A Case-Control Study of the Association between Vitamin D Levels and Gastric Incomplete Intestinal Metaplasia
Abstract
1. Introduction
2. Materials and Methods
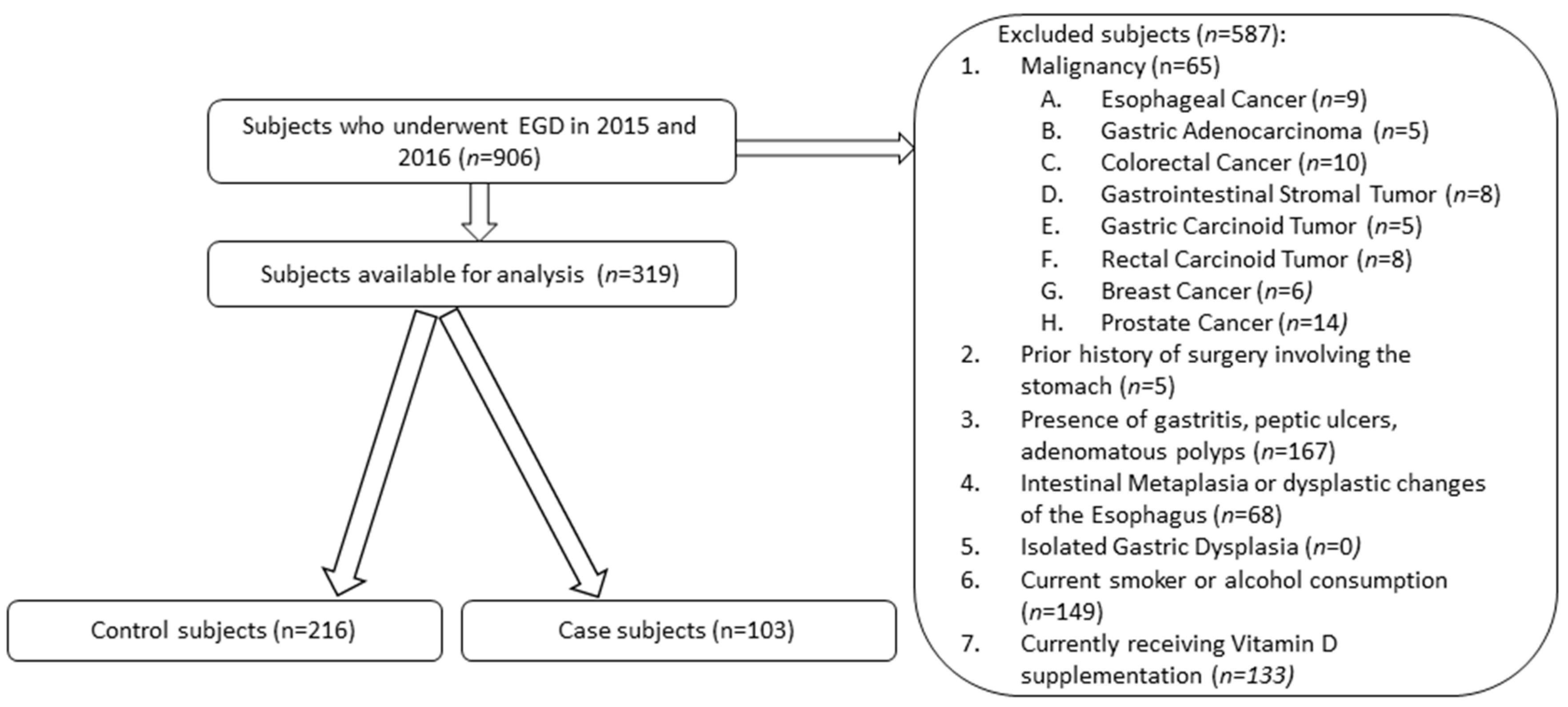
2.1. Subjects
2.2. Design
3. Results
3.1. Demographic Information
3.2. Analysis of Vitamin D Status in Intestinal Metaplasia
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- McLean, M.H.; El-Omar, E.M. Genetics of gastric cancer. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Rothenbacher, D.; Arndt, V. Epidemiology of stomach cancer. Methods Mol. Biol. 2009, 472, 467–477. [Google Scholar] [PubMed]
- Kneller, R.W.; You, W.C.; Chang, Y.S.; Liu, W.D.; Zhang, L.; Zhao, L.; Xu, G.W.; Fraumeni, J.F., Jr.; Blot, W.J. Cigarette smoking and other risk factors for progression of precancerous stomach lesions. J. Natl. Cancer Inst. 1992, 84, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Peleteiro, B.; La Vecchia, C.; Lunet, N. The role of helicobacter pylori infection in the web of gastric cancer causation. Eur. J. Cancer Prev. 2012, 21, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.; Piazuelo, M.B. The gastric precancerous cascade. J. Dig. Dis. 2012, 13, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.K.; Lin, S.R.; Ching, J.Y.; To, K.F.; Ng, E.K.; Chan, F.K.; Lau, J.Y.; Sung, J.J. Factors predicting progression of gastric intestinal metaplasia: Results of a randomized trial on helicobacter pylori eradication. Gut 2004, 53, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.M.; Wang, A.Y. Gastric intestinal metaplasia and early gastric cancer in the West: A changing paradigm. Gastroenterol. Hepatol. 2014, 10, 369–378. [Google Scholar]
- Leung, H.W.; Muo, C.H.; Liu, C.F.; Chan, A.L. Vitamin D3 intake dose and common cancer: A population-based case control study in a Chinese population. J. Cancer 2016, 7, 2028–2034. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, H.; Chen, W.; Li, D.; Yin, X.; Olsen, N.; Zheng, S.G. Vitamin D and chronic diseases. Aging Dis. 2017, 8, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Liu, Y.; Rimm, E.B.; Hollis, B.W.; Fuchs, C.S.; Stampfer, M.J.; Willett, W.C. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J. Nat. Cancer Inst. 2006, 98, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer 2002, 94, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Vyas, N.; Companioni, R.C.; Tiba, M.; Alkhawam, H.; Catalano, C.; Sogomonian, R.; Baum, J.; Walfish, A. Association between serum vitamin D levels and gastric cancer: A retrospective chart analysis. World J. Gastroenterol. 2016, 8, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.A.; Barry, E.L.; Mott, L.A. A Trial of Calcium and vitamin D for the prevention of colorectal adenomas. N. Eng. J. Med. 2015, 373, 1519–1530. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Qiu, M.Z.; Wang, D.S.; Luo, H.Y.; Zhang, D.S.; Wang, Z.Q.; Wang, F.H.; Li, Y.H.; Zhou, Z.W.; Xu, R.H. Prognostic effects of 25-hydroxyvitamin D levels in gastric cancer. J. Transl. Med. 2012, 10. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine society. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metabol. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, L.; Zhang, L.; Hu, W.; Shen, J.; Xiao, Z.; Wu, X.; Chan, F.L.; Cho, C.H. 1,25-Dihydroxyvitamin D3 suppresses gastric cancer cell growth through VDR- and mutant p53-mediated induction of p21. Life Sci. 2017, 179, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Matloob, A.F.; Du, J.; Pan, H.; Dong, Z.; Zhao, J.; Feng, Y.; Zhong, Y.; Huang, B.; Lu, J. Vitamin D stimulates apoptosis in gastric cancer cells in synergy with trichostatin A/sodium butyrate-induced and 5-aza-2′-deoxycytidine-induced PTEN upregulation. FEBS J. 2010, 277, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Park, M.R.; Lee, J.H.; Park, M.S.; Hwang, J.E.; Shim, H.J.; Cho, S.H.; Chung, I.J.; Bae, W.K. Suppressive effect of 19-nor-1α-25-dihydroxyvitamin D2 on gastric cancer cells and peritoneal metastasis model. J. Korean Med. Sci. 2012, 27, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Bao, A.; Li, Y.; Tong, Y.; Zheng, H.; Wu, W.; Wei, C. 1,25-Dihydroxyvitamin D3 and cisplatin synergistically induce apoptosis and cell cycle arrest in gastric cancer cells. Int. J. Mol. Med. 2014, 33, 1177–1184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Y.C.; Wang, J.L.; Kong, X.; Sun, T.T.; Chen, H.Y.; Hong, J.; Fang, J.Y. CD24 mediates gastric carcinogenesis and promotes gastric cancer progression via STAT3 activation. Apoptosis 2014, 19, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Lee, Y.S.; Shim, H.E.; Yoon, S.; Baek, S.Y.; Kim, B.S.; Oh, S.O. Vitamin D3 regulates cell viability in gastric cancer and cholangiocarcinoma. Anat. Cell Biol. 2011, 44, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Bao, A.; Li, Y.; Tong, Y.; Zheng, H.; Wu, W.; Wei, C. Tumor-suppressive effects of 1,25-dihydroxyvitamin D3 in gastric cancer cells. Hepatogastroenterology 2013, 60, 943–948. [Google Scholar] [PubMed]
- Chang, S.; Gao, L.; Yang, Y.; Tong, D.; Guo, B.; Liu, L.; Li, Z.; Song, T.; Huang, C. miR-145 mediates the antiproliferative and gene regulatory effects of vitamin D3 by directly targeting E2F3 in gastric cancer cells. Oncotarget 2015, 6, 7675–7685. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Da, M.; Zhang, Y.; Peng, L.; Yao, J.; Duan, Y. Alterations in vitamin D signaling pathway in gastric cancer progression: A study of vitamin D receptor expression in human normal, premalignant, and malignant gastric tissue. Int. J. Clin. Exp. Pathol. 2015, 8, 13176–13184. [Google Scholar] [PubMed]
- Fleet, J.C.; DeSmet, M.; Johnson, R.; Li, Y. Vitamin D and cancer: A review of molecular mechanisms. J. Biochem. 2012, 441, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Baloch, Z.; He, T.T.; Xia, X. Alcohol consumption and gastric cancer risk: A meta-analysis. Med. Sci. Monit. 2017, 23, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.; Rodrigues, S.; Amorim, L.; Peleteiro, B.; Lunet, N. Tobacco smoking and intestinal metaplasia: Systematic review and meta-analysis. Dig. Liver Dis. 2014, 46, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Fennerty, M.B.; Emerson, J.C.; Sampliner, R.E.; McGee, D.L.; Hixson, L.J.; Garewal, H.S. Gastric intestinal metaplasia in ethnic groups in the Southwestern United States. Cancer Epidemiol. Biomark. Prev. 1992, 1, 293–296. [Google Scholar]
- Olmez, S.; Aslan, M.; Erten, R.; Sayar, S.; Bayram, I. The prevalence of gastric intestinal metaplasia and distribution of helicobacter pylori infection, atrophy, dysplasia, and cancer in its subtypes. Gastroenterol. Res. Pract. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, N.K.; Kärkkäinen, P.A.; Färkkilä, M.A.; Arkkila, P.E. Prevalence and distribution of gastric intestinal metaplasia and its subtypes. Dig. Liver Dis. 2008, 40, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.; Fontham, E.T.; Bravo, J.C.; Bravo, L.E.; Ruiz, B.; Zarama, G.; Realpe, J.L.; Malcom, G.T.; Li, D.; Johnson, W.D.; et al. Chemoprevention of gastric dysplasia: Randomized trial of antioxidant supplements and anti-helicobacter pylori therapy. J. Nat. Cancer Inst. 2000, 92, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
| Intestinal Metaplasia (Cases) | No Intestinal Metaplasia (Controls) | p-Value | |
|---|---|---|---|
| Number of subjects (N) | 103 | 216 | |
| Age (years) | 59.4 ± 11.6 | 56.9 ± 13.6 | NS 1 |
| <60 years old | 49.5% (51) | 61.1% (132) | |
| >60 years old | 50.5% (52) | 38.9% (84) | |
| Gender | 0.01 | ||
| Male | 50.5% (52) | 32.4% (70) | |
| Female | 49.5% (51) | 67.5% (146) | |
| Ethnicity | NS | ||
| Hispanic | 68% (70) | 64.8% (140) | |
| African American | 23.3% (24) | 19.0% (41) | |
| Caucasian | 4.9% (5) | 13.0% (28) | |
| Asian | 3.9% (4) | 3.2% (7) | |
| Body mass index (BMI; kg/m2) | NS | ||
| Underweight | 3.9% (4) | 1.9% (4) | |
| Normal | 24.3% (25) | 24.1% (52) | |
| Overweight | 35.9% (37) | 34.7% (75) | |
| Obese (total) | 35.9% (37) | 39.3% (85) | |
| Obesity Class I | 24.3% (25) | 20.4% (44) | |
| Obesity Class II | 10.7% (11) | 12% (26) | |
| Obesity Class III | 1% (1) | 6.9% (15) | |
| History of Diabetes Mellitus | NS | ||
| Without Diabetes Mellitus | 87.4% (90) | 91.7% (198) | |
| With Diabetes Mellitus | 12.6% (13) | 8.3% (18) | |
| History of hypertension | <0.001 | ||
| Without Hypertension | 96.1% (99) | 81.9% (177) | |
| With hypertension | 3.9% (4) | 18.1% (39) | |
| Season of vitamin D measurement | 0.01 | ||
| Fall-Winter | 24.3% (25) | 38.4% (86) | |
| Spring-Summer | 75.7% (78) | 61.6% (133) |
| Vitamin D Status | Intestinal Metaplasia (Cases) | No Intestinal Metaplasia (Controls) |
|---|---|---|
| Normal vitamin D level (30–100 ng/dL) | 2.2% (7) | 52.0% (166) |
| Hypovitaminosis D (<30 ng/dL) | 97.8% (96) | 48.0% (50) |
| Vitamin D insufficiency (VDi) (20–30 ng/dL) | 43.8% (42) | 78% (39) |
| Vitamin D deficiency (VDd) (<20 ng/dL) | 56.3% (54) | 22% (11) |
| Odds ratio (95% CI) | p-value | |
| Normal vitamin D levels | ||
| versus | ||
| VDd and VDi | 54.1 (21.8–134.3) | <0.001 |
| VDd | 129.0 (43.7–381.2) | <0.001 |
| VDi | 31.0 (11.9–80.3) | <0.001 |
| VDd versus Vdi | 4.0 (1.7–9.6) | <0.001 |
| Intestinal Metaplasia (Cases) | No Intestinal Metaplasia (Controls) | p-Value | |
|---|---|---|---|
| Overall vitamin D level (ng/dL) | 19.7 ± 6.3 | 34.7 ± 10.0 | <0.001 |
| Variables | |||
| Age | |||
| <60 years old | 19.8 ± 6.5 | 35.2 ± 11.2 | <0.001 |
| >60 years old | 19.7 ± 6.3 | 34.0 ± 8.0 | <0.001 |
| Gender | |||
| Male | 20.4 ± 7.0 | 34.4 ± 10.4 | <0.001 |
| Female | 19.9 ± 6.6 | 34.8 ± 9.9 | <0.001 |
| BMI class | |||
| Underweight | 24.3 ± 2.6 | 42.0 ± 22.2 | 0.25 |
| Normal | 19.4 ± 6.5 | 34.5 ± 9.6 | <0.001 |
| Overweight | 19.7 ± 7.9 | 35.7 ± 10.8 | <0.001 |
| Obese (pooled) | 20.4 ± 6.1 | 33.5 ± 8.8 | <0.001 |
| Obesity class I | 21.1 ± 6.1 | 33.9 ± 8.9 | <0.001 |
| Obesity class II | 19.6 ± 6.4 | 33.1 ± 9.5 | 0.03 |
| Obesity class III | 20.5 * | 33.2 ± 7.4 | N/A * |
| Ethnicity | |||
| Hispanic | 19.3 ± 6.1 | 34.4 ± 10.8 | <0.001 |
| African American | 21.5 ± 6.6 | 34.6 ± 8.1 | <0.001 |
| Caucasian | 20.9 ± 7.8 | 36.2 ± 9.5 | <0.001 |
| Asian | 14.05 ± 3.8 | 35.2 ± 4.6 | <0.001 |
| Blood pressure | |||
| Normotensive patients | 19.6 ± 6.8 | 34.8 ± 10.1 | <0.001 |
| Hypertensive patients | 23.6 ± 6.3 | 34.4 ± 10.0 | <0.001 |
| History of diabetes mellitus | |||
| Without diabetes mellitus | 19.5 ± 6.3 | 34.4 ± 9.1 | <0.001 |
| With diabetes mellitus | 25.7 ± 5.0 | 38.4 ± 17.33 | NS |
| Timing of Vitamin D Collection | |||
| Fall-Winter | 22.0 ± 7.3 | 34.1 ± 9.9 | <0.001 |
| Spring-Summer | 19.6 ± 6.6 | 35.0 ± 10.1 | <0.001 |
| Variable | B | Standard Error | Wald Statistic | p-Value | OR, 95% CI |
|---|---|---|---|---|---|
| Hypovitaminosis D | 4.0 | 0.46 | 73.9 | <0.001 | 54.1 (21.8–134.3) |
| Age | 0.004 | 0.01 | 0.10 | 0.76 | 1.00 (0.98–1.03) |
| Ethnicity | −19.24 | 2 × 104 | 3.89 | 0.27 | 0.000 |
| Gender | −1.00 | 0.37 | 7.03 | 0.008 | 0.37 (0.18–0.77) |
| BMI | −0.06 | 0.14 | 0.17 | 0.68 | 0.95 (0.72–1.23) |
| Diabetes mellitus | −1.30 | 0.84 | 2.42 | 0.12 | 1.40 (0.05–1.40) |
| Hypertension | 0.22 | 0.51 | 0.18 | 0.67 | 1.24 (0.46–3.35) |
| Timing of vitamin D collection | 0.69 | 0.38 | 3.23 | 0.07 | 1.99 (0.94–4.23) |
| Constant | −2.92 | 1.73 | 2.86 | 0.1 | 0.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, K.; Gandhi, S.; Batool, R. A Case-Control Study of the Association between Vitamin D Levels and Gastric Incomplete Intestinal Metaplasia. Nutrients 2018, 10, 629. https://doi.org/10.3390/nu10050629
Singh K, Gandhi S, Batool R. A Case-Control Study of the Association between Vitamin D Levels and Gastric Incomplete Intestinal Metaplasia. Nutrients. 2018; 10(5):629. https://doi.org/10.3390/nu10050629
Chicago/Turabian StyleSingh, Kevin, Soren Gandhi, and Raffat Batool. 2018. "A Case-Control Study of the Association between Vitamin D Levels and Gastric Incomplete Intestinal Metaplasia" Nutrients 10, no. 5: 629. https://doi.org/10.3390/nu10050629
APA StyleSingh, K., Gandhi, S., & Batool, R. (2018). A Case-Control Study of the Association between Vitamin D Levels and Gastric Incomplete Intestinal Metaplasia. Nutrients, 10(5), 629. https://doi.org/10.3390/nu10050629





