The Anti-Periodontitis Effects of Ethanol Extract Prepared Using Lactobacillus paracasei subsp. paracasei NTU 101
Abstract
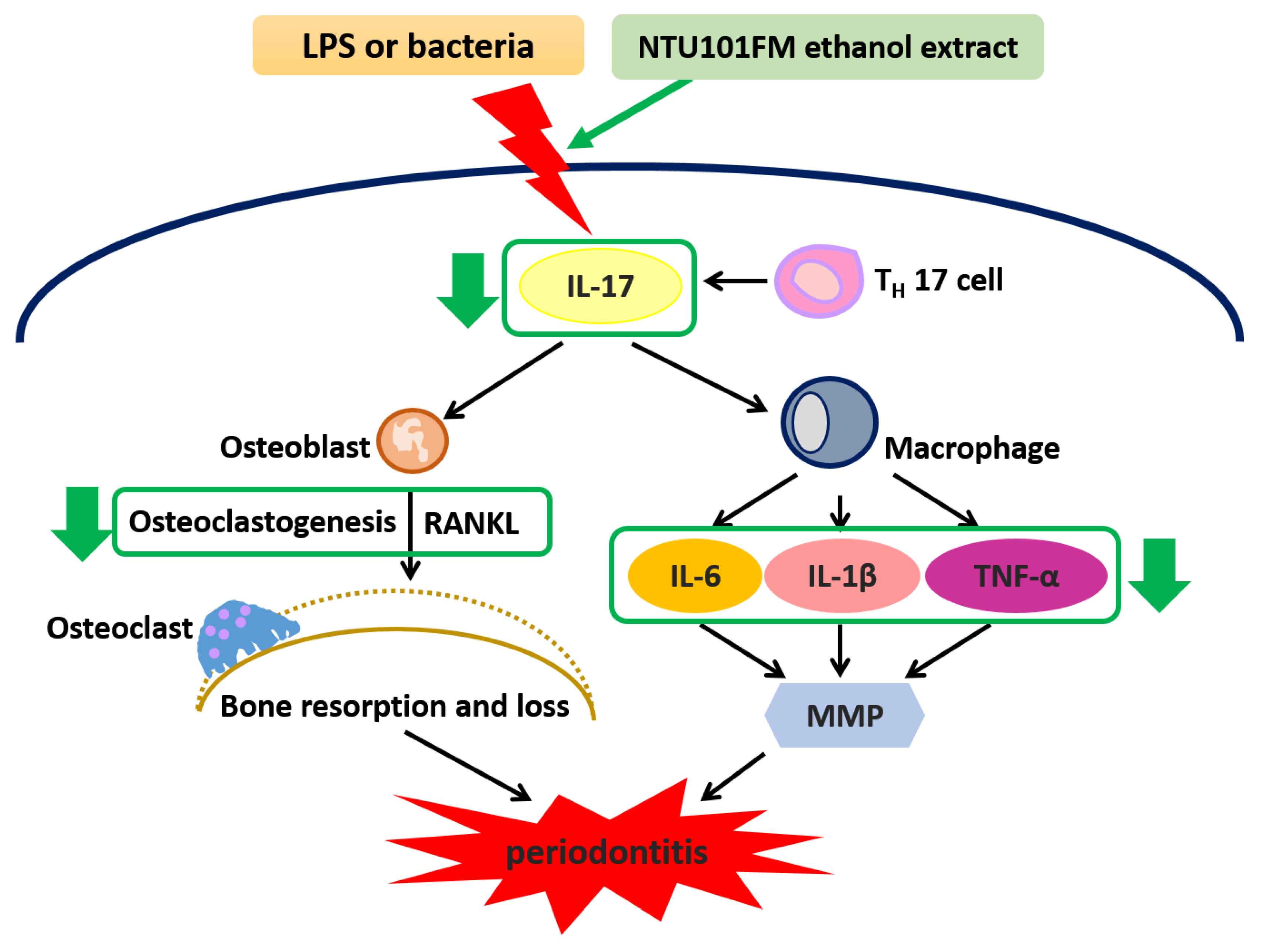
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fermented Skim Milk with NTU 101 and Extraction
2.3. Evaluation of the Anti-Oxidant Properties of NTU101FM
2.4. Anti-Microbial Activity of NTU101FM Ethanol Extract
2.5. Cell Culture and Cell Viability
2.6. Measurement of Nitric Oxide (NO) Production Levels
2.7. Measurement of Pro-Inflammatory Cytokine (IL-1β, IL-6, IL-17, and TNF-α) Levels
2.8. Osteoclast Differentiation, Tartrate-Resistant Acid Phosphatase (TRAP) Staining, and TRAP Activity
2.9. Assessment of Bone Resorptive Area by Pit Formation Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. The Anti-Oxidative Activities of NTU101FM
3.2. The Anti-Microbial Activities of NTU101FM
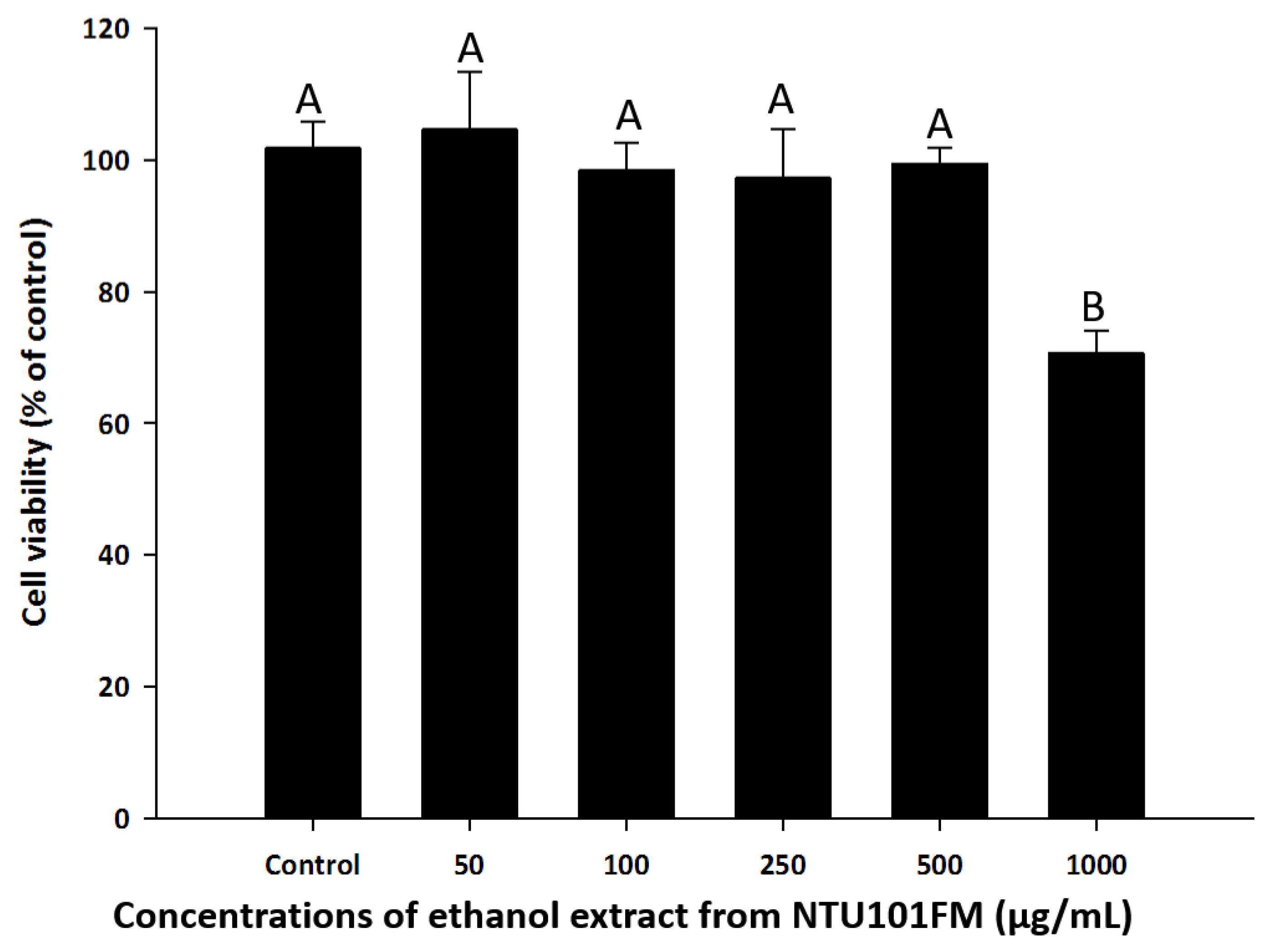
3.3. RAW 264.7 Cell Viability after Treatment with NTU101FM Ethanol Extract
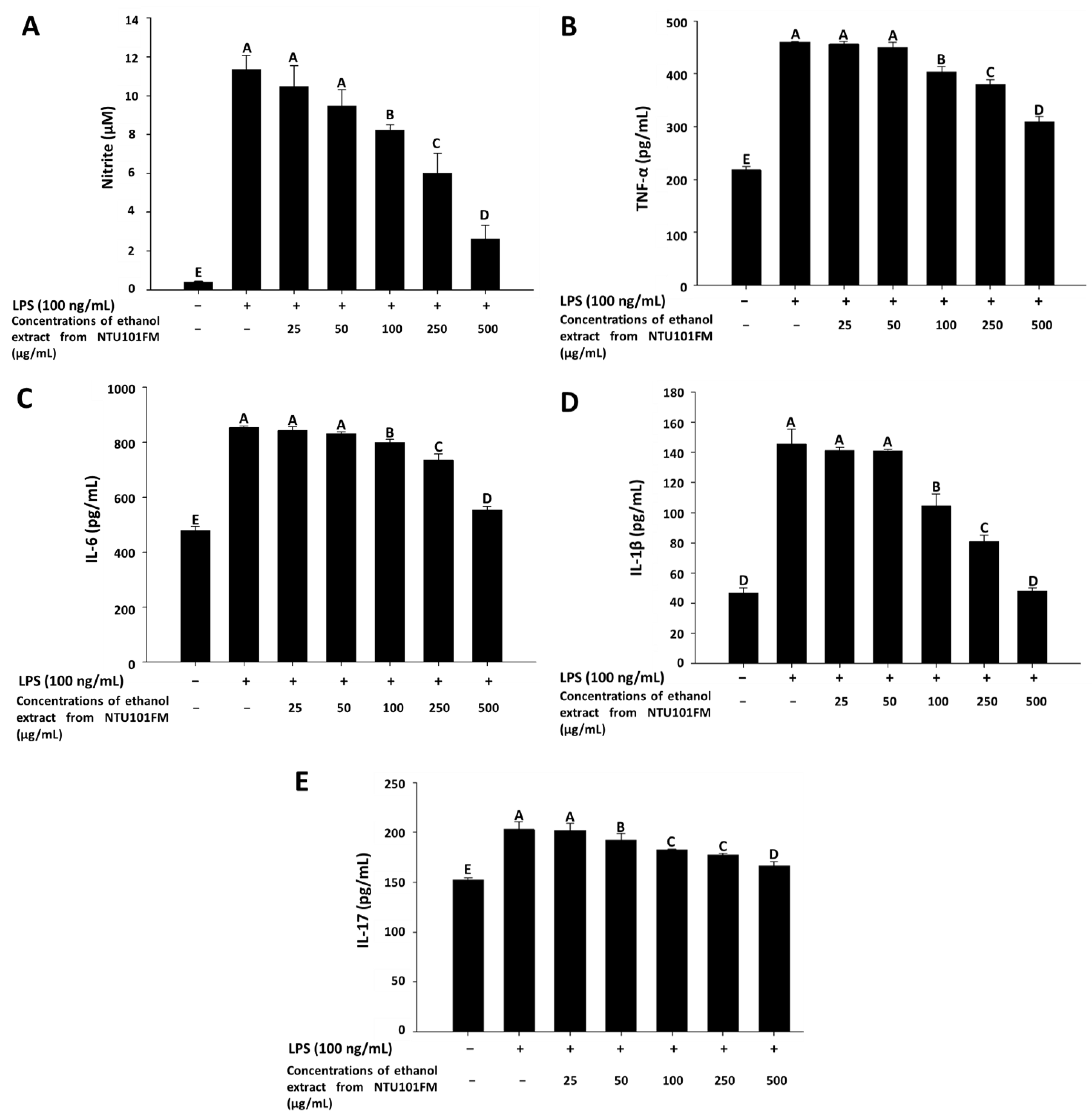
3.4. The Anti-Inflammatory Activities of NTU101FM Ethanol Extract on RAW 264.7 Cells
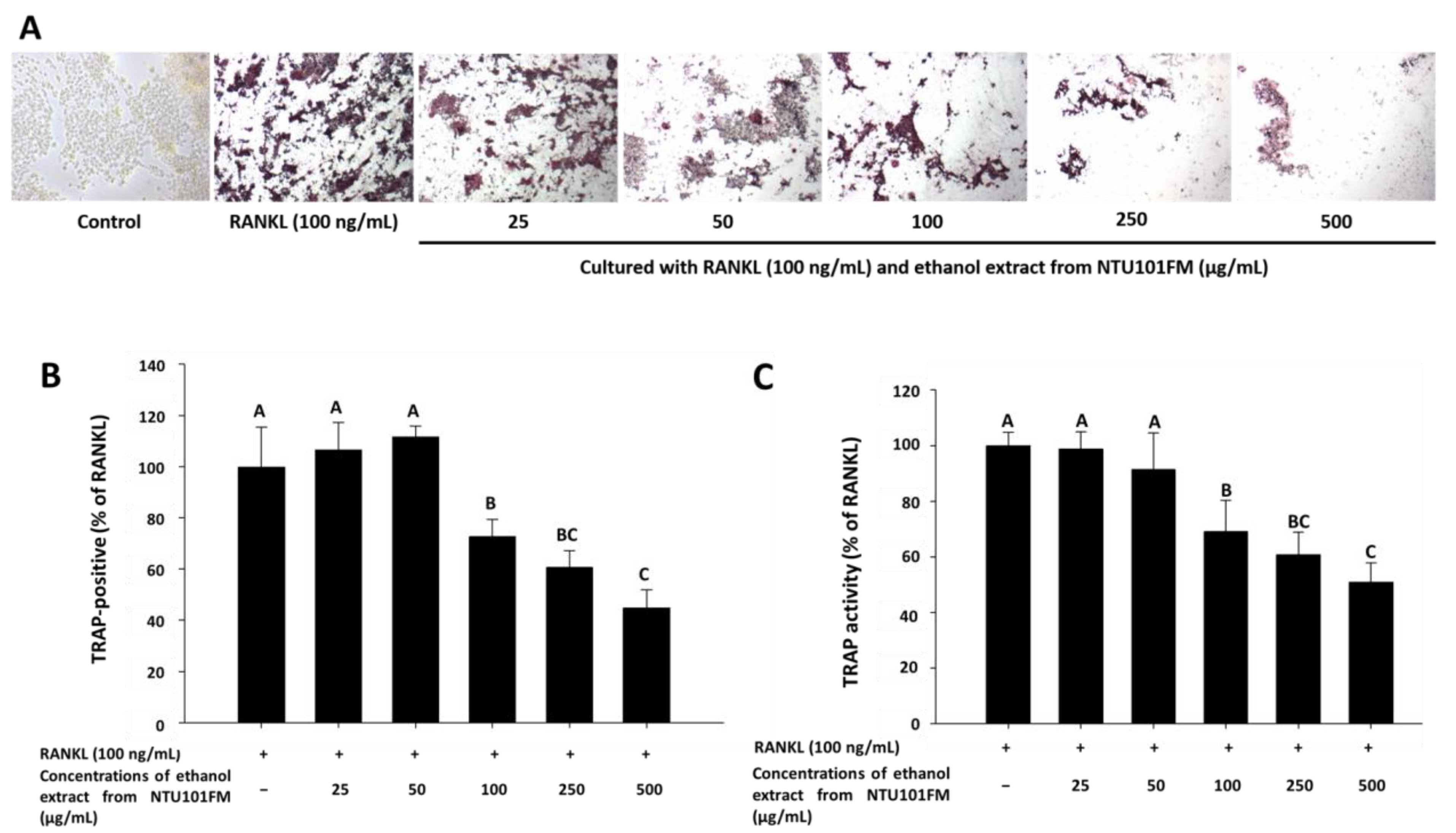
3.5. The Inhibitory Effects of NTU101FM Ethanol Extract on RANKL-Induced Osteoclastogenesis
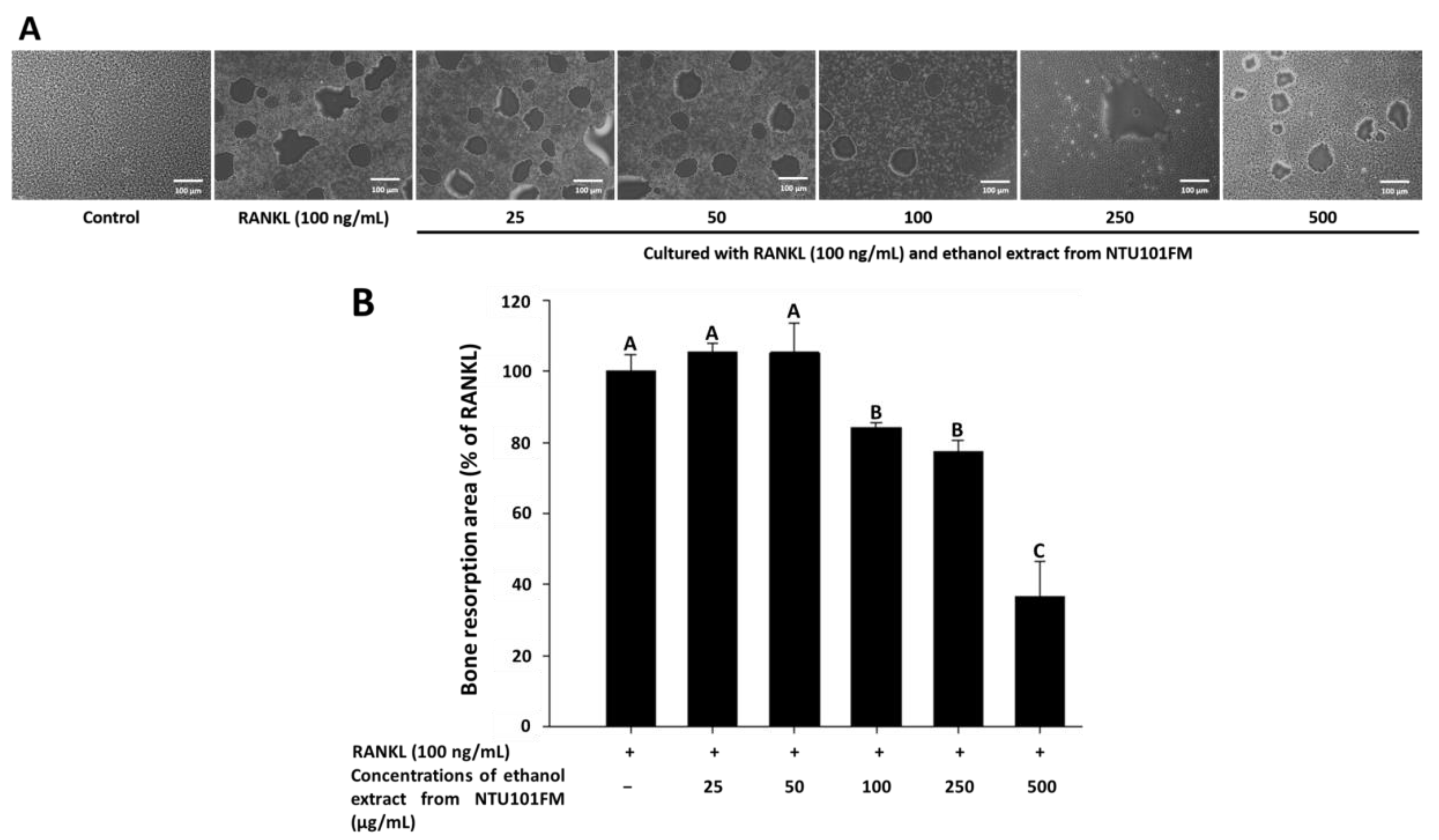
3.6. The Inhibitory Effects of NTU101FM Ethanol Extract on Bone Resorption
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABL | alveolar bone loss |
| ALP | alkaline phosphatase |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMSO | dimethyl sulfoxide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ELISA | enzyme-linked immunosorbent assay |
| FBS | fetal bovine serum |
| IL | interleukin |
| LAB | lactic acid bacteria |
| LPS | lipopolysaccharide |
| MBCs | minimum bactericidal concentrations |
| MICs | minimum inhibitory concentrations |
| MTT | 3-(4,5-dimethylthiazol)-2-yl-2,5-diphenyltetrazolium bromide |
| NO | nitric oxide |
| NTU 101 | Lactobacillus paracasei subsp. paracasei NTU 101 |
| NTU101FM | NTU 101-fermented skim milk |
| PBS | phosphate-buffered saline |
| RANKL | receptor activator of nuclear factor-κB ligand |
| ROS | reactive oxygen species |
| TCA | trichloroacetic acid |
| TNF | tumor necrosis factor |
| TRAP | tartrate-resistant acid phosphatase |
References
- Lindhe, J.; Ranney, R.; Lamster, I.; Charles, A.; Chung, C.P.; Flemmig, T.; Kinane, D.; Listgarten, M.; Löe, H.; Schoor, R.; et al. Consensus report: Chronic periodontitis. Ann. Periodontol. 1999, 4, 38. [Google Scholar] [CrossRef]
- Wiebe, C.B.; Putnins, E.E. The periodontal disease classification system of the American academy of periodontology—An update. J. Can. Dent. Assoc. 2000, 66, 594–597. [Google Scholar] [PubMed]
- Golpasand Hagh, L.; Zakavi, F.; Hajizadeh, F.; Saleki, M. The association between hyperlipidemia and periodontal infection. Iran. Red Crescent Med. J. 2014, 16, e6577. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tang, H.; Mattheos, N.; Yao, Y.; Jia, Y.; Ma, L.; Gong, P. In vivo osteoprotegerin gene therapy preventing bone loss induced by periodontitis. J. Periodontal. Res. 2015, 50, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Kobayashi, T.; Sakai, F.; Hosoya, T.; Yamamoto, M.; Kurita-Ochiai, T. Oral administration of Lactobacillus gasseri SBT2055 is effective in preventing Porphyromonas gingivalis-accelerated periodontal disease. Sci. Rep. 2017, 7, 545. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.; Ward, J.M.; Ready, D. Aggregatibacter (Actinobacillus) actinomycetemcomitans: A triple A* periodontopathogen? Periodontology 2000 2010, 54, 78–105. [Google Scholar] [CrossRef] [PubMed]
- Moradi, J.; Abbasipour, F.; Zaringhalam, J.; Maleki, B.; Ziaee, N.; Khodadoustan, A.; Janahmadi, M. Anethole, a medicinal plant compound, decreases the production of pro-inflammatory TNF-alpha and IL-1beta in a rat model of LPS-induced periodontitis. Iran. J. Pharm. Res. 2014, 13, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.A.; Lee, H.S.; Jung, Y.S.; Kim, S.W.; Lee, Y.W.; Chang, S.H.; Chung, H.J.; Kim, O.S.; Kim, Y.J. The effects of a novel botanical agent on lipopolysaccharide-induced alveolar bone loss in rats. J. Periodontol. 2013, 84, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.; Matthews, J.B. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontology 2000 2007, 43, 160–232. [Google Scholar] [CrossRef] [PubMed]
- Villa-Correa, Y.A.; Isaza-Guzman, D.M.; Tobon-Arroyave, S.I. Prognostic value of 8-hydroxy-2′-deoxyguanosine and human neutrophil elastase/alpha1-proteinase inhibitor complex as salivary biomarkers of oxidative stress in chronic periodontitis. J. Periodontol. 2015, 86, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, D.E.; Cha, J.H.; Bak, E.J.; Yoo, Y.J. Receptor activator of nuclear factor-κB ligand and sclerostin expression in osteocytes of alveolar bone in rats with ligature-induced periodontitis. J. Periodontol. 2014, 85, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Bizzini, B.; Pizzo, G.; Scapagnini, G.; Nuzzo, D.; Vasto, S. Probiotics and oral health. Curr. Pharm. Des. 2012, 18, 5522–5531. [Google Scholar] [CrossRef] [PubMed]
- Teanpaisan, R.; Dahlen, G. Use of polymerase chain reaction techniques and sodium dodecyl sulfate-polyacrylamide gel electrophoresis for differentiation of oral Lactobacillus species. Oral Microbiol. Immunol. 2006, 21, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Sookkhee, S.; Chulasiri, M.; Prachyabrued, W. Lactic acid bacteria from healthy oral cavity of Thai volunteers: Inhibition of oral pathogens. J. Appl. Microbiol. 2001, 90, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Krasse, P.; Carlsson, B.; Dahl, C.; Paulsson, A.; Nilsson, A.; Sinkiewicz, G. Decreased gum bleeding and reduced gingivitis by the probiotic Lactobacillus reuteri. Swed. Dent. J. 2006, 30, 55–60. [Google Scholar] [PubMed]
- Saha, S.; Tomaro-Duchesneau, C.; Tabrizian, M.; Prakash, S. Probiotics as oral health biotherapeutics. Expert Opin. Biol. Ther. 2012, 12, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.J.; Feng, X.P.; Zhang, X.L.; Le, K.Y. Effect of Porphyromonas gingivalis and Lactobacillus acidophilus on secretion of IL1β, IL6, and IL8 by gingival epithelial cells. Inflammation 2012, 35, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.M.; Chiu, C.H.; Pan, T.M. Fermentation of a milk-soymilk and Lycium chinense miller mixture using a new isolate of Lactobacillus paracasei subsp. paracasei NTU 101 and Bifidobacterium longum. J. Ind. Microbiol. Biotechnol. 2004, 31, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.F.; Pan, T.M. In vitro effects of lactic acid bacteria on cancer cell viability and antioxidant activity. J. Food Drug Anal. 2010, 18, 77–86. [Google Scholar]
- Chiang, S.S.; Pan, T.M. Beneficial effects of Lactobacillus paracasei subsp. paracasei NTU 101 and its fermented products. Appl. Microbiol. Biotechnol. 2012, 93, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.T.; Cheng, P.C.; Pan, T.M. The immunomodulatory effects of lactic acid bacteria for improving immune functions and benefits. Appl. Microbiol. Biotechnol. 2012, 96, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.H.; Pan, T.M. Inhibitory effect of Lactobacillus paracasei subsp. paracasei NTU 101 on rat dental caries. J. Funct. Foods 2014, 10, 223–231. [Google Scholar] [CrossRef]
- Lin, T.H.; Lin, C.H.; Pan, T.M. The implication of probiotics in the prevention of dental caries. Appl. Microbiol. Biotechnol. 2018, 102, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.T.; Tung, Y.T.; Chang, S.T. Antioxidant activities of ethanolic extracts from the twigs of Cinnamomum osmophloeum. Bioresour. Technol. 2008, 99, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Duh, P.D. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J. Agric. Food Chem. 1994, 42, 629–632. [Google Scholar] [CrossRef]
- Erdogan-Orhan, I.; Sever-Yilmaz, B.; Altun, M.L.; Saltan, G. Radical quenching activity, ferric-reducing antioxidant power, and ferrous ion-chelating capacity of 16 Ballota species and their total phenol and flavonoid contents. J. Med. Food 2010, 13, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Schillinger, U.; Lücke, F.K. Antibacterial activity of Lactobacillus sake isolated from meat. Appl. Environ. Microbiol. 1989, 55, 1901–1906. [Google Scholar] [PubMed]
- Kapadia, S.P.; Pudakalkatti, P.S.; Shivanaikar, S. Detection of antimicrobial activity of banana peel (Musa paradisiaca L.) on Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans: An in vitro study. Contemp. Clin. Dent. 2015, 6, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Venkatesalu, V. Antibacterial and antifungal activity of Syzygium jambolanum seeds. J. Ethnopharmacol. 2004, 91, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Studer, R.K.; Decker, K.; Melhem, S.; Georgescu, H. Nitric oxide inhibition of IGF-1 stimulated proteoglycan synthesis: Role of cGMP. J. Orthop. Res. 2003, 21, 914–921. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Hatipoglu, H.; Yaylak, F.; Gungor, Y. A brief review on the periodontal health in metabolic syndrome patients. Diabetes Metab. Syndr. 2015, 9, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Lo, M.T.; Wang, P.E.; Wang, T.T.; Chen, T.H.; Wu, G.H. A community-based epidemiological study of periodontal disease in Keelung, Taiwan: A model from Keelung community-based integrated screening programme (KCIS No. 18). J. Clin. Periodontol. 2007, 34, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, E.; Noro, K.; Yang, Z. Purification and identification of antimicrobial substances produced by two Lactobacillus casei strains. Int. Dairy J. 1995, 5, 503–513. [Google Scholar] [CrossRef]
- Pangsomboon, K.; Kaewnopparat, S.; Pitakpornpreecha, T.; Srichana, T. Antibacterial activity of a bacteriocin from Lactobacillus paracasei HL32 against Porphyromonas gingivalis. Arch. Oral Biol. 2006, 51, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Goldin, B.R. Health benefits of probiotics. Br. J. Nutr. 1998, 80, 203–207. [Google Scholar]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef] [PubMed]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S. Nitric oxide: Discovery and impact on clinical medicine. J. R. Soc. Med. 1999, 92, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.H.; Huang, R.Y.; Chou, T.C. Magnolol ameliorates ligature-induced periodontitis in rats and osteoclastogenesis: In vivo and in vitro study. Evid. Based Complement. Altern. Med. 2013, 2013, 634095. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Wei, C.; Zhou, L.; Qin, A.; Yang, M.; Tickner, J.; Huang, Y.; Zhao, J.; Xu, J. Luteoloside prevents lipopolysaccharide-induced osteolysis and suppresses RANKL-induced osteoclastogenesis through attenuating RANKL signaling cascades. J. Cell. Physiol. 2018, 233, 1723–1735. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhi, X.; Pan, P.; Cui, J.; Cao, L.; Weng, W.; Zhou, Q.; Wang, L.; Zhai, X.; Zhao, Q.; et al. Matrine prevents bone loss in ovariectomized mice by inhibiting RANKL-induced osteoclastogenesis. FASEB J. 2017, 31, 4855–4865. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.A.; Park, M.; Kim, Y.H.; Ryu, K.H.; Woo, S.Y. Mesenchymal stem cells inhibit RANKL-RANKL interactions between osteoclasts and TH17 cells via osteoprotegerin activity. Oncotarget 2017, 8, 83419–83431. [Google Scholar] [CrossRef] [PubMed]
- Ciucci, T.; Ibáñez, L.; Boucoiran, A.; Birgy-Barelli, E.; Pène, J.; Abou-Ezzi, G.; Arab, N.; Rouleau, M.; Hébuterne, X.; Yssel, H.; et al. Bone marrow TH17 TNFα cells induce osteoclast differentiation, and link bone destruction to IBD. Gut 2015, 64, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
| Concentration (mg/mL) | DPPH Eliminating Effect (%) | Reducing Power (OD700) | ||||||
|---|---|---|---|---|---|---|---|---|
| UF EE | UF WE | NTU 101 EE | NTU 101 WE | UF EE | UF WE | NTU 101 EE | NTU 101 WE | |
| 20.0 | 50.94 ± 2.06 Ac | 45.11 ± 4.24 Ac | 66.34 ± 3.57 Aa | 59.58 ± 4.17 Ab | 0.451 ± 0.03 Ac | 0.215 ± 0.01 Ad | 0.604 ± 0.02 Aa | 0.350 ± 0.01 Ab |
| 10.0 | 42.55 ± 1.51 Bc | 37.36 ± 1.03 Bd | 59.66 ± 1.64 Ba | 54.15 ± 1.55 Bb | 0.389 ± 0.00 Bb | 0.207 ± 0.00 ABd | 0.507 ± 0.02 Ba | 0.288 ± 0.01 Bc |
| 5.0 | 35.58 ± 0.57 Cc | 20.37 ± 0.92 Cd | 54.67 ± 1.65 Ca | 41.26 ± 1.70 Cb | 0.343 ± 0.00 Cb | 0.187 ± 0.00 Bd | 0.381 ± 0.00 Ca | 0.261 ± 0.01 BCc |
| 1.0 | 27.11 ± 2.49 Dc | N.D. | 45.36 ± 1.98 Da | 35.24 ± 1.01 Db | 0.276 ± 0.00 Da | 0.121 ± 0.01 Cb | 0.275 ± 0.01 Da | 0.251 ± 0.04 CDa |
| 0.1 | 16.06 ± 1.54 Ec | N.D. | 40.95 ± 2.09 Ea | 28.11 ± 2.21 Eb | 0.207 ± 0.00 Eb | 0.070 ± 0.02 Dc | 0.236 ± 0.01 Ea | 0.225 ± 0.01 Dab |
| Concentrations (mg/mL) | Inhibition Zone (Diameter, mm) a,b | |
|---|---|---|
| P. gingivalis BCRC 14417 | A. actinomycetemcomitans BCRC 14405 | |
| 200 | 16.50 ± 0.20 | 22.75 ± 0.35 |
| 100 | - | 19.50 ± 1.41 |
| 50 | - | 18.50 ± 0.71 |
| 25 | - | 11.75 ± 1.06 |
| Periodontal Pathogens | MIC (mg/mL) | MBC (mg/mL) |
|---|---|---|
| P. gingivalis BCRC 14417 | 30.00 | 30.00 |
| A. actinomycetemcomitans BCRC 14405 | 1.00 | 2.50 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.-H.; Tsai, T.-Y.; Pan, T.-M. The Anti-Periodontitis Effects of Ethanol Extract Prepared Using Lactobacillus paracasei subsp. paracasei NTU 101. Nutrients 2018, 10, 472. https://doi.org/10.3390/nu10040472
Liu T-H, Tsai T-Y, Pan T-M. The Anti-Periodontitis Effects of Ethanol Extract Prepared Using Lactobacillus paracasei subsp. paracasei NTU 101. Nutrients. 2018; 10(4):472. https://doi.org/10.3390/nu10040472
Chicago/Turabian StyleLiu, Te-Hua, Tsung-Yu Tsai, and Tzu-Ming Pan. 2018. "The Anti-Periodontitis Effects of Ethanol Extract Prepared Using Lactobacillus paracasei subsp. paracasei NTU 101" Nutrients 10, no. 4: 472. https://doi.org/10.3390/nu10040472
APA StyleLiu, T.-H., Tsai, T.-Y., & Pan, T.-M. (2018). The Anti-Periodontitis Effects of Ethanol Extract Prepared Using Lactobacillus paracasei subsp. paracasei NTU 101. Nutrients, 10(4), 472. https://doi.org/10.3390/nu10040472






