Effect of Dietary Sugar Intake on Biomarkers of Subclinical Inflammation: A Systematic Review and Meta-Analysis of Intervention Studies
Abstract
1. Introduction
2. Materials and Methods
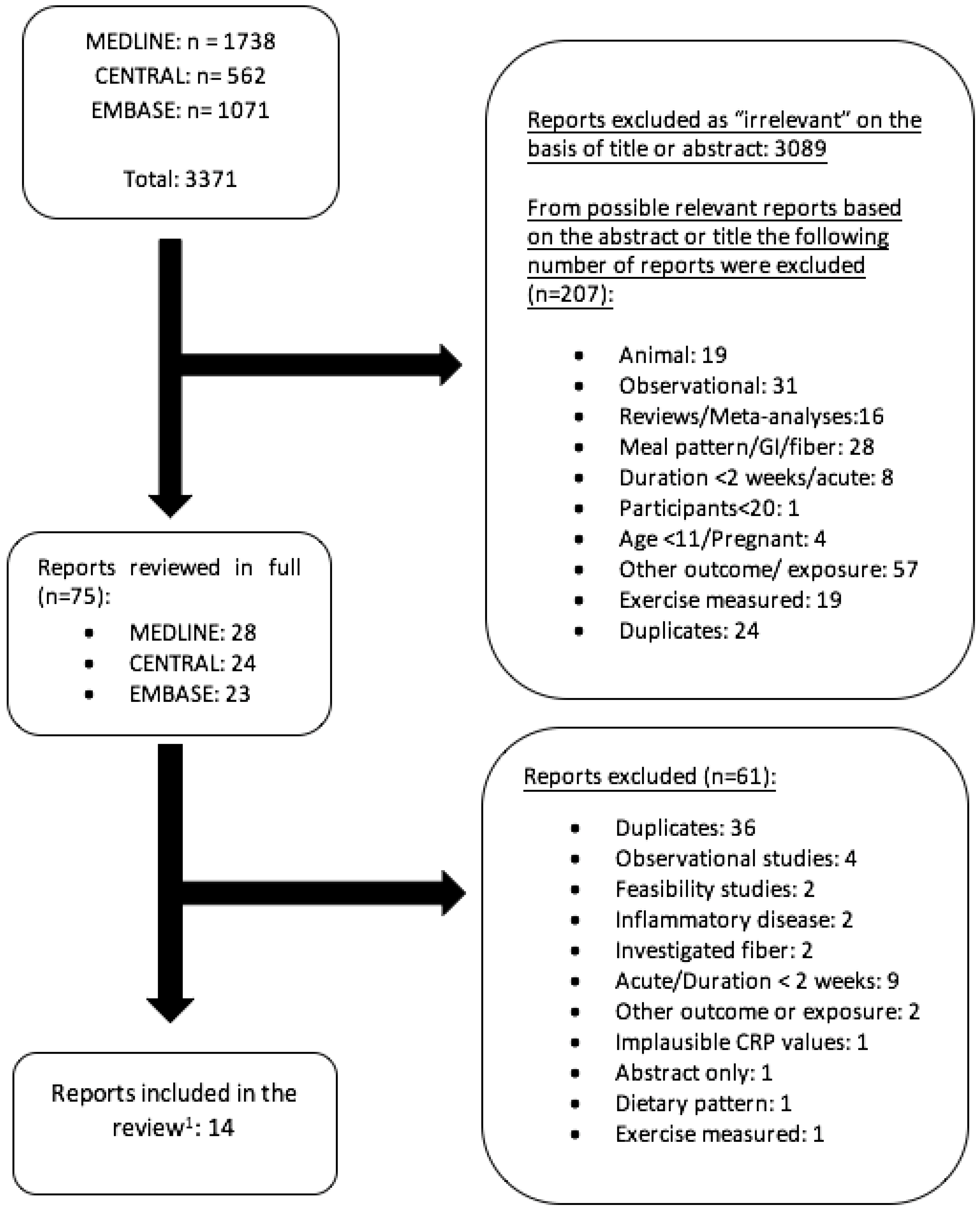
2.1. Study Selection
2.2. Data Extraction
2.3. Statistical Analysis
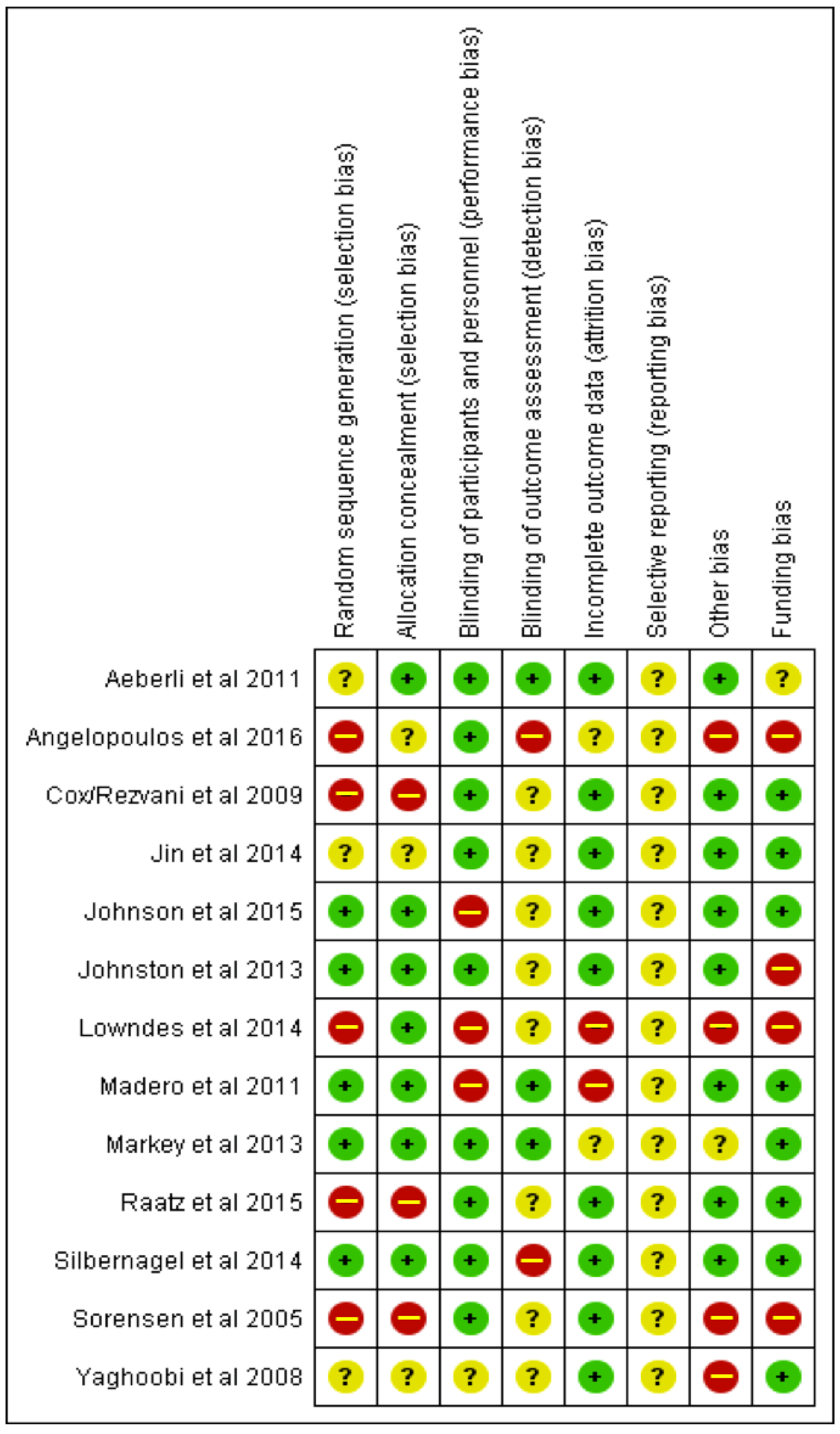
2.4. Assessment of Quality of Meta-Evidence
3. Results
3.1. Description of Studies
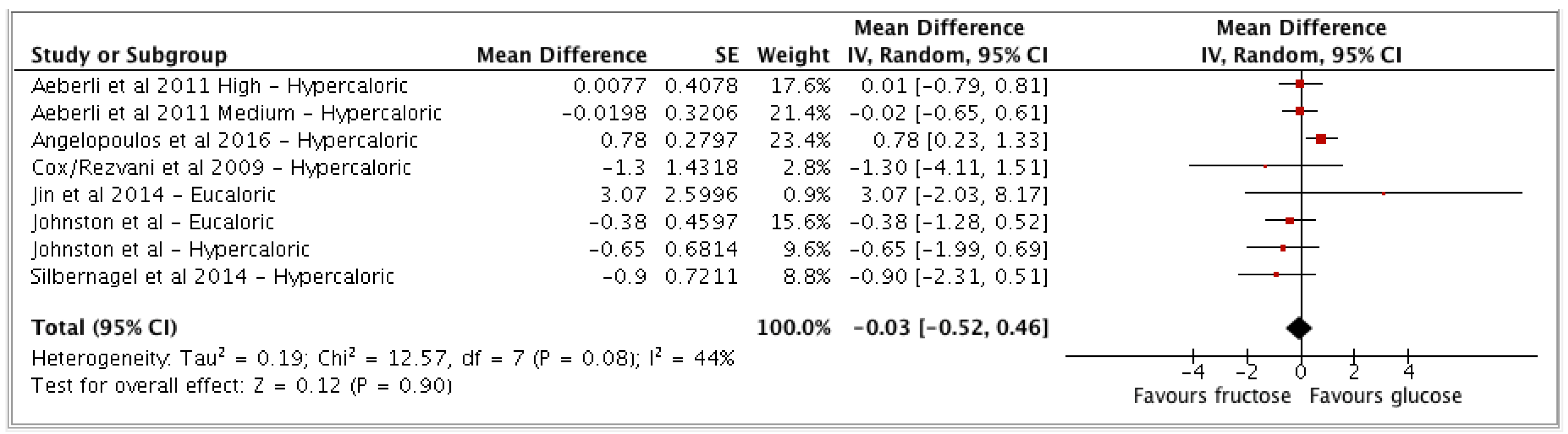
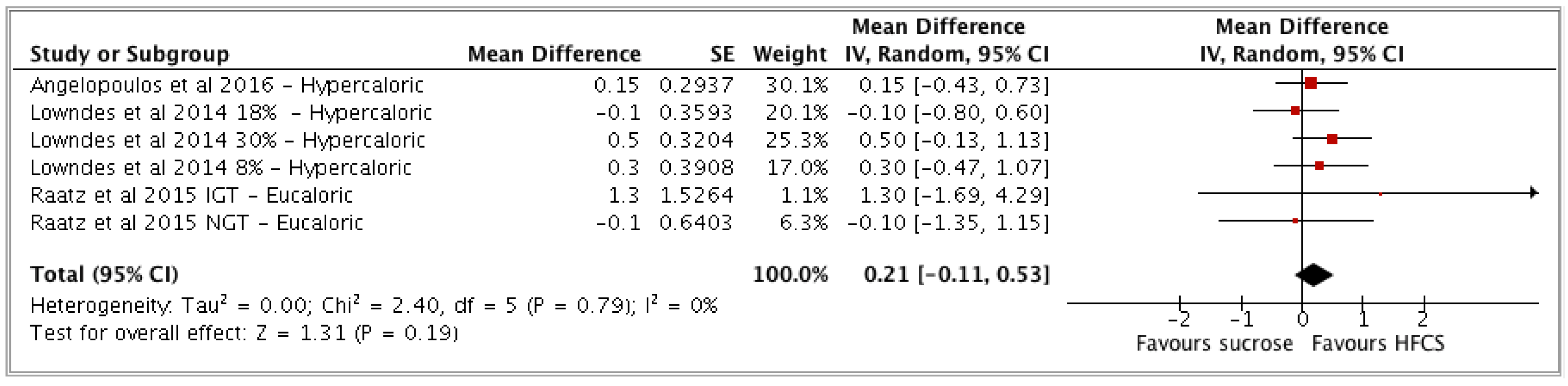
3.2. Meta-Analysis Results
- (a) Fructose vs glucose (n = 6 studies; n = 8 study arms);
- (b) HFCS vs sucrose (n = 3 studies; n = 6 study arms).
3.3. NutriGrade
3.4. Comprehensive Narrative Overview
4. Discussion
4.1. Implications Arising from the Study Design
4.2. Potentially Relevant Pathophysiological Mechanisms
4.3. Interpretation in the Context of Evidence from Observational Studies
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
References
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhou, Z.; Zhu, W.; Fan, X.; Liu, X. Plasma C-reactive protein is related to cognitive deterioration and dementia in patients with mild cognitive impairment. J. Neurol. Sci. 2009, 284, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Bettencourt, R.; Brenner, D.; Le, T.; Barrett-Connor, E.; Loomba, R. Association between Serum Interleukin-6 Concentrations and Mortality in Older Adults: The Rancho Bernardo Study. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- de Koning, L.; Malik, V.S.; Kellogg, M.D.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation 2012, 125, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Kosova, E.C.; Auinger, P.; Bremer, A.A. The Relationships between Sugar-Sweetened Beverage Intake and Cardiometabolic Markers in Young Children. J. Acad. Nutr. Diet. 2013, 113, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Hert, K.A.; Fisk, P.S.; Rhee, Y.S.; Brunt, A.R. Decreased consumption of sugar-sweetened beverages improved selected biomarkers of chronic disease risk among US adults: 1999 to 2010. Nutr. Res. 2014, 34, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Hoffmann, K.; Manson, J.E.; Willett, W.C.; Meigs, J.B.; Weikert, C.; Heidemann, C.; Colditz, G.A.; Hu, F.B. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am. J. Clin. Nutr. 2005, 82, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Newens, K.J.; Walton, J. A review of sugar consumption from nationally representative dietary surveys across the world. J. Hum. Nutr. Diet. 2016, 29, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Marriott, B.P.; Cole, N.; Lee, E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J. Nutr. 2009, 139, 1228s–1235s. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Nielsen, S.J.; Popkin, B.M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr. 2004, 79, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Sharma, A.J.; Grellinger, L.; Vos, M.B. Consumption of added sugars is decreasing in the United States. Am. J. Clin. Nutr. 2011, 94, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Public-Health-England. Consultation on draft SACN Carbohydrates and Health Report; Public-Health-England: London, UK, 2014. [Google Scholar]
- The World Health Organization. Guideline Recommendations; The World Health Organization: Geneva, Swizterland, 2015; Available online: http://www.who.int/mediacentre/news/releases/2015/sugar-guideline/en (accessed on 15 March 2018).
- Chung, M.; Ma, J.; Patel, K.; Berger, S.; Lau, J.; Lichtenstein, A.H. Fructose, high-fructose corn syrup, sucrose, and nonalcoholic fatty liver disease or indexes of liver health: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 833–849. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.; Sievenpiper, J.L.; de Souza, R.J.; Cozma, A.I.; Mirrahimi, A.; Carleton, A.J.; Ha, V.; Di Buono, M.; Jenkins, A.L.; Jenkins, D.J.; et al. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 2014, 68, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Softic, S.; Cohen, D.E.; Kahn, C.R. Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N.; Dixon, L.J.; Feldstein, A.E. Lipotoxicity in nonalcoholic fatty liver disease: Not all lipids are created equal. Expert Rev. Gastroenterol. Hepatol. 2009, 3, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Malhi, H.; Gores, G.J. Cellular and Molecular Mechanisms of Liver Injury. Gastroenterology 2008, 134, 1641–1654. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Knüppel, S.; Schwedhelm, C.; Hoffmann, G.; Missbach, B.; Stelmach-Mardas, M.; Dietrich, S.; Eichelmann, F.; Kontopantelis, E.; Boeing, H.; et al. Perspective: NutriGrade: A Scoring System to Assess and Judge the Meta-Evidence of Randomized Controlled Trials and Cohort Studies in Nutrition Research. Adv. Nutr. 2016, 7, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bao, W.; Liu, J.; Ouyang, Y.Y.; Wang, D.; Rong, S.; Xiao, X.; Shan, Z.L.; Zhang, Y.; Yao, P.; Liu, L.G. Inflammatory markers and risk of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2013, 36, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Herder, C.; Carstensen, M.; Ouwens, D.M. Anti-inflammatory cytokines and risk of type 2 diabetes. Diabetes Obes. Metab. 2013, 15 (Suppl. 3), 39–50. [Google Scholar] [CrossRef] [PubMed]
- Muris, D.M.; Houben, A.J.; Schram, M.T.; Stehouwer, C.D. Microvascular dysfunction is associated with a higher incidence of type 2 diabetes mellitus: A systematic review and meta-analysis. Arterioscler Thromb. Vasc. Biol. 2012, 32, 3082–3094. [Google Scholar] [CrossRef] [PubMed]
- Kaptoge, S.; Seshasai, S.R.; Gao, P.; Freitag, D.F.; Butterworth, A.S.; Borglykke, A.; Di Angelantonio, E.; Gudnason, V.; Rumley, A.; Danesh, J.; et al. Inflammatory cytokines and risk of coronary heart disease: New prospective study and updated meta-analysis. Eur. Heart J. 2014, 35, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Herder, C.; Baumert, J.; Thorand, B.; Koenig, W.; de Jager, W.; Meisinger, C.; Illig, T.; Martin, S.; Kolb, H. Chemokines as risk factors for type 2 diabetes: Results from the MONICA/KORA Augsburg study, 1984–2002. Diabetologia 2006, 49, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Herder, C.; Gala, T.; Carstensen-Kirberg, M.; Huth, C.; Zierer, A.; Wahl, S.; Sudduth-Klinger, J.; Kuulasmaa, K.; Peretz, D.; Ligthart, S.; Thorand, B.; et al. Circulating Levels of Interleukin 1-Receptor Antagonist and Risk of Cardiovascular Disease: Meta-Analysis of Six Population-Based Cohorts. Arterioscler Thromb. Vasc. Biol. 2017, 37, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Higgins JPT, G.S.e. Cochrane Handbook for Systematic Reviews of Interventions Version 510; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Higgins, J.P.; Higgins, J.P.T.; Simon, G.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, C.L.; Cox, B.; Havel, P.J.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.L.; Stanhope, K.L.; Schwarz, J.M.; Graham, J.L.; Hatcher, B.; Griffen, S.C.; Bremer, A.A.; Berglund, L.; McGahan, J.P.; Keim, N.L.; Havel, P.J. Circulating concentrations of monocyte chemoattractant protein-1, plasminogen activator inhibitor-1, and soluble leukocyte adhesion molecule-1 in overweight/obese men and women consuming fructose- or glucose-sweetened beverages for 10 weeks. J. Clin. Endocrinol. Metab. 2011, 96, E2034–E2038. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, R.; Cianflone, K.; McGahan, J.P.; Berglund, L.; Bremer, A.A.; Keim, N.L.; Griffen, S.C.; Havel, P.J.; Stanhope, K.L. Effects of sugar-sweetened beverages on plasma acylation stimulating protein, leptin and adiponectin: Relationships with metabolic outcomes. Obesity (Silver Spring) 2013, 21, 2471–2480. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Welsh, J.A.; Le, N.A.; Holzberg, J.; Sharma, P.; Martin, D.R.; Vos, M.B. Dietary fructose reduction improves markers of cardiovascular disease risk in Hispanic-American adolescents with NAFLD. Nutrients 2014, 6, 3187–3201. [Google Scholar] [CrossRef] [PubMed]
- Silbernagel, G.; Machann, J.; Häring, H.U.; Fritsche, A.; Peter, A. Plasminogen activator inhibitor-1, monocyte chemoattractant protein-1, e-selectin and C-reactive protein levels in response to 4-week very-high-fructose or -glucose diets. Eur. J. Clin. Nutr. 2014, 68, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.D.; Stephenson, M.C.; Crossland, H. No difference between high-fructose and high-glucose diets on liver triacylglycerol or biochemistry in healthy overweight men. Gastroenterology 2013, 145, 1016.e2–1025.e2. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulos, T.J.; Lowndes, J.; Sinnett, S.; Rippe, J.M. Fructose Containing Sugars at Normal Levels of Consumption Do Not Effect Adversely Components of the Metabolic Syndrome and Risk Factors for Cardiovascular Disease. Nutrients 2016, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Aeberli, I.; Gerber, P.A.; Hochuli, M.; Kohler, S.; Haile, S.R.; Gouni-Berthold, I.; Berthold, H.K.; Spinas, G.A.; Berneis, K. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 94, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.K.; Holven, K.B.; Nordstrand, N.; Mellembakken, J.R.; Tanbo, T.; Hjelmesæth, J. Fructose content of low calorie diets: Effect on cardiometabolic risk factors in obese women with polycystic ovarian syndrome: A randomized controlled trial. Endocr. Connect. 2015, 4, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Madero, M.; Arriaga, J.C.; Jalal, D.; Rivard, C.; McFann, K.; Pérez-Méndez, O.; Vázquez, A.; Ruiz, A.; Lanaspa, M.A.; Lozada, L.G.; et al. The effect of two energy-restricted diets, a low-fructose diet versus a moderate natural fructose diet, on weight loss and metabolic syndrome parameters: A randomized controlled trial. Metabolism 2011, 60, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Raatz, S.K.; Johnson, L.K.; Picklo, M.J. Consumption of Honey, Sucrose, and High-Fructose Corn Syrup Produces Similar Metabolic Effects in Glucose-Tolerant and -Intolerant Individuals. J. Nutr. 2015, 145, 2265–2272. [Google Scholar] [CrossRef] [PubMed]
- Lowndes, J.; Sinnett, S.; Yu, Z.; Rippe, J. The effects of fructose-containing sugars on weight, body composition and cardiometabolic risk factors when consumed at up to the 90th percentile population consumption level for fructose. Nutrients 2014, 6, 3153–3168. [Google Scholar] [CrossRef] [PubMed]
- Yaghoobi, N.; Al-Waili, N.; Ghayour-Mobarhan, M.; Parizadeh, S.M.; Abasalti, Z.; Yaghoobi, Z.; Yaghoobi, F.; Esmaeili, H.; Kazemi-Bajestani, S.M.; Aghasizadeh, R.; et al. Natural honey and cardiovascular risk factors; effects on blood glucose, cholesterol, triacylglycerole, CRP, and body weight compared with sucrose. Sci. World J. 2008, 8, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Markey, O.; Lejeune, J.; Lovegrove, J.A. Initial findings of the impact of an 8-week intervention of sugar reformulated product exchange on cardiovascular risk factors. Proc. Nutr. Soc. 2013, 72, E214. [Google Scholar] [CrossRef]
- Sorensen, L.B.; Raben, A.; Stender, S.; Astrup, A. Effect of sucrose on inflammatory markers in overweight humans. Am. J. Clin. Nutr. 2005, 82, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Sievenpiper, J.L. Controversies about sugars: Results from systematic reviews and meta-analyses on obesity, cardiometabolic disease and diabetes. Eur. J. Nutr. 2016, 55 (Suppl. 2), 25–43. [Google Scholar] [CrossRef] [PubMed]
- Litman, E.A.; Gortmaker, S.L.; Ebbeling, C.B.; Ludwig, D.S. Source of bias in sugar-sweetened beverage research: A systematic review. Public Health Nutr. 2018, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Di Gregorio, G.B.; Yao-Borengasser, A.; Rasouli, N.; Varma, V.; Lu, T.; Miles, L.M.; Ranganathan, G.; Peterson, C.A.; McGehee, R.E.; Kern, P.A. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: Association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes 2005, 54, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Szendroedi, J.; Kaul, K.; Jelenik, T.; Nowotny, P.; Jankowiak, F.; Herder, C.; Carstensen, M.; Krausch, M.; Trudo, W.; Roden, M.; et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015, 21, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.B.; Lavine, J.E. Dietary fructose in nonalcoholic fatty liver disease. Hepatology 2013, 57, 2525–2531. [Google Scholar] [CrossRef] [PubMed]
- Tiniakos, D.G.; Vos, M.B.; Brunt, E.M. Nonalcoholic fatty liver disease: Pathology and pathogenesis. Annu. Rev. Pathol. 2010, 5, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Spruss, A.; Kanuri, G.; Wagnerberger, S.; Haub, S.; Bischoff, S.C.; Bergheim, I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology 2009, 50, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Bergheim, I.; Weber, S.; Vos, M.; Krämer, S.; Volynets, V.; Kaserouni, S.; McClain, C.J.; Bischoff, S.C. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: Role of endotoxin. J. Hepatol. 2008, 48, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.B.; Pimentel-Nunes, P.; Roncon-Albuquerque, R. Jr.; Leite-Moreira, A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol. Int. 2010, 4, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Gersch, M.S.; Mu, W.; Cirillo, P.; Reungjui, S.; Zhang, L.; Roncal, C.; Sautin, Y.Y.; Johnson, R.J.; Nakagawa, T. Fructose, but not dextrose, accelerates the progression of chronic kidney disease. Am. J. Physiol. Renal Physiol. 2007, 293, F1256–F1261. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, P. Ketohexokinase-Dependent Metabolism of Fructose Induces Proinflammatory Mediators in Proximal Tubular Cells. J. Am. Soc. Nephrol. 2009, 20, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A. Postprandial hyperglycemia and diabetes complications: Is it time to treat? Diabetes 2005, 54, 1–7. [Google Scholar] [CrossRef] [PubMed]
- von Loeffelholz, C.; Horn, P.; Birkenfeld, A.L.; Claus, R.A.; Metzing, B.U.; Döcke, S.; Jahreis, G.; Heller, R.; Hoppe, S.; Stockmann, M.; Lock, J.F.; et al. Fetuin A is a Predictor of Liver Fat in Preoperative Patients with Nonalcoholic Fatty Liver Disease. J. Investig. Surg. 2016, 29, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Sievenpiper, J.L.; de Souza, R.J.; Mirrahimi, A.; Yu, M.E.; Carleton, A.J.; Beyene, J.; Chiavaroli, L.; Di Buono, M.; Jenkins, A.L.; Leiter, L.A.; et al. Effect of fructose on body weight in controlled feeding trials: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 156, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, J.M.; Sacks, F.M.; Smith, S.R.; LeBoff, M.S.; Rood, J.C.; Bray, G.A.; Ridker, P.M. Effect of dietary composition of weight loss diets on high-sensitivity c-reactive protein: The Randomized POUNDS LOST trial. Obesity (Silver Spring) 2013, 21, 681–689. [Google Scholar] [CrossRef] [PubMed]
- DeChristopher, L.R.; Uribarri, J.; Tucker, K.L. Intake of high-fructose corn syrup sweetened soft drinks, fruit drinks and apple juice is associated with prevalent arthritis in US adults, aged 20–30 years. Nutr. Diabetes 2016, 6, e199. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Costenbader, K.H.X.; Al-Daabil, M.; Sparks, J.A.; Solomon, D.A.; Hu, F.B.; Karlson, E.W.; Lu, B. Sugar-sweetened soda consumption and risk of developing rheumatoid arthritis in women. Am. J. Clin. Nutr. 2014, 100, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Fung, T.T.; Willett, W.C.; Stampfer, M.J.; Manson, J.E.; Hu, F.B. Dietary patterns and the risk of coronary heart disease in women. Arch. Intern. Med. 2001, 161, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Lutsey, P.L.; Steffen, L.M.; Stevens, J. Dietary intake and the development of the metabolic syndrome: The Atherosclerosis Risk in Communities study. Circulation 2008, 117, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Buyken, A.E.; M, D.J.; Dussort, P.; Johnson, I.T.; Macdonald, I.A.; Stowell, J.D.; Brouns, F.J.P.H. Dietary carbohydrates: A review of international recommendations and the methods used to derive them. Eur. J. Clin. Nutr. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
| First Author, Year, Country | Study Design | Participants’ Characteristics | Duration (weeks) | Intervention | Energy Intake | Sugar form § | Feeding Control ‡ |
|---|---|---|---|---|---|---|---|
| Aeberli et al. (2011) [39] Switzerland | Crossover Double-blind | 29 healthy males Age 20–50 years BMI 19–25 kg/m2 | 3 | Intervention:
| Hypercaloric * | Liquid | Supp/DA |
| Angelopoulos et al. (2016) [38] USA | Parallel Double-blind Randomized | 267 healthy participants (96 m/171 w) Age 37.7 ± 12.1 years BMI 26.3 ± 3.3 kg/m2 | 10 | Intervention:
| Hypercaloric * | Liquid | Supp/DA |
| Cox/Rezvani et al. † (2009) [33,34] USA | Parallel Blinded | 31 overweight/obese participants (16 m/15 w) Age 40–72 years BMI 25–35 kg/m2 | 10 | Intervention:
| Hypercaloric * | Liquid | Met/Supp |
| Jin et al. (2014) [35] USA | Parallel Double-blind Randomized | 24 overweight Hispanic-American adolescents with hepatic fat > 8%, Age 11–18 years BMI ≥85th percentile | 4 | Intervention:
| Eucaloric | Liquid | Supp |
| Johnson et al. (2015) [40] Finland | Parallel Randomized | 51 morbidly obese women with polycystic ovarian syndrome Age 18–40 years BMI ≥ 40 or 35–40 kg/m2 | 8 | Intervention:
| Hypocaloric | Liquid | Supp/DA |
| Johnston et al. (2013) [37] UK | Parallel Double-blind Randomized | 32 healthy overweight males Mean age 33–35 years BMI 25–32 kg/m2 | 2 | Intervention:
| Eucaloric and Hypercaloric * (2 weeks each) | Liquid | Supp/Met |
| Lowndes et al. (2014) [43] USA | Parallel Blinded Randomized | 355 overweight or obese participants (165 m/190 w) Age 20-–60 years BMI 23–35 kg/m2 | 10 | Intervention:
| Hypercaloric Sugars administered in milk medium. | Liquid | Supp |
| Madero et al. (2011) [41] Mexico | Parallel Randomized | 131 obese participants (102 w/29 m) Age 38.8 ± 8.8 years BMI 32.4 ± 4.5 kg/m2 | 6 | Intervention:
| Hypocaloric | Solid | DA |
| Markey et al. (2013) [45] UK | Crossover Double-blind Randomized | 50 normal or overweight participants (16 m/34 w) Age 20–49 years BMI 18.5–30 kg/m2 | 8 | Intervention:
| Eucaloric | Mixed | Supp/DA |
| Raatz et al. (2015) [42] USA | Crossover Randomized | 55 participants (39 w/ 16 m): group 1 with normal glucose tolerance (NGT) (n = 28), group 2 with impaired glucose tolerance (IGT) (n = 27) Mean age of NGT 39 years, Mean age of IGT 52 years BMI of NGT 26 kg/m2, BMI of IGT 31.5 kg/m2 | 2 | Intervention:
| Eucaloric | Liquid | Supp |
| Silbernagel et al. (2014) [36] Germany | Parallel Single-blinded Randomized | 20 healthy participants (12 m/8 w) Mean age 30 years BMI of 26 ± 0.5 kg/m2 | 4 | Intervention:
| Hypercaloric * | Liquid | Supp |
| Sorensen et al. (2005) [46] Denmark | Parallel | 41 overweight participants (6 m/35 w) Age 33–37 years BMI 27–28 kg/m2 | 10 | Intervention:
| Hypercaloric | Mixed | Supp |
| Yaghoobi et al. (2008) [44] Iran | Parallel Randomized | 55 overweight or obese participants (24 m/31 w) Age 20-–60 years BMI > 25 kg/m2 | ≈4 | Intervention:
| Eucaloric | Liquid | Supp |
| First Author, Year, Country | Outcome Baseline Concentrations 1 | Results | Funding Source †† | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Percent changes in inflammatory marker after completion of intervention | Statistical Tests Comment | |||||||||
| hsCRP/CRP | IL-6 | TNF-α | MCP-1 | sICAM-1 | sE-selectin | Adipo-nectin | ||||
| Aeberli et al. (2011) [39] Switzerland | hsCRP (ng/mL) 205.6 ± 430.7 Adiponectin (μg/mL) 6.44 ± 7.69 | High fructose: +109.19% * Moderate fructose:+82.2% High sucrose: +105.2% * High glucose: +89.74% * | N/A | N/A | N/A | N/A | N/A | High fructose: +18.6% * Moderate fructose: +14.8% High sucrose: +17.8% High glucose:+20.6% * | No treatment effect for hsCRP and adiponectin reported. hsCRP increased significantly after all of the interventions -highest increase observed in high fructose group | NR |
| Angelopoulos et al. (2016) [38] USA | CRP (mg/L) Fructose group: 1.74 ± 1.74 HFCS group: 1.92 ± 2.10 Sucrose group: 1.74 ± 1.78 Glucose group: 1.21 ± 1.43 | Fructose: +24.1% * HFCS: −3.1% Sucrose: −1.7% Glucose: +23.9% * | N/A | N/A | N/A | N/A | N/A | N/A | No significant between-group changes in CRP for fructose, HFCS, sucrose and glucose as compared to each other. p-Values not reported. | Industry |
| Cox/Rezvani et al. (2009) [33,34] USA | MCP-1 (pg/mL) 144.7 ± 18.8 sE-selectin (ng/dL) 45.0 ± 5.5 sICAM-1 (ng/mL) 221.9 ± 6.3 CRP (mg/L)3.7 ± 0.8 IL-6 (pg/mL) 3.5 ± 0.7 Adiponectin (ug/mL): 7.7 ± 1.1 TNF-α: NR | Fructose: −16.2% * Glucose: −22.8% * | Fructose: −11.4% * Glucose: +18.2% * | Fructose: −12.8% * Glucose: +0.3% * | Fructose: +37.7% * Glucose: −8.6% * | Fructose: +2.9% * Glucose: −1% * | Fructose: +14.4% * Glucose: −1.6% * | Fructose: −14.8% * Glucose: −9.1% * | Significant between-group change in MCP-1 (p = 0.03). Significant within-group change in sE-selectin (p = 0.048). But no significant between-group difference (p = 0.17). No significant between-group change in sICAM-1 (p = 0.22) CRP (p = 0.33) IL-6 (p = 0.31) adiponectin (p = 0.10) TNF-α (p = 0.42) | Agency |
| Jin et al. (2014) [35] USA | hsCRP (mg/L) 6.78 ± 3.16 | Fructose: +4.13% * Glucose: −23.4% * | N/A | N/A | N/A | N/A | N/A | N/A | Significant between-group change in hsCRP (p = 0.019). | Agency |
| Johnson et al. (2015) [40] Finland | CRP (mg/L) Low-fructose: 6.8 ± 7.4 Moderate-fructose: 10.9 ± 10.2 | Low-fructose: −8.8% Moderate-fructose: −29.3% | N/A | N/A | N/A | N/A | N/A | N/A | No significant between-group change in CRP (p = 0.278) Confounder (low-calorie diet → weight loss) | Agency |
| Johnston et al. (2013) [37] UK | CRP (mg/L) 1.01 ± 1.08 IL-6 (pg/mL) 3.56 ± 4.84 TNF-α (pg/mL)1.92 ± 0.5 | Isocaloric period: Fructose:−21.8% * Glucose: −11.4% * Hyper-caloric period: Fructose:−8.9% * Glucose:+40% * | Isocaloric period: Fructose:−4.2% * Glucose: −5.8% * Hyper-caloric period: Fructose:+23.8% * Glucose:−39.6% * | Isocaloric period: Fructose:−0.5% Glucose: −2.5% Hyper-caloric period: Fructose: −4.7% Glucose: −0.5% | N/A | N/A | N/A | N/A | No significant between-group change in CRP (p = 0.37), IL-6 (p = 0.23) or TNF-α (p = 0.36) in isocaloric or hypercaloric periods | Agency Industry—related conflict of interest |
| Lowndes et al. (2014) [38]USA | CRP (mg/L) HFCS 8%En intake: 1.9 ± 1.9 HFCS 18%En intake: 1.6 ± 1.6 HFCS 30%En intake: 2.1 ± 2.1 Sucrose 8%En intake: 1.5 ± 1.6 Sucrose 18%En intake: 2.0 ± 1.8 Sucrose 30%En intake: 1.5 ± 1.8 | HFCS: 8%En: +26.3% 18%En: +25% 30%En: 0% Sucrose: 8%En: +40% 18%En: +5% 30%En: +6.7% | N/A | N/A | N/A | N/A | N/A | N/A | No significant between-group change (HFCS vs. sucrose) (p = 0.679)No significant between-group changes in CRP between various intake amounts (8% vs. 18% vs. 30%) (p = 0.597) Percent increases in the 30%En groups were low/lowest. | Industry |
| Madero et al. (2011) [41] Mexico | sICAM (ng/dL) Low-fructose: 4.44 ± 0.11 Moderate-fructose: 4.37 ± 0.11 | N/A | N/A | N/A | N/A | Low-fructose: −6.3% Moderate-fructose: −9.6% | N/A | N/A | No significant between-group change in sICAM-1 (P = 0.19) Significant within-group decrease for sICAM-1 in low-fructose (p = 0.01) and moderate-fructose (p < 0.0001). Confounder (low-calorie diet → weight loss) | Agency |
| Markey et al. (2013) [45] UK | CRP (mg/L) Regular sugar intake: 0.93 ± 0.94 Reduced sugar intake: 1.05 ± 1.35 | Regular sugar: +6.5% Re-formulated sugar: +15.2% | N/A | N/A | N/A | N/A | N/A | N/A | No treatment effect for sucrose (p = 0.593) | Agency |
| Raatz et al. (2015) [42] USA | hsCRP (mg/L) Glucose tolerant (NGT): 2.2 ± 0.5 Glucose impaired (IGT): 4.6± 0.8 IL-6 (pg/mL) Glucose tolerant (NGT): 1.6 ± 0.2 Glucose impaired (IGT): 2.6 ± 0.5 | HFCS: NGT: −5% IGT: +29.6% Sucrose: NGT: −20% IGT: +15.8% Honey: NGT: +8.7% IGT: +41.2% | HFCS: NGT: +7.7% NGT: +6.7% Sucrose: IGT: −22.2% NGT: +3.5% Honey: NGT: +23.1% IGT: +19.4% | N/A | N/A | N/A | N/A | N/A | No treatment effect for hsCRP or IL-6. | Agency |
| Silbernagel et al. (2014) [36] Germany | CRP (mg/dL) 0.13 ± 0.06 MCP-1 (pg/mL) 275 ± 34 E-selectin (ng/mL) 31.8 ±5.1 | Fructose: −7.7% * Glucose: +57% * | N/A | N/A | Fructose: −16.7% * Glucose: −9.1% * | N/A | Fructose: −7.8% * Glucose: +3.5% * | N/A | No significant between-group change in CRP (P = 0.284), MCP-1 (p = 0.803) or E-selectin (p = 0.311) | Agency |
| Sorensen et al. (2005) [46]Denmark | CRP (mg/L) 1.8 (0.9–3.0) | Sucrose: +6% Artificial sweetener: −26% | N/A | N/A | N/A | N/A | N/A | N/A | No significant between-group change (p = 0.1) Percent changes reported after excluding 4 subjects with CRP > 10 mg/L | Industry |
| Yaghoobi et al. (2008) [44] Iran | hsCRP (mg/dL) Healthy subjects (normal hsCRP levels): 4.8 ± 3.2 Subjects with elevated hsCRP 9.9 ± 3.6 | Sucrose: −1% Honey: −3.3% | N/A | N/A | N/A | N/A | N/A | N/A | No significant between-group effect observed (p > 0.5). | Agency |
| hs(CRP) (mg/L) Intervention vs. Control | No. of Studies | No. of Participants | MD | 95% CI | p-Value | I2 (%) (95% CI) 1 | Quality of Meta-Evidence (NutriGrade) 2 |
|---|---|---|---|---|---|---|---|
| Fructose vs glucose | 6 | 403 | −0.03 | −0.52, 0.46 | (p = 0.90) | 44 (0, 75) | Low |
| HFCS vs sucrose | 3 | 677 | 0.21 | −0.11, 0.53 | (p = 0.19) | 0 (0, 75) | Low |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Corte, K.W.; Perrar, I.; Penczynski, K.J.; Schwingshackl, L.; Herder, C.; Buyken, A.E. Effect of Dietary Sugar Intake on Biomarkers of Subclinical Inflammation: A Systematic Review and Meta-Analysis of Intervention Studies. Nutrients 2018, 10, 606. https://doi.org/10.3390/nu10050606
Della Corte KW, Perrar I, Penczynski KJ, Schwingshackl L, Herder C, Buyken AE. Effect of Dietary Sugar Intake on Biomarkers of Subclinical Inflammation: A Systematic Review and Meta-Analysis of Intervention Studies. Nutrients. 2018; 10(5):606. https://doi.org/10.3390/nu10050606
Chicago/Turabian StyleDella Corte, Karen W., Ines Perrar, Katharina J. Penczynski, Lukas Schwingshackl, Christian Herder, and Anette E. Buyken. 2018. "Effect of Dietary Sugar Intake on Biomarkers of Subclinical Inflammation: A Systematic Review and Meta-Analysis of Intervention Studies" Nutrients 10, no. 5: 606. https://doi.org/10.3390/nu10050606
APA StyleDella Corte, K. W., Perrar, I., Penczynski, K. J., Schwingshackl, L., Herder, C., & Buyken, A. E. (2018). Effect of Dietary Sugar Intake on Biomarkers of Subclinical Inflammation: A Systematic Review and Meta-Analysis of Intervention Studies. Nutrients, 10(5), 606. https://doi.org/10.3390/nu10050606





