Vitamin D3 Status and the Association with Human Cathelicidin Expression in Patients with Different Clinical Forms of Active Tuberculosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Patient Materials
2.3. Tuberculin Skin Test (TST)
2.4. QuantiFERON-TB Gold In-Tube
2.5. 25(OH)D3 Concentrations in Plasma
2.6. mRNA Extraction and Quantitative Real-Time PCR
2.7. Immunohistochemistry and Quantitative Image Analysis of Frozen Tissue Sections
2.8. Statistical Analysis
3. Results
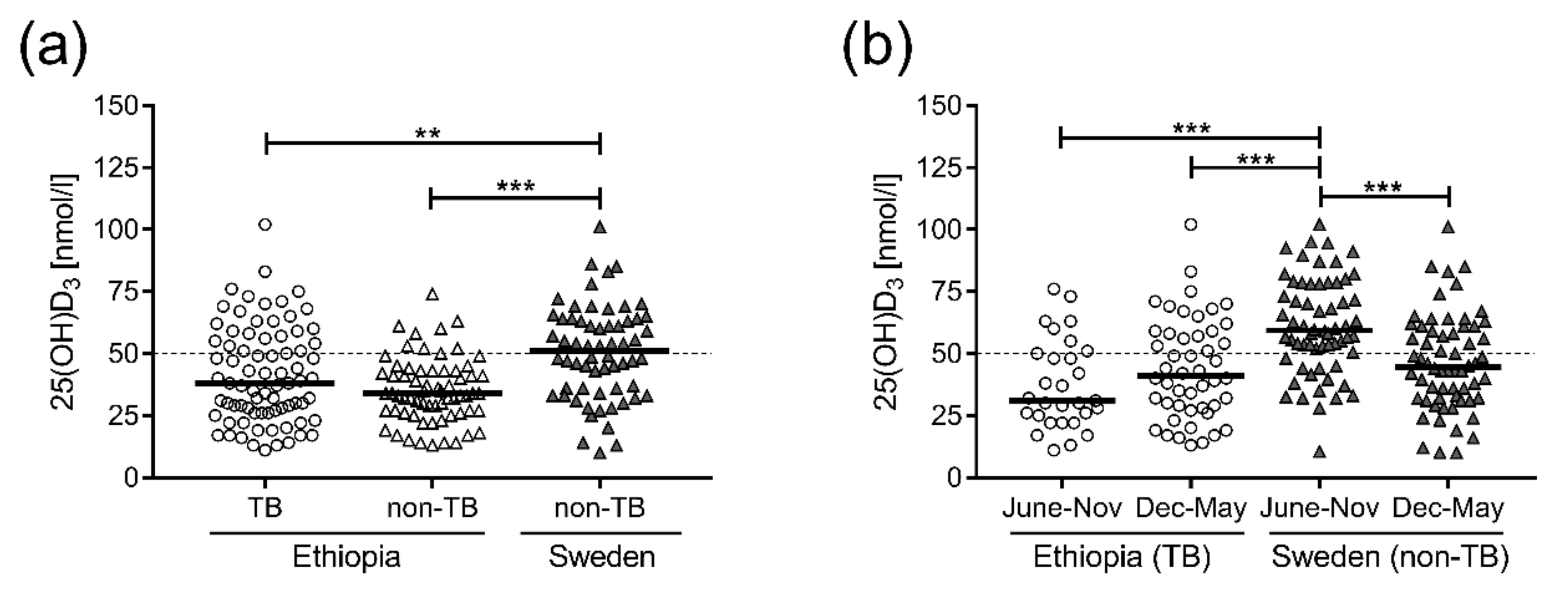
3.1. VitD3 Levels in Plasma from Ethiopian TB Patients as Well as Non-TB Controls were Significantly Lower Compared to Swedish Non-TB Controls
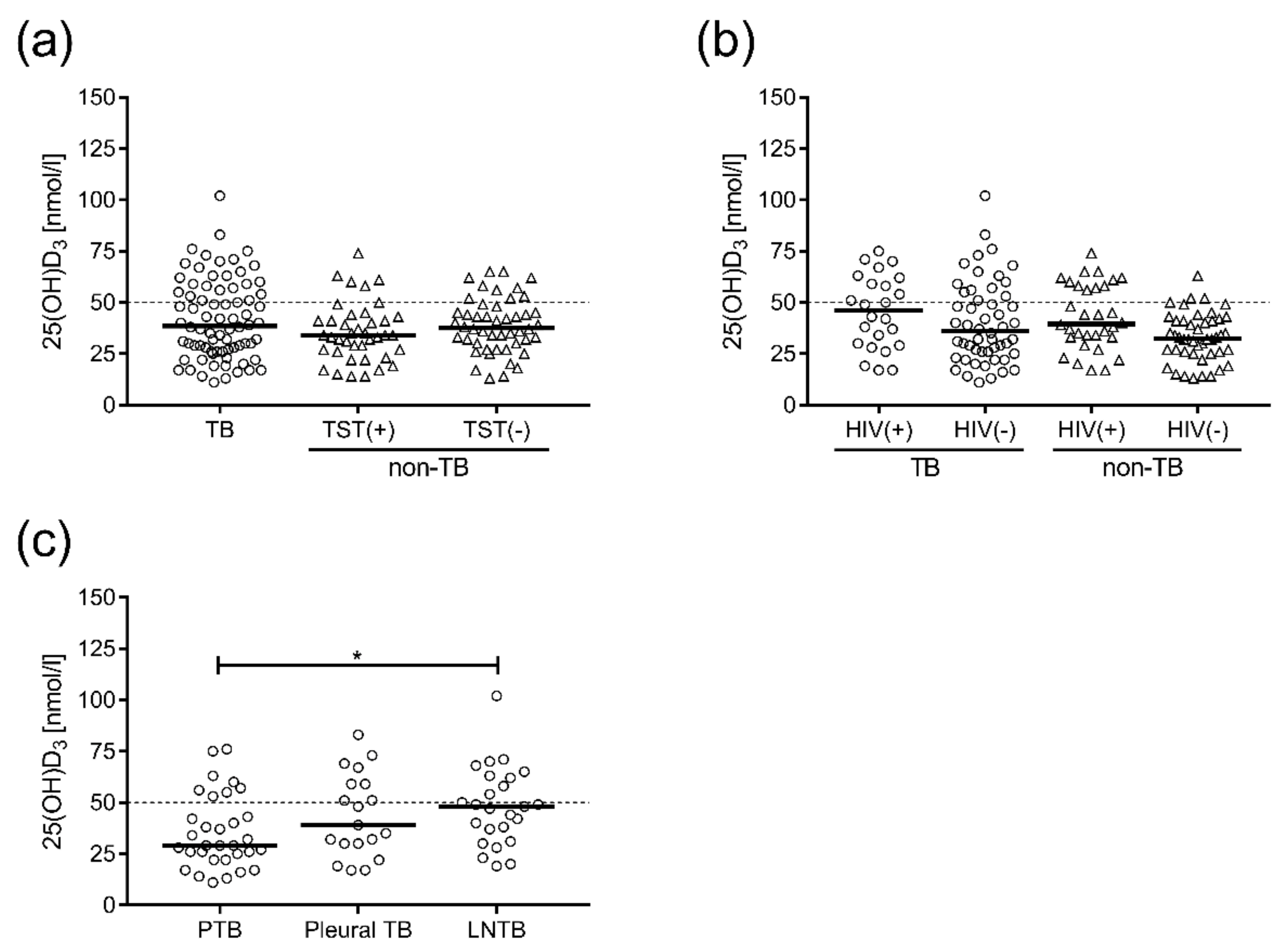
3.2. VitD3 Status in Ethiopian TB Patients and Non-TB Controls was not Affected by Latent TB or HIV Infection but was Associated with the Clinical Form of TB Disease
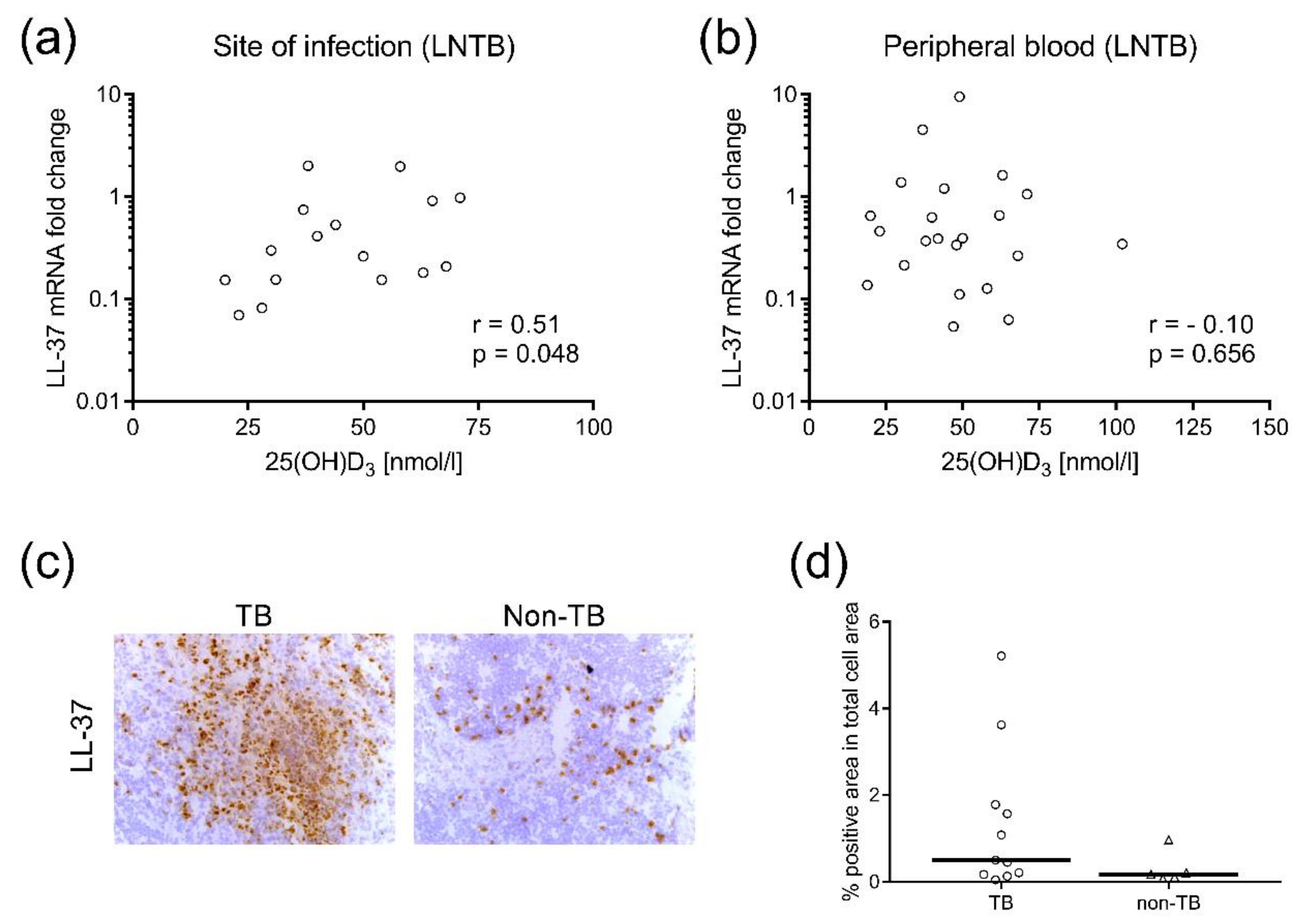
3.3. LL-37 mRNA Expression was Elevated in the Peripheral Blood of TB Patients Compared to non-TB Controls and also Systemically in PTB and Pleural TB Compared to the Site of Mtb Infection
3.4. LL-37 Expression in Mtb-Infected Lymph Nodes Correlated to Plasma levels of 25(OH)D3
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Global Tuberculosis Report 2016; WHO/HTM/TB/2016.13; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Zittermann, A.; Pilz, S.; Hoffmann, H.; Marz, W. Vitamin D and airway infections: A European perspective. Eur. J. Med. Res. 2016, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Pareek, M.; Innes, J.; Sridhar, S.; Grass, L.; Connell, D.; Woltmann, G.; Wiselka, M.; Martineau, A.R.; Kon, O.M.; Dedicoat, M.; et al. Vitamin d deficiency and tb disease phenotype. Thorax 2015, 70, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Gois, P.H.F.; Ferreira, D.; Olenski, S.; Seguro, A.C. Vitamin d and infectious diseases: Simple bystander or contributing factor? Nutrients 2017, 9, E651. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, H.S.; Nel, E.D.; Beyers, N.; Gie, R.P.; Scott, F.; Donald, P.R. A decade of experience with mycobacterium tuberculosis culture from children: A seasonal influence on incidence of childhood tuberculosis. Tuber. Lung Dis. 1996, 77, 43–46. [Google Scholar] [CrossRef]
- Martineau, A.R.; Nhamoyebonde, S.; Oni, T.; Rangaka, M.X.; Marais, S.; Bangani, N.; Tsekela, R.; Bashe, L.; de Azevedo, V.; Caldwell, J.; et al. Reciprocal seasonal variation in vitamin d status and tuberculosis notifications in cape town, south Africa. Proc. Natl. Acad. Sci. USA 2011, 108, 19013–19017. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.A.; Gowda, U.; Renzaho, A.M. The prevalence of vitamin d deficiency among dark-skinned populations according to their stage of migration and region of birth: A meta-analysis. Nutrition 2016, 32, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Stead, W.W.; Senner, J.W.; Reddick, W.T.; Lofgren, J.P. Racial differences in susceptibility to infection by mycobacterium tuberculosis. N. Engl. J. Med. 1990, 322, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.O.; Aspinall, R. Vitamin d status and the host resistance to infections: What it is currently (not) understood. Clin. Ther. 2017, 39, 930–945. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Wilkinson, K.A.; Newton, S.M.; Floto, R.A.; Norman, A.W.; Skolimowska, K.; Davidson, R.N.; Sorensen, O.E.; Kampmann, B.; Griffiths, C.J.; et al. IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: The role of cathelicidin LL-37. J. Immunol. 2007, 178, 7190–7198. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Chow, L.N.; Mookherjee, N. Cationic host defence peptides: Multifaceted role in immune modulation and inflammation. J. Innate Immun. 2012, 4, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Santiago, B.; Hernandez-Pando, R.; Carranza, C.; Juarez, E.; Contreras, J.L.; Aguilar-Leon, D.; Torres, M.; Sada, E. Expression of cathelicidin LL-37 during mycobacterium tuberculosis infection in human alveolar macrophages, monocytes, neutrophils, and epithelial cells. Infect. Immun. 2008, 76, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T.; Stenger, S.; Tang, D.H.; Modlin, R.L. Cutting edge: Vitamin d-mediated human antimicrobial activity against mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol. 2007, 179, 2060–2063. [Google Scholar] [CrossRef] [PubMed]
- Rekha, R.S.; Rao Muvva, S.S.; Wan, M.; Raqib, R.; Bergman, P.; Brighenti, S.; Gudmundsson, G.H.; Agerberth, B. Phenylbutyrate induces LL-37-dependent autophagy and intracellular killing of mycobacterium tuberculosis in human macrophages. Autophagy 2015, 11, 1688–1699. [Google Scholar] [CrossRef] [PubMed]
- Torres-Juarez, F.; Cardenas-Vargas, A.; Montoya-Rosales, A.; Gonzalez-Curiel, I.; Garcia-Hernandez, M.H.; Enciso-Moreno, J.A.; Hancock, R.E.; Rivas-Santiago, B. LL-37 immunomodulatory activity during mycobacterium tuberculosis infection in macrophages. Infect. Immun. 2015, 83, 4495–4503. [Google Scholar] [CrossRef] [PubMed]
- Elenius, V.; Palomares, O.; Waris, M.; Turunen, R.; Puhakka, T.; Ruckert, B.; Vuorinen, T.; Allander, T.; Vahlberg, T.; Akdis, M.; et al. The relationship of serum vitamins a, d, e and ll-37 levels with allergic status, tonsillar virus detection and immune response. PLoS ONE 2017, 12, e0172350. [Google Scholar] [CrossRef] [PubMed]
- Bivona, G.; Agnello, L.; Ciaccio, M. Vitamin d and immunomodulation: Is it time to change the reference values? Ann. Clin. Lab. Sci. 2017, 47, 508–510. [Google Scholar] [PubMed]
- Hoe, E.; Nathanielsz, J.; Toh, Z.Q.; Spry, L.; Marimla, R.; Balloch, A.; Mulholland, K.; Licciardi, P.V. Anti-inflammatory effects of vitamin d on human immune cells in the context of bacterial infection. Nutrients 2016, 8, 806. [Google Scholar] [CrossRef] [PubMed]
- Ashenafi, S.; Aderaye, G.; Bekele, A.; Zewdie, M.; Aseffa, G.; Hoang, A.T.; Carow, B.; Habtamu, M.; Wijkander, M.; Rottenberg, M.; et al. Progression of clinical tuberculosis is associated with a Th2 immune response signature in combination with elevated levels of SOC S3. Clin. Immunol. 2014, 151, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Ashenafi, S.; Aderaye, G.; Zewdie, M.; Raqib, R.; Bekele, A.; Magalhaes, I.; Lema, B.; Habtamu, M.; Rekha, R.S.; Aseffa, G.; et al. BCG-specific igg-secreting peripheral plasmablasts as a potential biomarker of active tuberculosis in HIV negative and HIV positive patients. Thorax 2013, 68, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.; Norlin, A.C.; Hansen, S.; Rekha, R.S.; Agerberth, B.; Bjorkhem-Bergman, L.; Ekstrom, L.; Lindh, J.D.; Andersson, J. Vitamin D3 supplementation in patients with frequent respiratory tract infections: A randomised and double-blind intervention study. BMJ Open 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.; Samarina, A.; Fink, J.; Rahman, S.; Grundstrom, S. Impaired expression of perforin and granulysin in CD8+ T cells at the site of infection in human chronic pulmonary tuberculosis. Infect. Immun. 2007, 75, 5210–5222. [Google Scholar] [CrossRef] [PubMed]
- Brighenti, S.; Andersson, J. Local immune responses in human tuberculosis: Learning from the site of infection. J. Infect. Dis. 2012, 205 (Suppl. 2), S316–S324. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.S.; Lovell, M.A.; Gorbsky, G.J. Stability of nuclear segments in human neutrophils and evidence against a role for microfilaments or microtubules in their genesis during differentiation of HL60 myelocytes. J. Leukoc. Biol. 1995, 58, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Nnoaham, K.E.; Clarke, A. Low serum vitamin d levels and tuberculosis: A systematic review and meta-analysis. Int. J. Epidemiol. 2008, 37, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Wu, G.; Yang, W.; Gu, X.; Liang, W.; Yao, Y.; Song, Y. A serum vitamin d level <25 nmol/L pose high tuberculosis risk: A meta-analysis. PLoS ONE 2015, 10, e0126014. [Google Scholar]
- Keflie, T.S.; Nolle, N.; Lambert, C.; Nohr, D.; Biesalski, H.K. Vitamin d deficiencies among tuberculosis patients in Africa: A systematic review. Nutrition 2015, 31, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.J.; Wang, X.H.; Liu, Z.D.; Cao, W.L.; Han, Y.; Ma, A.G.; Xu, S.F. Vitamin d deficiency and the risk of tuberculosis: A meta-analysis. Drug Des. Devel. Ther. 2017, 11, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Workineh, M.; Mathewos, B.; Moges, B.; Gize, A.; Getie, S.; Stendahl, O.; Schon, T.; Abate, E. Vitamin D deficiency among newly diagnosed tuberculosis patients and their household contacts: A comparative cross-sectional study. Arch. Public Health 2017, 75, 25. [Google Scholar] [CrossRef] [PubMed]
- Tessema, B.; Moges, F.; Habte, D.; Hiruy, N.; Yismaw, S.; Melkieneh, K.; Kassie, Y.; Girma, B.; Melese, M.; Suarez, P.G. Vitamin D deficiency among smear positive pulmonary tuberculosis patients and their tuberculosis negative household contacts in northwest Ethiopia: A case-control study. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Arnedo-Pena, A.; Juan-Cerdan, J.V.; Romeu-Garcia, M.A.; Garcia-Ferrer, D.; Holguin-Gomez, R.; Iborra-Millet, J.; Pardo-Serrano, F. Vitamin D status and incidence of tuberculosis infection conversion in contacts of pulmonary tuberculosis patients: A prospective cohort study. Epidemiol. Infect. 2015, 143, 1731–1741. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, D.; Gupta, N.; Mukherjee, A.; Lodha, R.; Singh, V.; Grewal, H.M.; Bhatnagar, S.; Singh, S.; Kabra, S.K.; Delhi Pediatric, T.B.S.G. Vitamin d levels in Indian children with intrathoracic tuberculosis. Indian J. Med. Res. 2014, 140, 531–537. [Google Scholar] [PubMed]
- Musarurwa, C.; Zijenah, L.S.; Duri, D.Z.; Mateveke-Dangaiso, K.; Mhandire, K.; Chipiti, M.M.; Munjoma, M.W.; Mujaji, W.B. Association of high serum vitamin D concentrations with active pulmonary TB in an HIV co-endemic setting, Harare, Zimbabwe. BMC Infect. Dis. 2017, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Owolabi, O.; Agbla, S.; Owiafe, P.; Donkor, S.; Togun, T.; Sillah, A.K.; Ota, M.O.; Sutherland, J.S. Elevated serum 25-hydroxy (OH) vitamin D levels are associated with risk of TB progression in Gambian adults. Tuberculosis 2016, 98, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Friis, H.; Range, N.; Changalucha, J.; Praygod, G.; Jeremiah, K.; Faurholt-Jepsen, D.; Krarup, H.; Molgaard, C.; Andersen, A. Vitamin D status among pulmonary TB patients and non-TB controls: A cross-sectional study from Mwanza, Tanzania. PLoS ONE 2013, 8, e81142. [Google Scholar] [CrossRef] [PubMed]
- Wejse, C.; Olesen, R.; Rabna, P.; Kaestel, P.; Gustafson, P.; Aaby, P.; Andersen, P.L.; Glerup, H.; Sodemann, M. Serum 25-hydroxyvitamin d in a west African population of tuberculosis patients and unmatched healthy controls. Am. J. Clin. Nutr. 2007, 86, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.R.; Kline, L.; Holick, M.F. Influence of season and latitude on the cutaneous synthesis of vitamin D3: Exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J. Clin. Endocrinol. Metab. 1988, 67, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Fares, A. Seasonality of tuberculosis. J. Glob. Infect. Dis. 2011, 3, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Friis, H.; Range, N.; Pedersen, M.L.; Molgaard, C.; Changalucha, J.; Krarup, H.; Magnussen, P.; Soborg, C.; Andersen, A.B. Hypovitaminosis d is common among pulmonary tuberculosis patients in Tanzania but is not explained by the acute phase response. J. Nutr. 2008, 138, 2474–2480. [Google Scholar] [CrossRef] [PubMed]
- Korthals Altes, H.; Kremer, K.; Erkens, C.; van Soolingen, D.; Wallinga, J. Tuberculosis seasonality in the Netherlands differs between natives and non-natives: A role for vitamin d deficiency? Int. J. Tuberc. Lung Dis. 2012, 16, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Feleke, Y.; Abdulkadir, J.; Mshana, R.; Mekbib, T.A.; Brunvand, L.; Berg, J.P.; Falch, J.A. Low levels of serum Calcidiol in an African population compared to a north European population. Eur J Endocrinol 1999, 141, 358–360. [Google Scholar] [CrossRef] [PubMed]
- Nansera, D.; Graziano, F.M.; Friedman, D.J.; Bobbs, M.K.; Jones, A.N.; Hansen, K.E. Vitamin d and calcium levels in Ugandan adults with human immunodeficiency virus and tuberculosis. Int. J. Tuberc. Lung Dis. 2011, 15, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Steenhoff, A.P.; Redwood, A.; Pettifor, J.M.; Hove, J.; Bisson, G.P.; Mosepele, M.; Pusoesele, P.; Thakur, R.; Kovarik, C.; Gross, R. Vitamin D status in HIV-infected patients with and without tuberculosis: A pilot study. J. Acquir. Immune Defic. Syndr. 2012, 61, e21–e23. [Google Scholar] [CrossRef] [PubMed]
- Conesa-Botella, A.; Goovaerts, O.; Massinga-Loembe, M.; Worodria, W.; Mazakpwe, D.; Luzinda, K.; Mayanja-Kizza, H.; Colebunders, R.; Kestens, L.; TB IRIS Study Group. Low prevalence of vitamin d deficiency in Ugandan HIV-infected patients with and without tuberculosis. Int. J. Tuberc. Lung Dis. 2012, 16, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Syal, K.; Banerjee, D.; Hota, D.; Gupta, D.; Kaul, D.; Chakrabarti, A. Low plasma levels of cholecalciferol and 13-cis-retinoic acid in tuberculosis: Implications in host-based chemotherapy. Nutrition 2013, 29, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Rehn, A.; Rahman, J.; Andersson, J.; Svensson, M.; Brighenti, S. Pulmonary tuberculosis patients with a vitamin D deficiency demonstrate low local expression of the antimicrobial peptide LL-37 but enhanced FoxP3+ regulatory T cells and IgG-secreting cells. Clin Immunol 2015, 156, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Afsal, K.; Harishankar, M.; Banurekha, V.V.; Meenakshi, N.; Parthasarathy, R.T.; Selvaraj, P. Effect of 1,25-dihydroxy vitamin D3 on cathelicidin expression in patients with and without cavitary tuberculosis. Tuberculosis 2014, 94, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Uitterlinden, A.G.; Fang, Y.; Van Meurs, J.B.; Pols, H.A.; Van Leeuwen, J.P. Genetics and biology of vitamin d receptor polymorphisms. Gene 2004, 338, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Song, G.G. Vitamin D receptor gene Foki, Taqi, Bsmi, and Apai polymorphisms and susceptibility to pulmonary tuberculosis: A meta-analysis. Genet. Mol. Res. 2015, 14, 9118–9129. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, X.; Cao, Z.; Cheng, X. Association of vitamin d receptor gene Taqi polymorphisms with tuberculosis susceptibility: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 10187–10203. [Google Scholar] [PubMed]
- Wilkinson, R.J.; Llewelyn, M.; Toossi, Z.; Patel, P.; Pasvol, G.; Lalvani, A.; Wright, D.; Latif, M.; Davidson, R.N. Influence of vitamin d deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: A case-control study. Lancet 2000, 355, 618–621. [Google Scholar] [CrossRef]

| Variable | Total Active TB (n = 77) | PTB (n = 32) | Pleural TB (n = 20) | LNTB (n = 25) | Ethiopian Non-TB Controls (n = 78) | Swedish Non-TB Controls (n = 62) |
|---|---|---|---|---|---|---|
| Gender: | ||||||
| Male (M)–No (%) | 42 (55%) | 18 (56%) | 10 (50%) | 14 (56%) | 41 (54%) | 17 (27%) |
| Female (F)–No (%) | 35 (45%) | 14 (44%) | 10 (50%) | 11 (44%) | 35 (46%) | 45 (73%) |
| Age, median (range) | 27 (18–72) | 28.5 (18–54) | 27 (19–72) | 25.5 (18–57) | 29 (18–68) | 51.5 (21–72) |
| HIV infection, No (%) | 24 (31%) | 11 (34%) | 5 (25%) | 8 (32%) | 32 (41%) | 0 (0%) |
| Positive TST, No (%) | 65 (84%) | 26 (81%) | 16 (80%) | 23 (92%) | 40 (51%) | ND |
| Weight loss, No (%) | 55 (71%) | 28 (88%) | 15 (75%) | 12 (48%) | ND | ND |
| Occupation, No (%) | 33 (43%) | 17 (53%) | 9 (45%) | 7 (28%) | ND | ND |
| Other illness, No (%) a | 6 (8%) | 3 (9%) | 2 (10%) | 1 (4%) | ND | ND |
| 25(OH)D3 nmol/L (median) | 38.5 | 29.0 | 39.0 | 48.0 | 35.0 | 51.0 |
| 25(OH)D3 < 25 nmol/L–No (%) | 15 (20%) | 8 (25%) | 4 (20%) | 3 (12%) | 14 (18%) | 4 (6%) |
| 25(OH)D3 < 50 nmol/L–No (%) b | 50 (66%) | 24 (75%) | 11 (58%) | 15 (60%) | 63 (81%) | 31 (50%) |
| 25(OH)D3 50–75 nmol/L–No (%) | 23 (30%) | 7 (22%) | 7 (37%) | 9 (36%) | 15 (19%) | 26 (42%) |
| 25(OH)D3 > 75 nmol/L–No (%) | 3 (4%) | 1 (3%) | 1 (5%) | 1 (4%) | 0 (0%) | 5 (8%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashenafi, S.; Mazurek, J.; Rehn, A.; Lemma, B.; Aderaye, G.; Bekele, A.; Assefa, G.; Chanyalew, M.; Aseffa, A.; Andersson, J.; et al. Vitamin D3 Status and the Association with Human Cathelicidin Expression in Patients with Different Clinical Forms of Active Tuberculosis. Nutrients 2018, 10, 721. https://doi.org/10.3390/nu10060721
Ashenafi S, Mazurek J, Rehn A, Lemma B, Aderaye G, Bekele A, Assefa G, Chanyalew M, Aseffa A, Andersson J, et al. Vitamin D3 Status and the Association with Human Cathelicidin Expression in Patients with Different Clinical Forms of Active Tuberculosis. Nutrients. 2018; 10(6):721. https://doi.org/10.3390/nu10060721
Chicago/Turabian StyleAshenafi, Senait, Jolanta Mazurek, Anders Rehn, Beede Lemma, Getachew Aderaye, Amsalu Bekele, Getachew Assefa, Menberework Chanyalew, Abraham Aseffa, Jan Andersson, and et al. 2018. "Vitamin D3 Status and the Association with Human Cathelicidin Expression in Patients with Different Clinical Forms of Active Tuberculosis" Nutrients 10, no. 6: 721. https://doi.org/10.3390/nu10060721
APA StyleAshenafi, S., Mazurek, J., Rehn, A., Lemma, B., Aderaye, G., Bekele, A., Assefa, G., Chanyalew, M., Aseffa, A., Andersson, J., Bergman, P., & Brighenti, S. (2018). Vitamin D3 Status and the Association with Human Cathelicidin Expression in Patients with Different Clinical Forms of Active Tuberculosis. Nutrients, 10(6), 721. https://doi.org/10.3390/nu10060721






