gerontol. Int. 2017, 17, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Nagai, T.; Iwakami, N.; Sugano, Y.; Honda, S.; Okada, A.; Asaumi, Y.; Aiba, T.; Noguchi, T.; Kusano, K.; et al. Usefulness of geriatric nutritional risk index for assessing nutritional status and its prognostic impact in patients aged >/=65 years with acute heart failure. Am. J. Cardiol. 2016, 118, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Forrest, L.M.; McMillan, D.C.; McArdle, C.S.; Angerson, W.J.; Dunlop, D.J. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br. J. Cancer 2003, 89, 1028–1030. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.R.; Cook, E.J.; Goulder, F.; Justin, T.A.; Keeling, N.J. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J. Surg. Oncol. 2005, 91, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Paramanathan, A.; Saxena, A.; Morris, D.L. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg. Oncol. 2014, 23, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, G.J.; Charles, K.A.; Roxburgh, C.S.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; Ace, O.; McNamara, M.G.; Al-Mubarak, M.; Vera-Badillo, F.E.; Hermanns, T.; Seruga, B.; Ocana, A.; Tannock, I.F.; Amir, E. Prognostic role of platelet to lymphocyte ratio in solid tumors: A systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tao, L.; Lu, M.; Xiu, D. Prognostic role of platelet to lymphocyte ratio in pancreatic cancers: A meta-analysis including 3028 patients. Medicine 2018, 97, 9616. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Fu, Y.; Su, Q.; Wang, H. Prognostic role of platelet-lymphocyte ratio in colorectal cancer: A systematic review and meta-analysis. Medicine 2016, 95, 3837. [Google Scholar] [CrossRef] [PubMed]
- Toyokawa, G.; Kozuma, Y.; Matsubara, T.; Haratake, N.; Takamori, S.; Akamine, T.; Takada, K.; Katsura, M.; Shimokawa, M.; Shoji, F.; et al. Prognostic impact of controlling nutritional status score in resected lung squamous cell carcinoma. J. Thorac. Dis. 2017, 9, 2942–2951. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.F.; Li, J.H.; Li, M.; Yang, Y.; Liu, Y.H. The prognostic role of controlling nutritional status scores in patients with solid tumors. Clin. Chim. Acta 2017, 474, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, D.; Sawayama, H.; Kurashige, J.; Iwatsuki, M.; Eto, T.; Tokunaga, R.; Kitano, Y.; Yamamura, K.; Ouchi, M.; Nakamura, K.; et al. Controlling nutritional status (conut) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer 2018, 21, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Harimoto, N.; Yoshizumi, T.; Sakata, K.; Nagatsu, A.; Motomura, T.; Itoh, S.; Harada, N.; Ikegami, T.; Uchiyama, H.; Soejima, Y.; et al. Prognostic significance of preoperative controlling nutritional status (conut) score in patients undergoing hepatic resection for hepatocellular carcinoma. World J. Surg. 2017, 41, 2805–2812. [Google Scholar] [CrossRef] [PubMed]
- Iseki, Y.; Shibutani, M.; Maeda, K.; Nagahara, H.; Ohtani, H.; Sugano, K.; Ikeya, T.; Muguruma, K.; Tanaka, H.; Toyokawa, T.; et al. Impact of the preoperative controlling nutritional status (conut) score on the survival after curative surgery for colorectal cancer. PLoS ONE 2015, 10, e0132488. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.W.; Kim, I.H. Comparison of different nutritional assessments in detecting malnutrition among gastric cancer patients. World J. Gastroenterol. 2010, 16, 3310–3317. [Google Scholar] [CrossRef] [PubMed]
- Pablo, A.M.; Izaga, M.A.; Alday, L.A. Assessment of nutritional status on hospital admission: Nutritional scores. Eur. J. Clin. Nutr. 2003, 57, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Sucher, K.; Hollenbeck, C.B. Comparison of ideal body weight equations and published height-weight tables with body mass index tables for healthy adults in the united states. Nutr. Clin. Pract. 2006, 21, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.E.; Rogowski, W.H.; Leidl, R.; Stollenwerk, B. Transparency vs. Closed-door policy: Do process characteristics have an impact on the outcomes of coverage decisions? A statistical analysis. Health Policy 2013, 112, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Roxburgh, C.S.; McMillan, D.C. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010, 6, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Andreyev, H.J.; Norman, A.R.; Oates, J.; Cunningham, D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur. J. Cancer 1998, 34, 503–509. [Google Scholar] [CrossRef]
- Forrest, L.M.; McMillan, D.C.; McArdle, C.S.; Angerson, W.J.; Dunlop, D.J. Comparison of an inflammation-based prognostic score (gps) with performance status (ecog) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br. J. Cancer 2004, 90, 1704–1706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ye, B.; Liang, W.; Ren, Y. Preoperative prognostic nutritional index is a powerful predictor of prognosis in patients with stage iii ovarian cancer. Sci. Rep. 2017, 7, 9548. [Google Scholar] [CrossRef] [PubMed]
- Osugi, J.; Muto, S.; Matsumura, Y.; Higuchi, M.; Suzuki, H.; Gotoh, M. Prognostic impact of the high-sensitivity modified glasgow prognostic score in patients with resectable non-small cell lung cancer. J. Cancer Res. Ther. 2016, 12, 945–951. [Google Scholar] [PubMed]
- McMillan, D.C.; Crozier, J.E.; Canna, K.; Angerson, W.J.; McArdle, C.S. Evaluation of an inflammation-based prognostic score (gps) in patients undergoing resection for colon and rectal cancer. Int. J. Colorectal Dis. 2007, 22, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, S.; Ginoya, S.; Tandon, P.; Gohel, T.D.; Guirguis, J.; Vallabh, H.; Jevenn, A.; Hanouneh, I. Malnutrition: Laboratory markers vs nutritional assessment. Gastroenterol. Rep. 2016, 4, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Elmore, M.F.; Wagner, D.R.; Knoll, D.M.; Eizember, L.; Oswalt, M.A.; Glowinski, E.A.; Rapp, P.A. Developing an effective adult nutrition screening tool for a community hospital. J. Am. Diet. Assoc. 1994, 94, 1113–1121. [Google Scholar] [CrossRef]
- Seltzer, M.H.; Bastidas, J.A.; Cooper, D.M.; Engler, P.; Slocum, B.; Fletcher, H.S. Instant nutritional assessment. JPEN J. Parenter Enteral Nutr. 1979, 3, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Gonda, K.; Harada, M.; Tanisaka, Y.; Arai, S.; Mashimo, Y.; Iwano, H.; Sato, H.; Ryozawa, S.; Takahashi, T.; et al. Increased neutrophil-to-lymphocyte ratio is a novel marker for nutrition, inflammation and chemotherapy outcome in patients with locally advanced and metastatic esophageal squamous cell carcinoma. Biomed. Rep. 2017, 7, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Merrill, R.D.; Burke, R.M.; Northrop-Clewes, C.A.; Rayco-Solon, P.; Flores-Ayala, R.; Namaste, S.M.; Serdula, M.K.; Suchdev, P.S. Factors associated with inflammation in preschool children and women of reproductive age: Biomarkers reflecting inflammation and nutritional determinants of anemia (brinda) project. Am. J. Clin. Nutr. 2017, 106, 348S–358S. [Google Scholar] [PubMed]
- Raiten, D.J.; Sakr Ashour, F.A.; Ross, A.C.; Meydani, S.N.; Dawson, H.D.; Stephensen, C.B.; Brabin, B.J.; Suchdev, P.S.; van Ommen, B. Inflammation and nutritional science for programs/policies and interpretation of research evidence (inspire). J. Nutr. 2015, 145, 1039S–1108S. [Google Scholar] [CrossRef] [PubMed]
- Suchdev, P.S.; Boivin, M.J.; Forsyth, B.W.; Georgieff, M.K.; Guerrant, R.L.; Nelson, C.A., III. Assessment of neurodevelopment, nutrition, and inflammation from fetal life to adolescence in low-resource settings. Pediatrics 2017, 139, S23–S37. [Google Scholar] [CrossRef] [PubMed]
- Crumley, A.B.; McMillan, D.C.; McKernan, M.; McDonald, A.C.; Stuart, R.C. Evaluation of an inflammation-based prognostic score in patients with inoperable gastro-oesophageal cancer. Br. J. Cancer 2006, 94, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, M.; Nagata, H.; Takagi, K.; Horie, T.; Kubota, K. Inflammation-based prognostic score is a novel predictor of postoperative outcome in patients with colorectal cancer. Ann. Surg. 2007, 246, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Crumley, A.B.; Stuart, R.C.; McKernan, M.; McDonald, A.C.; McMillan, D.C. Comparison of an inflammation-based prognostic score (gps) with performance status (ecog-ps) in patients receiving palliative chemotherapy for gastroesophageal cancer. J. Gastroenterol. Hepatol. 2008, 23, e325–e329. [Google Scholar] [CrossRef] [PubMed]
- Roxburgh, C.S.; Crozier, J.E.; Maxwell, F.; Foulis, A.K.; Brown, J.; McKee, R.F.; Anderson, J.H.; Horgan, P.G.; McMillan, D.C. Comparison of tumour-based (petersen index) and inflammation-based (glasgow prognostic score) scoring systems in patients undergoing curative resection for colon cancer. Br. J. Cancer 2009, 100, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, A.; Onoda, H.; Imai, N.; Iwaku, A.; Oishi, M.; Fushiya, N.; Koike, K.; Nishino, H.; Tajiri, H. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br. J. Cancer 2012, 107, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Huang, D. The value of the systematic inflammation-based glasgow prognostic score in patients with gastric cancer: A literature review. J. Cancer Res. Ther. 2014, 10, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Matsumine, A.; Asanuma, K.; Matsubara, T.; Sudo, A. The value of the high-sensitivity modified glasgow prognostic score in predicting the survival of patients with a soft-tissue sarcoma. Bone Jt. J. 2015, 97, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Okuma, T.; Oka, H.; Hirai, T.; Ohki, T.; Ikegami, M.; Sawada, R.; Shinoda, Y.; Akiyama, T.; Sato, K.; et al. Neutrophil-to-lymphocyte ratio after pazopanib treatment predicts response in patients with advanced soft-tissue sarcoma. Int. J. Clin. Oncol. 2018, 23, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Jiang, S.; Situ, D.; Lin, Y.; Yang, H.; Li, Y.; Long, H.; Zhou, Z. Prognostic value of monocyte and neutrophils to lymphocytes ratio in patients with metastatic soft tissue sarcoma. Oncotarget 2015, 6, 9542–9550. [Google Scholar] [CrossRef] [PubMed]
- Szkandera, J.; Gerger, A.; Liegl-Atzwanger, B.; Stotz, M.; Samonigg, H.; Friesenbichler, J.; Stojakovic, T.; Leithner, A.; Pichler, M. The derived neutrophil/lymphocyte ratio predicts poor clinical outcome in soft tissue sarcoma patients. Am. J. Surg. 2015, 210, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Que, Y.; Qiu, H.; Li, Y.; Chen, Y.; Xiao, W.; Zhou, Z.; Zhang, X. Preoperative platelet-lymphocyte ratio is superior to neutrophil-lymphocyte ratio as a prognostic factor for soft-tissue sarcoma. BMC Cancer 2015, 15, 648. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Usui, S.; Kikuchi, S.; Goto, Y.; Sakai, M.; Onizuka, M.; Sato, Y. Preoperative lymphocyte count is an independent prognostic factor in node-negative non-small cell lung cancer. Lung Cancer 2012, 75, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Kusumanto, Y.H.; Dam, W.A.; Hospers, G.A.; Meijer, C.; Mulder, N.H. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis 2003, 6, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, S.; Schrum, A.G.; Cho, H.I.; Celis, E.; Gabrilovich, D.I. Mechanism of t cell tolerance induced by myeloid-derived suppressor cells. J. Immunol. 2010, 184, 3106–3116. [Google Scholar] [CrossRef] [PubMed]
- Gasic, G.J.; Gasic, T.B.; Stewart, C.C. Antimetastatic effects associated with platelet reduction. Proc. Natl. Acad. Sci. USA 1968, 61, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hutterer, E.; Isenring, E.; Kaasa, S.; et al. Espen guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Van Bokhorst-de van der Schueren, M.A.E.; Guaitoli, P.R.; Jansma, E.P.; de Vet, H.C.W. A systematic review of malnutrition screening tools for the nursing home setting. J. Am. Med. Dir. Assoc. 2014, 15, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Virizuela, J.A.; Camblor-Alvarez, M.; Luengo-Perez, L.M.; Grande, E.; Alvarez-Hernandez, J.; Sendros-Madrono, M.J.; Jimenez-Fonseca, P.; Cervera-Peris, M.; Ocon-Breton, M.J. Nutritional support and parenteral nutrition in cancer patients: An expert consensus report. Clin. Transl. Oncol. 2018, 20, 619–629. [Google Scholar] [CrossRef] [PubMed]
| Variables | |
|---|---|
| Proportion of female | 48.5% (50/103) |
| Diagnosis age | 64 (52–73) |
| WBC (/µL) | 6060 (4855–7530) |
| Plate (×104/µL) | 24.5 (20.1–29.4) |
| T-cholesterol (mg/dL) | 191.5 ± 39.1 |
| GPS | 0.0 (0.0–1.0) |
| GNRI | 102.7 (95.3–107.5) |
| NLR | 2.3 (1.6–3.3) |
| PLR | 15.0 (12.3–19.2) |
| CONUT score | 1.0 (0.0–2.0) |
| Maximum diameter of tumor | 70.0 (48.5–100.0) |
| Proportion of trunk onset | 20.4% (21/103) |
| Stage (cases) | 2 (32); 3 (60); 4 (11) |
| Proportion of resectable STS | 90.3% (93/103) |
| Survival time (months) | 60.6 ± 39.6 |
| Survival rate at one year | 85.4% (88/103) |
| Factor | Death within 1 Year | 1 Year Survival | p Value |
|---|---|---|---|
| Number | 15 | 88 | |
| Proportion of female | 40.0% (6/15) | 50.0% (44/88) | 0.581 |
| Diagnosis age | 72 (64.5–81.5) | 64 (51.0–70.5) | 0.009 ** |
| WBC (/µL) | 6220 (5570–9005) | 5950 (4783–7393) | 0.217 |
| Plate (×104/µL) | 26.3 (21.9–30.9) | 24.2 (19.8–28.6) | 0.231 |
| T- cholesterol (mg/dl) | 204.1 ± 35.7 | 189.3 ± 39.4 | 0.178 |
| GPS | 1.0 (1.0–2.0) | 0.0 (0.0–0.0) | <0.001 ** |
| GNRI | 89.3 (86.0–95.3) | 104.2 (98.2–108.7) | <0.001 ** |
| NLR | 4.0 (2.6–5.8) | 2.2 (1.6–3.0) | 0.003 ** |
| PLR | 19.6 (15.5–26.7) | 14.6 (11.7–17.7) | 0.003 ** |
| CONUT score | 3.0 (2.0–4.5) | 1.0 (0.0–2.0) | <0.001 ** |
| Maximum diameter of tumor | 112.0 (94.0–150.0) | 65.5 (40.8–94.3) | <0.001 ** |
| Proportion of trunk onset | 26.7% (4/15) | 19.3% (17/88) | 0.50 |
| Stage (cases) | 1(0)/2(1)/3(9)/4(5) | 1(0)/2(31)/3(51)/4(6) | 0.002 ** |
| Proportion of resectable STS | 53.3% (8/15) | 96.6% (85/88) | <0.001 ** |
| Coefficient of Determination R2:0.640 | ||
|---|---|---|
| Variables | HR (95% CI) | p Value |
| Female | 0.074 (0.006–0.974) | 0.048 * |
| Diagnosis age | 1.090 (1.009–1.177) | 0.030 * |
| GPS | 8.660 (1.986–37.245) | 0.004 ** |
| NLR | 1.368 (0.842–2.221) | 0.206 |
| Stage | 27.512 (1.974–383.486) | 0.014 * |
| Resectable STS | 0.010 (0.001–0.175) | 0.002 ** |
| Cox Proportional Hazards Model | ||
|---|---|---|
| HR (95% CI) | p Value | |
| NLR | 1.229 (1.032–1.462) | 0.020 * |
| PLR | 1.016 (1.002–1.031) | 0.028 * |
| Maximum diameter of Tumor | 1.004 (1.001–1.007) | 0.006 ** |
| Stage | 2.779 (1.424–5.422) | 0.003 ** |
| Resectable STS | 0.131 (0.051–0.338) | <0.001 ** |
| Variables | |
|---|---|
| Proportion of female | 47.3% (44/93) |
| Diagnosis age | 64 (51–73) |
| WBC (/µL) | 6110 (4900–7600) |
| Plate (×104/µL) | 24.9 (20.2–29.5) |
| GPS | 0.0 (0.0–0.0) |
| GNRI | 104.2 (96.8–108.5) |
| NLR | 2.3 (1.6–3.2) |
| PLR | 15.0 (11.8–19.0) |
| CONUT score | 1.0 (0.0–2.0) |
| Maximum diameter of tumor | 69.0 (42.0–100.0) |
| Proportion of trunk onset | 18.3% (17/93) |
| Proportion of deep onset | 47.3% (44/93) |
| Stage (cases) | 2 (31); 3 (55); 4 (7) |
| Survival time (months) | 65.4 ± 38.2 |
| Cox Proportional Hazards Model | ||
|---|---|---|
| HR (95% CI) | p Value | |
| Female | 0.313 (0.128–0.767) | 0.011 * |
| Age | 1.024 (0.993–1.055) | 0.126 |
| GPS | 2.098 (1.299–3.388) | 0.002 ** |
| Trunk onset | 0.316 (0.073–1.375) | 0.125 |
| Stage | 3.336 (1.405–7.924) | 0.006 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaki, H.; Nagano, S.; Komiya, S.; Taniguchi, N.; Setoguchi, T. Validation of Different Nutritional Assessment Tools in Predicting Prognosis of Patients with Soft Tissue Spindle-Cell Sarcomas. Nutrients 2018, 10, 765. https://doi.org/10.3390/nu10060765
Sasaki H, Nagano S, Komiya S, Taniguchi N, Setoguchi T. Validation of Different Nutritional Assessment Tools in Predicting Prognosis of Patients with Soft Tissue Spindle-Cell Sarcomas. Nutrients. 2018; 10(6):765. https://doi.org/10.3390/nu10060765
Chicago/Turabian StyleSasaki, Hiromi, Satoshi Nagano, Setsuro Komiya, Noboru Taniguchi, and Takao Setoguchi. 2018. "Validation of Different Nutritional Assessment Tools in Predicting Prognosis of Patients with Soft Tissue Spindle-Cell Sarcomas" Nutrients 10, no. 6: 765. https://doi.org/10.3390/nu10060765
APA StyleSasaki, H., Nagano, S., Komiya, S., Taniguchi, N., & Setoguchi, T. (2018). Validation of Different Nutritional Assessment Tools in Predicting Prognosis of Patients with Soft Tissue Spindle-Cell Sarcomas. Nutrients, 10(6), 765. https://doi.org/10.3390/nu10060765




