The In Ovo Feeding Administration (Gallus Gallus)—An Emerging In Vivo Approach to Assess Bioactive Compounds with Potential Nutritional Benefits
Abstract
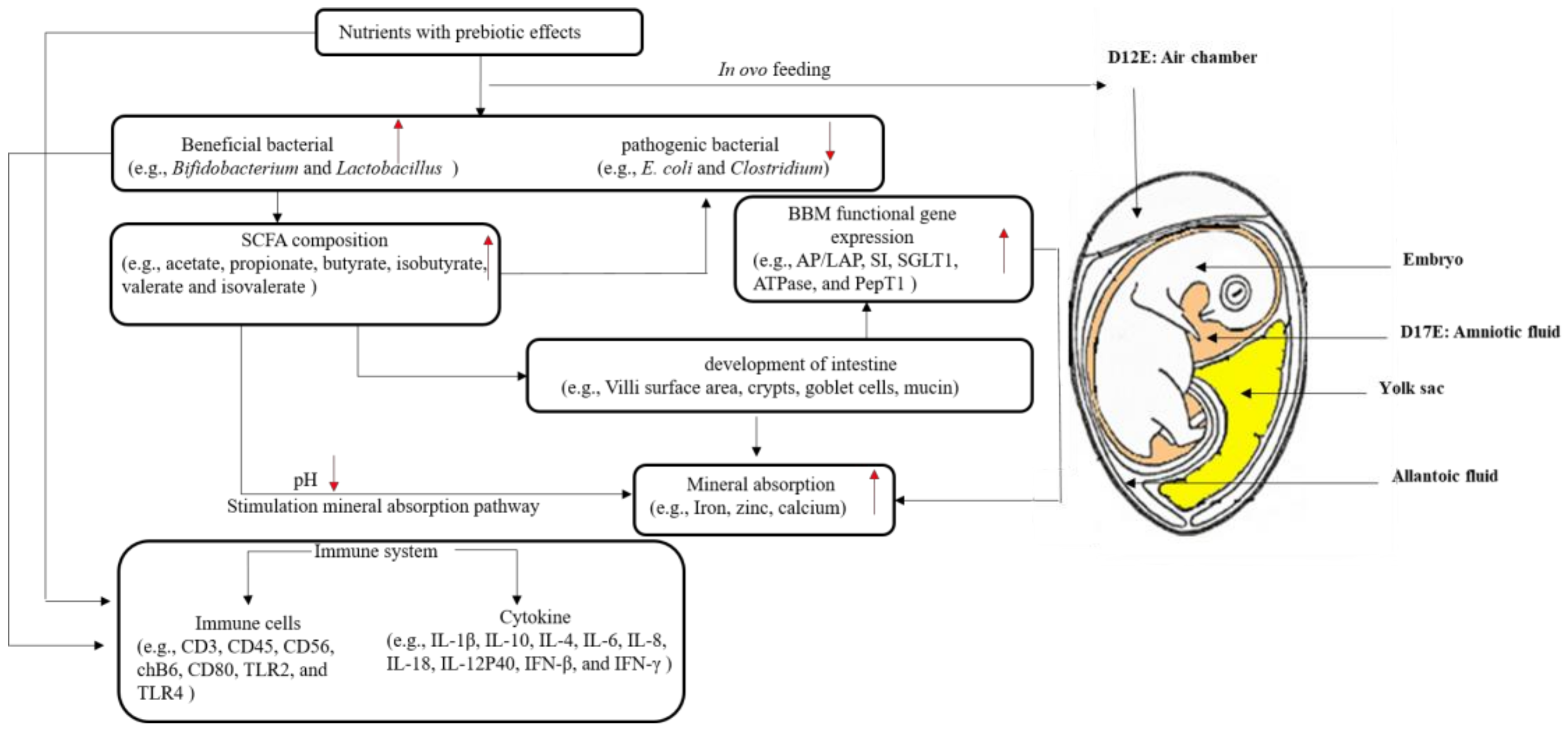
1. Introduction
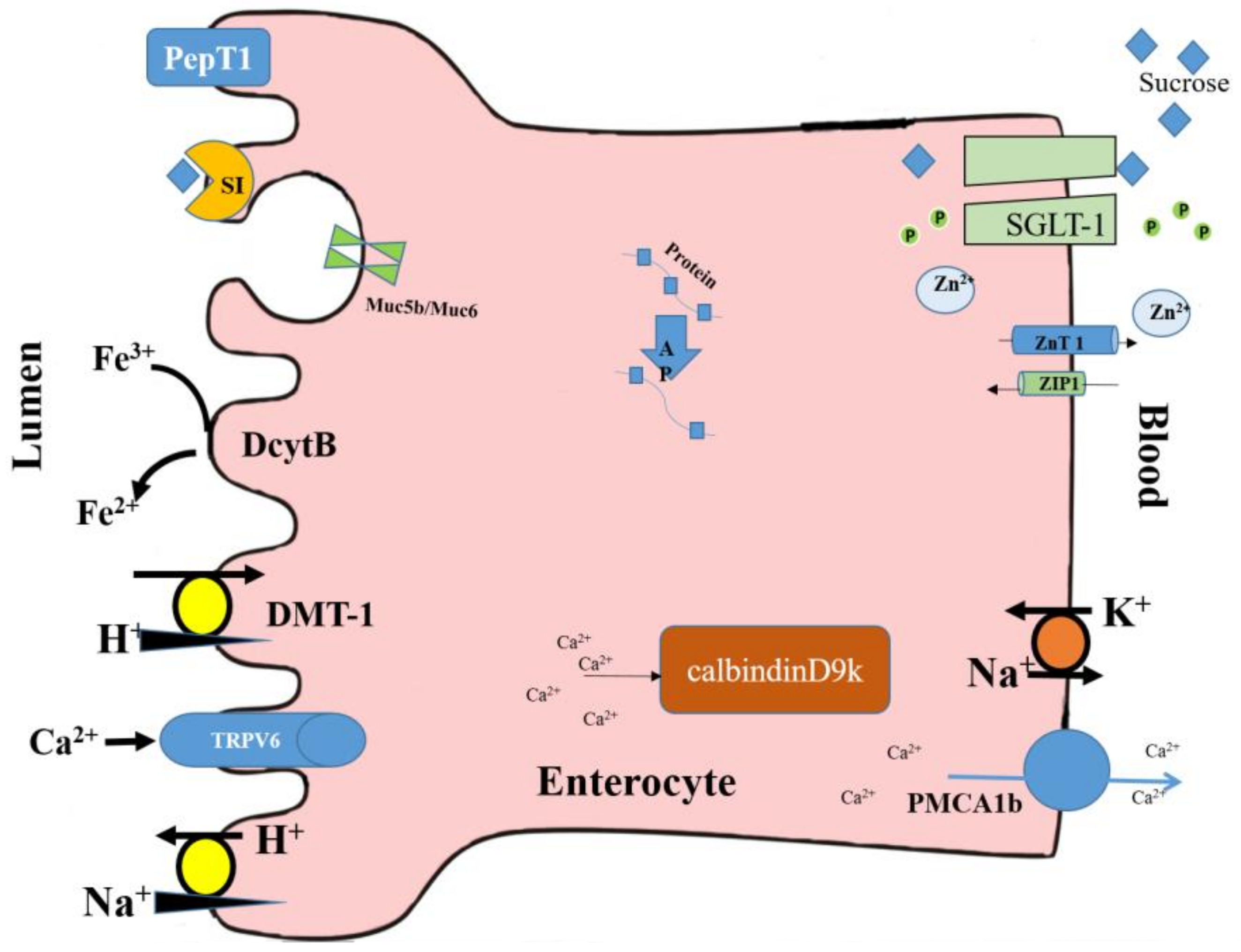
2. In Ovo Administration and Mineral Absorption
2.1. Iron Status
2.2. Zinc Status
2.3. Calcium Status
3. In Ovo Administration and Small Intestinal Morphology
3.1. Intestinal Morphometric Parameters
3.2. Microbial Populations
3.3. Short-Chain Fatty Acid Composition
3.4. Brush Border Membrane (BBM) Gene Expressions
4. In Ovo Administration and the Immune System
5. Conclusions and Future work
Author Contributions
Conflicts of Interest
References
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Yusof, R.M.; Hassan, F.A. Prebiotics as functional foods: A review. J. Funct. Foods 2013, 5, 1542–1553. [Google Scholar] [CrossRef]
- Orsolic, N.; Goluza, E.; Dikic, D.; Lisicic, D.; Sasilo, K.; Rodak, E.; Jelec, Z.; Lazarus, M.V.; Orct, T. Role of flavonoids on oxidative stress and mineral contents in the retinoic acid-induced bone loss model of rat. Eur. J. Nutr. 2014, 53, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Whisner, C.M.; Martin, B.R.; Nakatsu, C.H.; McCabe, G.P.; McCabe, L.D.; Peacock, M.; Weaver, C.M. Soluble maize fibre affects short-term calcium absorption in adolescent boys and girls: A randomised controlled trial using dual stable isotopic tracers. Br. J. Nutr. 2014, 112, 446–456. [Google Scholar] [CrossRef] [PubMed]
- García-Vieyra, M.I.; del Real, A.; López, M.G. Agave fructans: Their effect on mineral absorption and bone mineral content. J. Med. Food 2014, 17, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F. Functional foods: Their role in health promotion and disease prevention. J. Food Sci. 2004. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018, 245, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Srichaikul, K.; Wong, J.M.W.; Kendall, C.W.C.; Bashyam, B.; Vidgen, E.; Lamarche, B.; Rao, A.V.; Jones, P.J.H.; Josse, R.G.; et al. Supplemental barley protein and casein similarly affect serum lipids in hypercholesterolemic women and men. J. Nutr. 2010, 140, 1633–1637. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Jung, C.; Choi, H.; Kim, C.; Ha, H. Effectiveness of phosvitin peptides on enhancing bioavailability of calcium and its accumulation in bones. Food Chem. 2005, 93, 577–583. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Prebiotics and probiotics: Are they functional foods? Am. J. Chin. Nutr. 2000, 71, 1682S–1687S. [Google Scholar] [CrossRef]
- Catry, E.; Bindels, L.B.; Tailleux, A.; Lestavel, S.; Neyrinck, A.M.; Goossens, J.-F.; Lobysheva, I.; Plovier, H.; Essaghir, A.; Demoulin, J.-B.; et al. Targeting the gut microbiota with inulin-type fructans: Preclinical demonstration of a novel approach in the management of endothelial dysfunction. Gut 2018, 67, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Chunchai, T.; Thunapong, W.; Yasom, S.; Wanchai, K.; Eaimworawuthikul, S.; Metzler, G.; Lungkaphin, A.; Pongchaidecha, A.; Sirilun, S.; Chaiyasut, C.; et al. Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J. Neuroinflamm. 2018. [Google Scholar] [CrossRef] [PubMed]
- Park, S.F.; Kroll, R.G. Expression of listeriolysin and phosphatidylinositol-specific phospholipase C is repressed by the plant-derived molecule cellobiose in Listeria monocytogenes. Mol. Microbiol. 1993, 8, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Roller, M.; Rechkemmer, G.; Watzl, B. Prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis modulates intestinal immune functions in rats. J. Nutr. 2004, 134, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Bornet, F.; Brouns, F.; Tashiro, Y.; Duvillier, V. Nutritional aspects of short-chain fructooligosaccharides: Natural occurrence, chemistry, physiology and health implications. Dig. Liver Dis. 2002, 34, S111–S120. [Google Scholar] [CrossRef]
- Burns, A.; Rowland, I. Anti-carcinogenicity of probiotics and prebiotics. Curr. Issues Intest. Microbiol. 2000, 1, 13–24. [Google Scholar] [PubMed]
- McBain, A.; Macfarlane, G. Modulation of genotoxic enzyme activities by non-digestible oligosaccharide metabolism in in-vitro human gut bacterial ecosystems. J. Med. Microbiol. 2001, 50, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Bryk, G.; Coronel, M.Z.; Pellegrini, G.; Mandalunis, P.; Rio, M.E.; de Portela, M.L.P.M.; Zeni, S.N. Effect of a combination GOS/FOS® prebiotic mixture and interaction with calcium intake on mineral absorption and bone parameters in growing rats. Eur. J. Nutr. 2015, 54, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.B.; Cumps, J.; Devogelaer, J. Dietary chicory inulin increases whole-body bone mineral density in growing male rats. J. Nutr. 2002, 132, 3599–3602. [Google Scholar] [CrossRef] [PubMed]
- Tenorio, M.D.; Espinosa-Martos, I.; Préstamo, G.; Rupérez, P. Soybean whey enhance mineral balance and caecal fermentation in rats. Eur. J. Nutr. 2010, 49, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M. Functional food concept and its application to prebiotics. Dig. Liver Dis. 2002, 34, S105–S110. [Google Scholar] [CrossRef]
- Holloway, L.; Moynihan, S.; Abrams, S.A.; Kent, K.; Hsu, A.R.; Friedlander, A.L. Effects of oligofructose-enriched inulin on intestinal absorption of calcium and magnesium and bone turnover markers in postmenopausal women. Br. J. Nutr. 2007, 97, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Martin, B.R.; Nakatsu, C.H.; Armstrong, A.P.; Clavijo, A.; McCabe, L.D.; McCabe, G.P.; Duignan, S.; Schoterman, M.H.; van den Heuvel, E.G. Galactooligosaccharides improve mineral absorption and bone properties in growing rats through gut fermentation. J. Agric. Food Chem. 2011, 59, 6501–6510. [Google Scholar] [CrossRef] [PubMed]
- Le Blay, G.; Michel, C.; Blottière, H.M.; Cherbut, C. Prolonged intake of fructo-oligosaccharides induces a short-term elevation of lactic acid-producing bacteria and a persistent increase in cecal butyrate in rats. J. Nutr. 1999, 129, 2231–2235. [Google Scholar] [CrossRef] [PubMed]
- Kruger, M.C.; Brown, K.E.; Collett, G.; Layton, L.; Schollum, L.M. The effect of fructooligosaccharides with various degrees of polymerization on calcium bioavailability in the growing rat. Exp. Biol. Med. 2003, 228, 683–688. [Google Scholar] [CrossRef]
- Silvi, S.; Rumney, C.; Cresci, A.; Rowland, I. Resistant starch modifies gut microflora and microbial metabolism in human flora-associated rats inoculated with faeces from Italian and UK donors. J. Appl. Microbiol. 1999, 86, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, K.; Nakatsu, Y.; Sato, M.; Sedarnawati, Y.; Sugano, M. Effects of xylooligosaccharides on blood glucose, serum and liver lipids and cecum short-chain fatty acids in diabetic rats. Agric. Biol. Chem. 1991, 55, 199–205. [Google Scholar]
- Tahiri, M.; Tressol, J.C.; Arnaud, J.; Bornet, F.R.; Bouteloup-Demange, C.; Feillet-Coudray, C.; Brandolini, M.; Ducros, V.; Pépin, D.; Brouns, F.; et al. Effect of short-chain fructooligosaccharides on intestinal calcium absorption and calcium status in postmenopausal women: A stable-isotope study. Am. J. Chin. Nutr. 2003, 77, 449–457. [Google Scholar] [CrossRef]
- López-Huertas, E.; Teucher, B.; Boza, J.J.; Martínez-Férez, A.; Majsak-Newman, G.; Baró, L.; Carrero, J.J.; González-Santiago, M.; Fonollá, J.; Fairweather-Tait, S. Absorption of calcium from milks enriched with fructo-oligosaccharides, caseinophosphopeptides, tricalcium phosphate, and milk solids. Am. J. Chin. Nutr. 2006, 83, 310–316. [Google Scholar] [CrossRef]
- Griffin, I.; Davila, P.; Abrams, S. Non-digestible oligosaccharides and calcium absorption in girls with adequate calcium intakes. Br. J. Nutr. 2002, 87, S187–S191. [Google Scholar] [CrossRef] [PubMed]
- Abrams, S.A.; Griffin, I.J.; Hawthorne, K.M.; Liang, L.; Gunn, S.K.; Darlington, G.; Ellis, K.J. A combination of prebiotic short- and long-chain inulin-type fructans enhances calcium absorption and bone mineralization in young adolescents. Am. J. Chin. Nutr. 2005, 82, 471–476. [Google Scholar] [CrossRef]
- Ito, M.; Deguchi, Y.; Miyamori, A.; Matsumoto, K.; Kikuchi, H.; Matsumoto, K.; Kobayashi, Y.; Yajima, T.; Kan, T. Effects of administration of galactooligosaccharides on the human faecal microflora, stool weight and abdominal sensation. Microb. Ecol. Health Dis. 1990, 3, 285–292. [Google Scholar] [CrossRef]
- Sharma, J.; Burmester, B. Resistance of marek’s disease at hatching in chickens vaccinated as embryos with the turkey herpesvirus. Avian. Dis. 1982, 26, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Kadam, M.M.; Barekatain, M.R.; K Bhanja, S.; Iji, P.A. Prospects of in ovo feeding and nutrient supplementation for poultry: The science and commercial applications—A review. J. Sci. Food Agric. 2013, 93, 3654–3661. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Kidd, M. Optimum site for in ovo amino acid injection in broiler breeder eggs. Poult. Sci. 2001, 80, 1425–1429. [Google Scholar]
- Tako, E.; Ferket, P.; Uni, Z. Effects of in ovo feeding of carbohydrates and beta-hydroxy-beta-methylbutyrate on the development of chicken intestine. Poult. Sci. 2004, 83, 2023–2028. [Google Scholar] [CrossRef] [PubMed]
- Effect of in Ovo Injection of Vitamins on the Chick Weight and Post-Hatch Growth Performance in Broiler Chickens. Available online: https://www.cabi.org/animalscience/worlds-poultry-science-association-wpsa/wpsa-france-2007/ (accessed on 24 February 2018).
- Sobolewska, A.; Elminowska-Wenda, G.; Bogucka, J.; Dankowiakowska, A.; Kułakowska, A.; Szczerba, A.; Stadnicka, K.; Szpinda, M.; Bednarczyk, M. The influence of in ovo injection with the prebiotic DiNovo® on the development of histomorphological parameters of the duodenum, body mass and productivity in large-scale poultry production conditions. J. Anim. Sci. Biotechnol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Villaluenga, C.M.; Wardeńska, M.; Pilarski, R.; Bednarczyk, M.; Gulewicz, K. Utilization of the chicken embryo model for assessment of biological activity of different oligosaccharides. Folia Boil. 2004, 52, 135–142. [Google Scholar] [CrossRef]
- Uni, Z.; Ferket, P.R. Enhancement of Development of Oviparous Species by in Ovo Feeding. U.S. Patent 6,592,878, 15 July 2003. [Google Scholar]
- Salahi, A.; Mozhdeh, M.; Seyed, N. Optimum time of in ovo injection in eggs of young broiler breeder flock. In Proceedings of the 18th Eur. Symp. on Poultry Nutrition, Izmir, Turkey, 31 October–4 November 2011; World’s Poultry Science Association: Izmir, Turkey, 2011; pp. 557–559. [Google Scholar]
- Pacifici, S.; Song, J.; Zhang, C.; Wang, Q.; Glahn, R.P.; Kolba, N.; Tako, E. Intra amniotic administration of raffinose and stachyose affects the intestinal brush border functionality and alters gut microflora populations. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Kolba, N.; Glahn, R.P.; Tako, E. Intra-amniotic administration (Gallus Gallus) of cicer arietinum and lens culinaris prebiotics extracts and duck egg white peptides affects calcium status and intestinal functionality. Nutrients 2017, 9. [Google Scholar] [CrossRef]
- Bednarczyk, M.; Stadnicka, K.; Kozłowska, I.; Abiuso, C.; Tavaniello, S.; Dankowiakowska, A.; Sławińska, A.; Maiorano, G. Influence of different prebiotics and mode of their administration on broiler chicken performance. Animal 2016, 10, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, G.; Stadnicka, K.; Tavaniello, S.; Abiuso, C.; Bogucka, J.; Bednarczyk, M. In ovo validation model to assess the efficacy of commercial prebiotics on broiler performance and oxidative stability of meat. Poult. Sci. 2017, 96, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Slawinska, A.; Plowiec, A.; Siwek, M.; Jaroszewski, M.; Bednarczyk, M. Long-term transcriptomic effects of prebiotics and synbiotics delivered in ovo in broiler chickens. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Berrocoso, J.; Kida, R.; Singh, A.; Kim, Y.; Jha, R. Effect of in ovo injection of raffinose on growth performance and gut health parameters of broiler chicken. Poult. Sci. 2016, 96, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Zarei, A.; Morovat, M.; Chamani, M.; Sadeghi, A.; Dadvar, P. Effect of in ovo feeding and dietary feeding of silybum marianum extract on performance, immunity and blood cation-anion balance of broiler chickens exposed to high temperatures. Iran. J. Appl. Anim. Sci. 2016, 6, 697–705. [Google Scholar]
- Calik, A.; Ceylan, A.; Ekim, B.; Adabi, S.G.; Dilber, F.; Bayraktaroglu, A.G.; Tekinay, T.; Özen, D.; Sacakli, P. The effect of intra-amniotic and posthatch dietary synbiotic administration on the performance, intestinal histomorphology, cecal microbial population, and short-chain fatty acid composition of broiler chickens. Poult. Sci. 2016, 96, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Miśta, D.; Króliczewska, B.; Pecka-Kiełb, E.; Kapuśniak, V.; Zawadzki, W.; Graczyk, S.; Kowalczyk, A.; Łukaszewicz, E.; Bednarczyk, M. Effect of in ovo injected prebiotics and synbiotics on the caecal fermentation and intestinal morphology of broiler chickens. Anim. Prod. Sci. 2016. [Google Scholar] [CrossRef]
- Płowiec, A.; Sławińska, A.; Siwek, M.Z.; Bednarczyk, M.F. Effect of in ovo administration of inulin and Lactococcus lactis on immune-related gene expression in broiler chickens. Am. J. Vet. Res. 2015, 76, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Pruszynska-Oszmalek, E.; Kolodziejski, P.; Stadnicka, K.; Sassek, M.; Chalupka, D.; Kuston, B.; Nogowski, L.; Mackowiak, P.; Maiorano, G.; Jankowski, J.; et al. In ovo injection of prebiotics and synbiotics affects the digestive potency of the pancreas in growing chickens. Poult. Sci. 2015, 94, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Tako, E.; Glahn, R.P.; Knez, M.; Stangoulis, J.C.R. The effect of wheat prebiotics on the gut bacterial population and iron status of iron deficient broiler chickens. Nutr. J. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Hartono, K.; Reed, S.; Ankrah, N.A.; Glahn, R.P.; Tako, E. Alterations in gut microflora populations and brush border functionality following intra-amniotic daidzein administration. RSC Adv. 2015, 5, 6407–6412. [Google Scholar] [CrossRef]
- Tako, E.; Glahn, R. Intra-amniotic administration and dietary inulin affect the iron status and intestinal functionality of iron-deficient broiler chickens. Poult. Sci. 2012, 91, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, G.; Sobolewska, A.; Cianciullo, D.; Walasik, K.; Elminowska-Wenda, G.; Sławińska, A.; Tavaniello, S.; Żylińska, J.; Bardowski, J.; Bednarczyk, M. Influence of in ovo prebiotic and synbiotic administration on meat quality of broiler chickens. Poult. Sci. 2012, 91, 2963–2969. [Google Scholar] [CrossRef] [PubMed]
- Cheled-Shoval, S.; Amit-Romach, E.; Barbakov, M.; Uni, Z. The effect of in ovo administration of mannan oligosaccharide on small intestine development during the pre-and posthatch periods in chickens. Poult. Sci. 2011, 90, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, A.; Tako, E.; Ferket, P.; Uni, Z. Mucin gene expression and mucin content in the chicken intestinal goblet cells are affected by in ovo feeding of carbohydrates. Poult. Sci. 2006, 85, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Tako, E.; Ferket, P.R.; Uni, Z. Changes in chicken intestinal zinc exporter mRNA expression and small intestinal functionality following intra-amniotic zinc-methionine administration. J. Nutr. Biochem. 2005, 16, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.; Rocha, S.; Fernandes, R. Iron metabolism: From health to disease. J. Chin. Lab. Anal. 2014, 28, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, M.D.S.B.; Campos Hankins, N.A.; Arruda, S.F. Effect of vitamin A supplementation on iron status in humans: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Young, I.; Parker, H.M.; Rangan, A.; Prvan, T.; Cook, R.L.; Donges, C.E.; Steinbeck, K.S.; O’Dwyer, N.J.; Cheng, H.L.; Franklin, J.L.; et al. Association between haem and non-haem iron intake and serum ferritin in healthy young women. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, D.; Sun, P.; Bucheli, P.; Li, L.; Hou, Y.; Wang, J. Effects of soluble tea polysaccharides on hyperglycemia in alloxan-diabetic mice. J. Agric. Food Chem. 2007, 55, 5523–5528. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Sahrawat, K.; Puppala, N.; Ortiz, R. Plant prebiotics and human health: Biotechnology to breed prebiotic-rich nutritious food crops. Electr. J. Biotechnol. 2014, 17, 238–245. [Google Scholar] [CrossRef]
- Cian, R.E.; Garzon, A.G.; Betancur Ancona, D.; Chel Guerrero, L.; Drago, S.R. Chelating properties of peptides from red seaweed pyropia columbina and its effect on iron bio-accessibility. Plant. Foods Hum. Nutr. 2016, 71, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Eckert, E.; Lu, L.; Unsworth, L.D.; Chen, L.; Xie, J.; Xu, R. Biophysical and in vitro absorption studies of iron chelating peptide from barley proteins. J. Funct. Foods 2016, 25, 291–301. [Google Scholar] [CrossRef]
- Zhou, J.; Mao, X.-Y.; Wang, X.; Ai, T.; Ma, J.-J.; Li, Y.-H. Anti-anaemia efficacy of β-lactoglobulin hydrolysate-iron complex on iron-deficient anaemic rats. Eur. J. Nutr. 2014, 53, 877–884. [Google Scholar] [CrossRef] [PubMed]
- De Cássia Freitas, K.; Amancio, O.M.S.; de Morais, M.B. High-performance inulin and oligofructose prebiotics increase the intestinal absorption of iron in rats with iron deficiency anaemia during the growth phase. Br. J. Nutr. 2012, 108, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Tako, E.; Rutzke, M.; Glahn, R. Using the domestic chicken (Gallus Gallus) as an in vivo model for iron bioavailability. Poult. Sci. 2010, 89, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Gangola, M.P.; Jaiswal, S.; Kannan, U.; Gaur, P.M.; Baga, M.; Chibbar, R.N. Galactinol synthase enzyme activity influences raffinose family oligosaccharides (RFO) accumulation in developing chickpea (Cicer arietinum L.) seeds. Phytochemistry 2016, 125, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Lindeboom, N.; Baga, M.; Vandenberg, A.; Chibbar, R.N. Composition and correlation between major seed constituents in selected lentil (Lens culinaris. Medik) genotypes. Can. J. Plant. Sci. 2011, 91, 825–835. [Google Scholar] [CrossRef]
- Gaither, L.A.; Eide, D.J. Eukaryotic zinc transporters and their regulation. In Zinc Biochemistry, Physiology, and Homeostasis; Springer: Dordrecht, The Netherlands, 2001; pp. 65–84. [Google Scholar]
- Salgueiro, M.A.J.; Zubillaga, M.B.; Lysionek, A.E.; Caro, R.A.; Weill, R.; Boccio, J.R. The role of zinc in the growth and development of children. Nutrition 2002, 18, 510–519. [Google Scholar] [CrossRef]
- Tian, K.; Wang, Y.-X.; Li, L.-X.; Liu, Y.-Q. Neuronal death/apoptosis induced by intracellular zinc deficiency associated with changes in amino-acid neurotransmitters and glutamate receptor subtypes. J. Inorg. Biochem. 2018, 179, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Trijatmiko, K.R.; Dueñas, C.; Tsakirpaloglou, N.; Torrizo, L.; Arines, F.M.; Adeva, C.; Balindong, J.; Oliva, N.; Sapasap, M.V.; Borrero, J.; et al. Biofortified indica rice attains iron and zinc nutrition dietary targets in the field. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Gaither, L.A.; Eide, D.J. Functional expression of the human hzip2 zinc transporter. J. Biol. Chem. 2000, 275, 5560–5564. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D.; Findley, S.D. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J. 1995, 14, 639–649. [Google Scholar] [PubMed]
- Reed, S.; Neuman, H.; Moscovich, S.; Glahn, R.P.; Koren, O.; Tako, E. Chronic zinc deficiency alters chick gut microbiota composition and function. Nutrients 2015, 7, 9768–9784. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.; Qin, X.; Ran-Ressler, R.; Brenna, J.T.; Glahn, R.P.; Tako, E. Dietary zinc deficiency affects blood linoleic acid: Dihomo-γ-linolenic acid (la: Dgla) ratio; a sensitive physiological marker of zinc status in vivo (Gallus Gallus). Nutrients 2014, 6, 1164–1180. [Google Scholar] [CrossRef] [PubMed]
- Knez, M.; Tako, E.; Glahn, R.P.; Kolba, N.; de Courcy-Ireland, E.; Stangoulis, J.C.R. Linoleic acid: Dihomo-γ-linolenic acid ratio predicts the efficacy of Zn-biofortified wheat in chicken (Gallus Gallus). J. Agric. Food Chem. 2018, 66, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Purali, N. Fast calcium transients translate the distribution and conduction of neural activity in different regions of a single sensory neuron. Invert. Neurosci. 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Noda, K.; Yang, Y.; Shen, Z.; Chen, Z.; Ogata, Y. Calcium hydroxide regulates transcription of the bone sialoprotein gene via a calcium-sensing receptor in osteoblast-like ROS 17/2.8 cells. Eur. J. Oral Sci. 2018, 126, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Liu, W.; Shi, W.; Ma, Z.; He, H. Desalted duck egg white peptides promote calcium uptake by counteracting the adverse effects of phytic acid. Food Chem. 2017, 219, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.C.; Barbosa Castro, A.S.; Rodrigues, V.C.; Fernandes, S.A.; Filomeno Fontes, E.A.; de Oliveira, T.T.; Duarte Martino, H.S.; de Luces Fortes Ferreira, C.L. Yacon flour and bifidobacterium longum modulate bone health in rats. J. Med. Food 2012, 15, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Scholz-Ahrens, K.E.; Ade, P.; Marten, B.; Weber, P.; Timm, W.; Aςil, Y.; Glüer, C.-C.; Schrezenmeir, J. Prebiotics, probiotics, and synbiotics affect mineral absorption, bone mineral content, and bone structure. J. Nutr. 2007, 137, 838S–846S. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zeng, T.; Wang, S.-E.; Wang, W.; Wang, Q.; Yu, H.-X. Fructo-oligosaccharides enhance the mineral absorption and counteract the adverse effects of phytic acid in mice. Nutrition 2010, 26, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Scholz-Ahrens, K.E.; Schaafsma, G.; van den Heuvel, E.G.; Schrezenmeir, J. Effects of prebiotics on mineral metabolism. Am. J. Chin. Nutr. 2001, 73, 459s–464s. [Google Scholar] [CrossRef]
- Scholz-Ahrens, K.E.; Schrezenmeir, J. Inulin, oligofructose and mineral metabolism-experimental data and mechanism. Br. J. Nutr. 2002, 87, 179S–S186. [Google Scholar] [CrossRef]
- Afsana, K.; Shiga, K.; Ishizuka, S.; Hara, H. Ingestion of an indigestible saccharide, difructose anhydride III, partially prevents the tannic acid-induced suppression of iron absorption in rats. J. Nutr. 2003, 133, 3553–3560. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Whisner, C.M.; Castillo, L.F. Prebiotics, bone and mineral metabolism. Calcif. Tissue Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- McCabe, L.; Britton, R.A.; Parameswaran, N. Prebiotic and probiotic regulation of bone health: Role of the intestine and its microbiome. Curr. Osteoporos. Rep. 2015, 13, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.-E. Review on chicken intestinal villus histological alterations related with intestinal function. J. Poult. Sci. 2002, 39, 229–242. [Google Scholar] [CrossRef]
- Potten, C.S.; Loeffler, M. A comprehensive model of the crypts of the small intestine of the mouse provides insight into the mechanisms of cell migration and the proliferation hierarchy. J. Theor. Biol. 1987, 127, 381–391. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, C.; Xia, M.; Zhan, X.; Wang, M. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003, 82, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Croom, J.; Christensen, V.; Black, B.; Bird, A.; Daniel, L.; McBride, B.; Eisen, E. Jejunal glucose uptake and oxygen consumption in turkey poults selected for rapid growth. Poult. Sci. 1997, 76, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Samanya, M.; Yamauchi, K.-E. Histological alterations of intestinal villi in chickens fed dried bacillus subtilis var. Natto. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 133, 95–104. [Google Scholar] [CrossRef]
- Bäckhed, F.; Fraser, C.M.; Ringel, Y.; Sanders, M.E.; Sartor, R.B.; Sherman, P.M.; Versalovic, J.; Young, V.; Finlay, B.B. Defining a healthy human gut microbiome: Current concepts, future directions, and clinical applications. Cell. Host Microb. 2012, 12, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-K.; Liao, J.-W.; Chung, Y.-C.; Hsieh, C.-P.; Chan, Y.-C. Xylooligosaccharides and fructooligosaccharides affect the intestinal microbiota and precancerous colonic lesion development in rats. J. Nutr. 2004, 134, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; van den Brandt, P.A.; Stobberingh, E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Consortium, H.M.P. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486. [Google Scholar] [CrossRef]
- Partida-Rodriguez, O.; Serrano-Vazquez, A.; Nieves-Ramirez, M.E.; Moran, P.; Rojas, L.; Portillo, T.; Gonzalez, E.; Hernandez, E.; Finlay, B.B.; Ximenez, C. Human intestinal microbiota: Interaction between parasites and the host immune response. Arch. Med. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, D.; Refaey, M.M.; Xu, W. High spatial and temporal variations of microbial community along the southern catfish gastrointestinal tract: Insights into dynamic food digestion. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Zhong, T.; Pandya, Y.; Joerger, R.D. 16s rRNA-based analysis of microbiota from the cecum of broiler chickens. Appl. Environ. Microbiol. 2002, 68, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Yegani, M.; Korver, D.R. Factors affecting intestinal health in poultry. Poult. Sci. 2008, 87, 2052–2063. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Chin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Lutz, T.; Scharrer, E. Effect of short-chain fatty acids on calcium absorption by the rat colon. Exp. Physiol. 1991, 76, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Martin, B.R.; Story, J.A.; Hutchinson, I.; Sanders, L. Novel fibers increase bone calcium content and strength beyond efficiency of large intestine fermentation. J. Agric. Food Chem. 2010, 58, 8952–8957. [Google Scholar] [CrossRef] [PubMed]
- Trinidad, T.P.; Wolever, T.; Thompson, L.U. Effect of acetate and propionate on calcium absorption from the rectum and distal colon of humans. Am. J. Chin. Nutr. 1996, 63, 574–578. [Google Scholar] [CrossRef]
- Yang, L.-C.; Wu, J.-B.; Lu, T.-J.; Lin, W.-C. The prebiotic effect of anoectochilus formosanus and its consequences on bone health. Br. J. Nutr. 2013, 109, 1779–1788. [Google Scholar] [CrossRef]
- Rinttilä, T.; Apajalahti, J. Intestinal microbiota and metabolites—Implications for broiler chicken health and performance1. J. Appl. Poult. Res. 2013, 22, 647–658. [Google Scholar] [CrossRef]
- Uni, Z.; Tako, E.; Gal-Garber, O.; Sklan, D. Morphological, molecular, and functional changes in the chicken small intestine of the late-term embryo. Poult. Sci. 2003, 82, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Noy, Y.; Sklan, D. Yolk utilisation in the newly hatched poult. Br. Poult. Sci. 1998, 39, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Modulation of Immunity Genes through in Ovo Supplemented Amino Acids in Broiler Chickens. Available online: https://www.researchgate.net/publication/258255600_Modulation_of_Immunity_Genes_through_in_ovo_Supplemented_Amino_Acids_in_Broiler_Chickens (accessed on 26 March 2018).
- Bhattacharya, D.; Boppana, V.; Roy, R.; Roy, J. Method for Automated Design of Integrated Circuits with Targeted Quality Objectives Using Dynamically Generated Building Blocks. U.S. Patent 7,225,423, 29 May 2007. [Google Scholar]
- Schley, P.; Field, C. The immune-enhancing effects of dietary fibres and prebiotics. Br. J. Nutr. 2002, 87, S221–S230. [Google Scholar] [CrossRef] [PubMed]
- Galdeano, C.M.; Perdigon, G. Role of viability of probiotic strains in their persistence in the gut and in mucosal immune stimulation. J. Appl. Microbiol. 2004, 97, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Bernot, A.; Auffray, C. Primary structure and ontogeny of an avian CD3 transcript. Proc. Natl. Acad. Sci. USA 1991, 88, 2550–2554. [Google Scholar] [CrossRef] [PubMed]
- Igyártó, B.; Nagy, N.; Magyar, A.; Oláh, I. Identification of the avian B-cell-specific Bu-1 alloantigen by a novel monoclonal antibody. Poult. Sci. 2008, 87, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Madej, J.; Bednarczyk, M. Effect of in ovo-delivered prebiotics and synbiotics on the morphology and specific immune cell composition in the gut-associated lymphoid tissue. Poult. Sci. 2016, 95, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Foye, O.; Ferket, P.; Uni, Z. The effects of in ovo feeding arginine, β-hydroxy-β-methyl-butyrate, and protein on jejunal digestive and absorptive activity in embryonic and neonatal turkey poults. Poult. Sci. 2007, 86, 2343–2349. [Google Scholar] [CrossRef] [PubMed]
| Injected Substances | Aims | Injected Target | Infection Time | References |
|---|---|---|---|---|
| Extract of Laminaria species of seaweed | development of duodenum | air chamber | Day 12 | [37] |
| Raffinose and stachyose | iron bioavailability, BBM functionality, gut microflora population | amniotic fluid | Day 17 | [41] |
| Extract of chickpea and lentil, duck egg white peptides | calcium bioavailability, BBM functionality, gut microflora population | amniotic fluid | Day 17 | [42] |
| Extract of beta-glucans, Transgalactooligosaccharides | hatchability, gut microflora population | air chamber | Day 12 | [43] |
| Extract containing laminarin and fucoidan; Transgalactooligosaccharides from milk lactose | muscle, lipid oxidation of meat | air chamber | Day 12 | [44] |
| Inulin, Galactooligosaccharides (GOS), Lactococcus lactis | transcriptomic prolife of spleen, cecal tonsils, and large intestine | air chamber | Day 12 | [45] |
| Raffinose | gut health and immune system | air chamber | Day 12 | [46] |
| Silybum marianum extract | immune system | amniotic fluid | Day 17 | [47] |
| Inulin, Enterococcus faecium | BBM functionality, gut microflora population, short-chain fatty acid content | amniotic fluid | Day 17 | [48] |
| Inulin, transgalactooligosaccharides, Lactococcus lactis | gut health and short-chain fatty acid content | air chamber | Day 12 | [49] |
| Inulin, Lactococcus lactis | immune-related gene expression | air chamber | Day 12 | [50] |
| Inulin, transgalactooligosaccharides, Lactococcus lactis | digestive potency of pancreas | air chamber | Day 12 | [51] |
| Wheat prebiotics | iron bioavailability, gut microflora population | amniotic fluid | Day 17 | [52] |
| Daidzein | BBM functionality, gut microflora population | amniotic fluid | Day 17 | [53] |
| Inulin | iron bioavailability, gut functionality | amniotic fluid | Day 17 | [54] |
| Raffinose, Lactococcus lactis | muscle fiber | air chamber | Day 12 | [55] |
| Mannan oligosaccharides | small intestine development | amniotic fluid | Day 17 | [56] |
| Dextrin, maltose, sucrose | mucin gene expression | amniotic fluid | Day 17 | [57] |
| Zinc-methionine | zinc status, small intestine development | amniotic fluid | Day 17 | [58] |
| β-hydroxy-β-methyl butyrate, Dextrin, maltose, sucrose | small intestine development | amniotic fluid | Day 17 | [35] |
| Gene | References |
|---|---|
| Section A: Functional Gene Expression | |
| Aminopeptidase (AP)/leucine aminopeptidase (LAP) | [41,42,53,56,58,122] |
| Sucrose isomaltase (SI) | [35,41,42,53,56,58,122] |
| Sodium glucose transporter 1 (SGLT1) | [41,53,56,58] |
| ATPase | [53,58] |
| Peptide transporter 1 (PepT1) | [56] |
| Section B: Immune system | |
| CD3, CD45, CD56, chB6 | [46] |
| CD80 | [50] |
| TLR2, TLR4 | [46,56] |
| Section C: Cytokine | |
| IL-1β, IL-10 | [46] |
| IL-4, IL-6, IL-8, IL-18, IL-12P40 | [50] |
| IFN-β, IFN-γ | [50] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, T.; Tako, E. The In Ovo Feeding Administration (Gallus Gallus)—An Emerging In Vivo Approach to Assess Bioactive Compounds with Potential Nutritional Benefits. Nutrients 2018, 10, 418. https://doi.org/10.3390/nu10040418
Hou T, Tako E. The In Ovo Feeding Administration (Gallus Gallus)—An Emerging In Vivo Approach to Assess Bioactive Compounds with Potential Nutritional Benefits. Nutrients. 2018; 10(4):418. https://doi.org/10.3390/nu10040418
Chicago/Turabian StyleHou, Tao, and Elad Tako. 2018. "The In Ovo Feeding Administration (Gallus Gallus)—An Emerging In Vivo Approach to Assess Bioactive Compounds with Potential Nutritional Benefits" Nutrients 10, no. 4: 418. https://doi.org/10.3390/nu10040418
APA StyleHou, T., & Tako, E. (2018). The In Ovo Feeding Administration (Gallus Gallus)—An Emerging In Vivo Approach to Assess Bioactive Compounds with Potential Nutritional Benefits. Nutrients, 10(4), 418. https://doi.org/10.3390/nu10040418





