Spray-Dried Potato Juice as a Potential Functional Food Component with Gastrointestinal Protective Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Material
2.2. Spray-Dried Potato Juice Analysis
2.3. In Vitro Experiments
2.3.1. Cell Cultures
2.3.2. Cytotoxicity Assay
2.3.3. Transepithelial Electrical Resistance
2.3.4. Quantification of Proinflammatory Gene Expression Using Real-Time PCR
2.3.5. Determination of TNF-α and IL-6
2.4. In Vivo Experiment
2.4.1. Experimental Design
2.4.2. Ulcer Index
2.4.3. Inflammatory Cytokines (TNF-α and IL-6)
2.5. Statistical Analysis
3. Results
3.1. Spray-Dried Potato Juice Characteristics
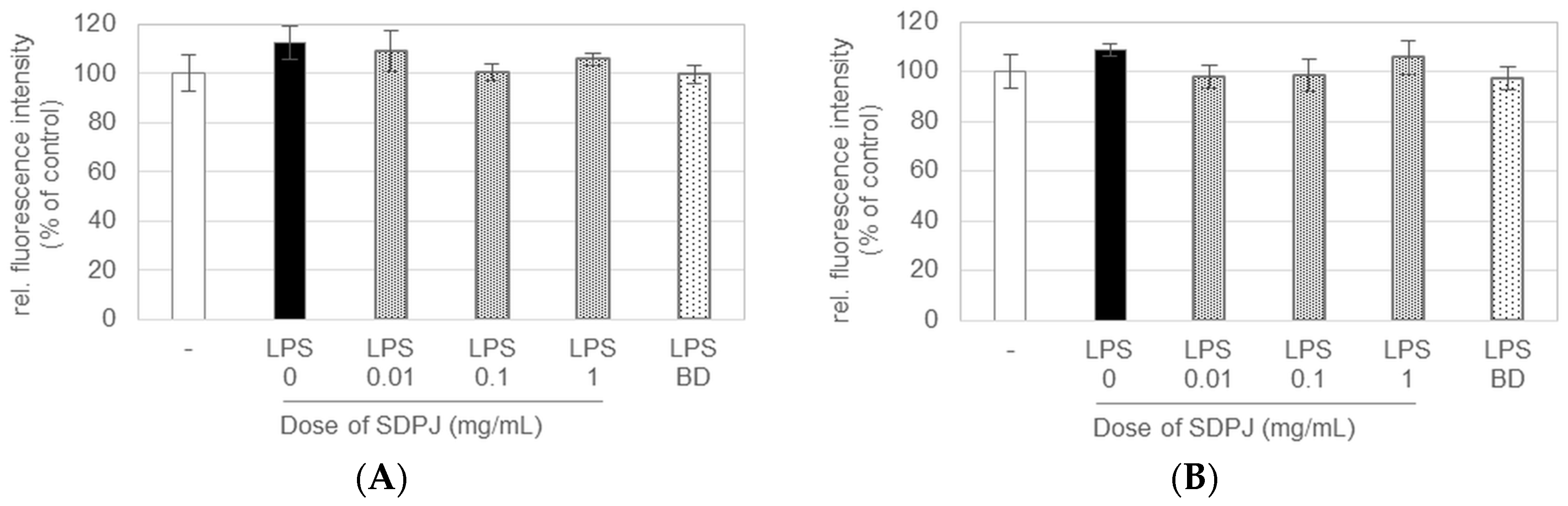
3.2. Effect of Spray-Dried Potato Juice on Intestinal and Macrophage Cell Viability
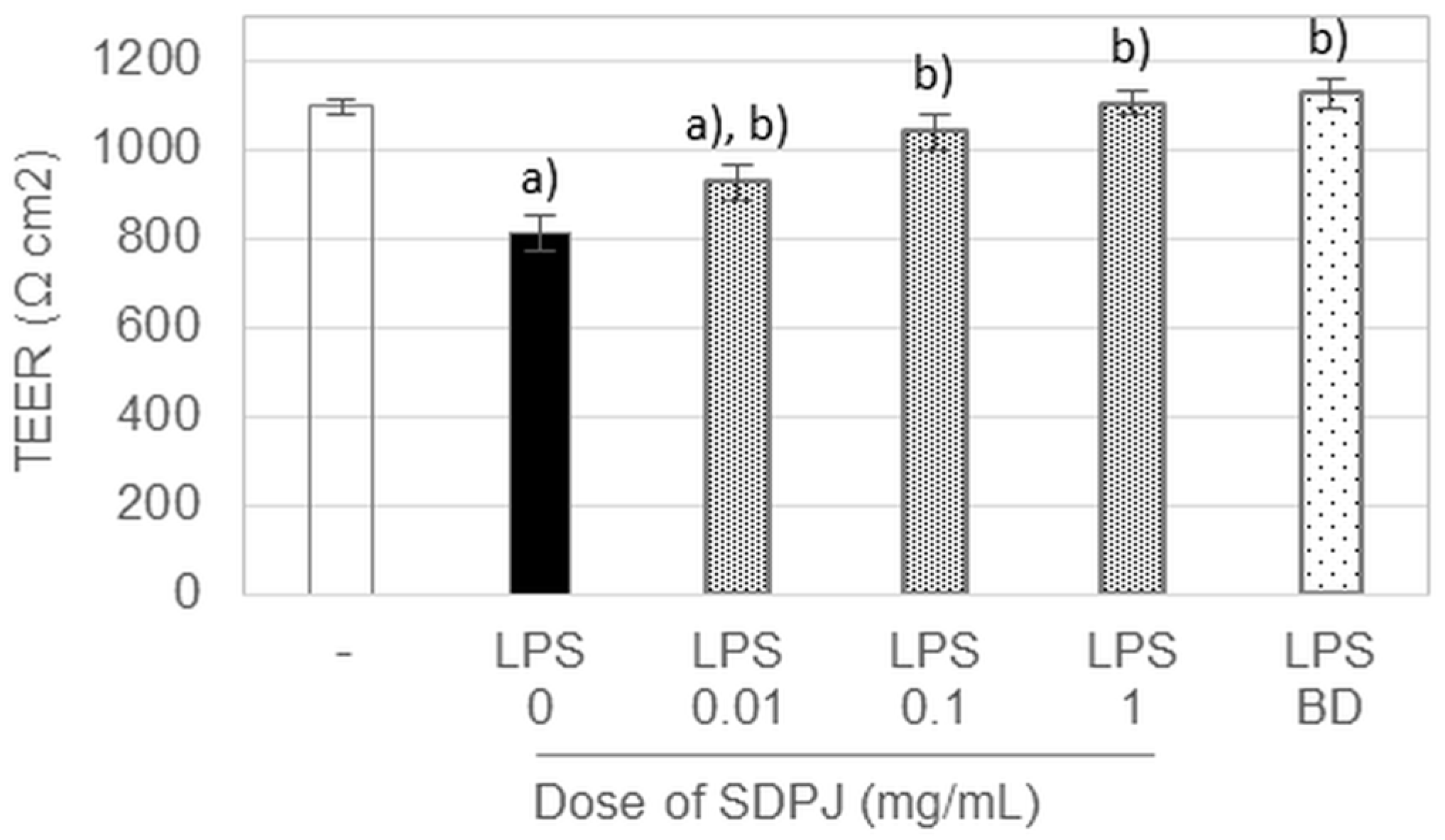
3.3. Effects of Spray-Dried Potato Juice on TEER in the Caco-2/RAW264.7 Co-Culture System
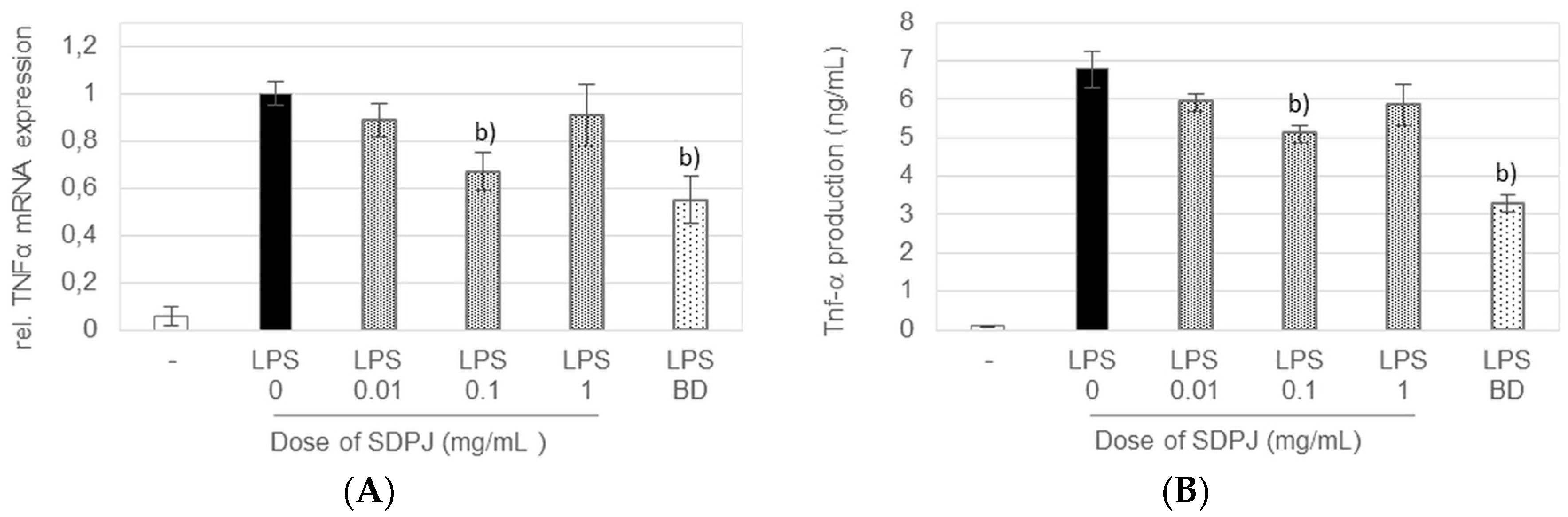
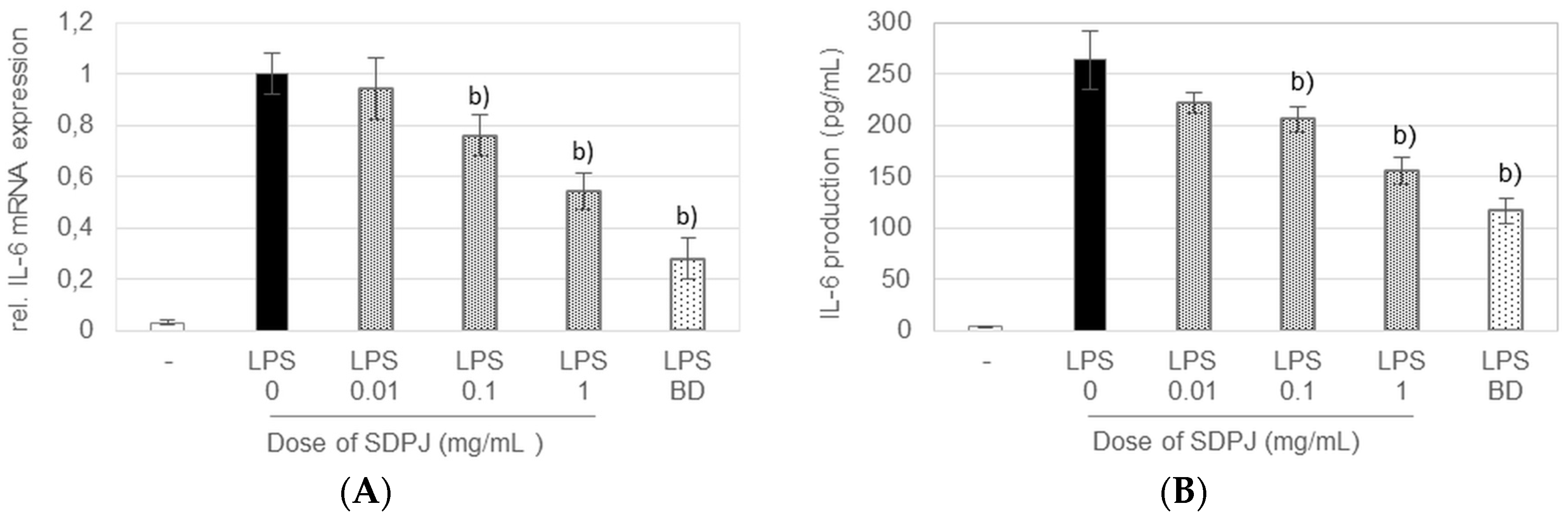
3.4. Anti-Inflammatory Effects of Spray-Dried Potato Juice in the Caco-2/RAW264.7 Co-Culture System
3.5. Anti-Ulcerogenic Effect of Spray-Dried Potato Juice in Rats
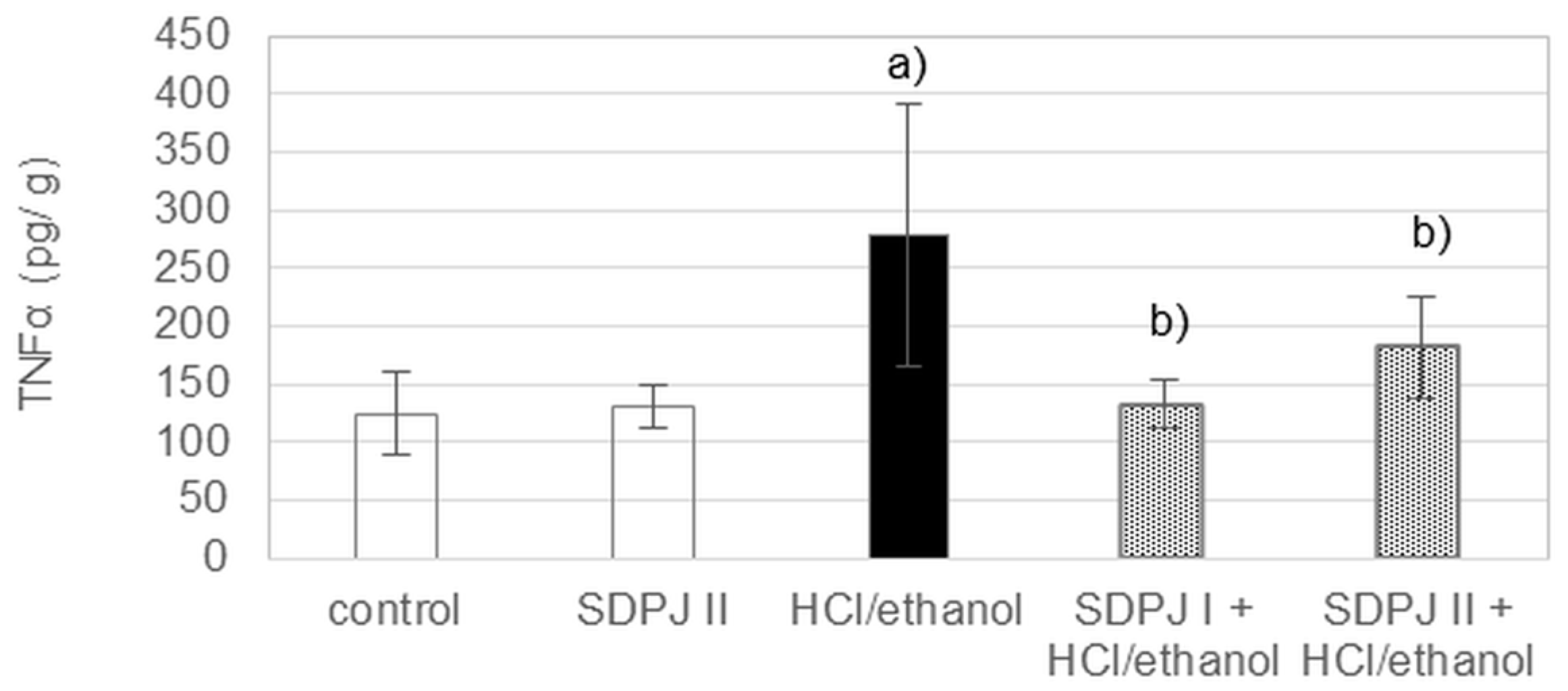
3.6. Anti-Inflammatory Effects of Spray-Dried Potato Juice in Rats
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, X.Y.; Mo, H.Y.; Huang, Y. Letter: Risk factor and mortality of peptic ulcer disease. Aliment. Pharm. Therap. 2016, 44, 424–425. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Yoshino, J.; Akamatsu, T.; Itoh, T.; Kato, M.; Kamada, T.; Takagi, A.; Chiba, T.; Nomura, S.; Mizokami, Y.; et al. Evidence-based clinical practice guidelines for peptic ulcer disease 2015. J. Gastroenterol. 2016, 51, 177–194. [Google Scholar] [CrossRef] [PubMed]
- Marcus, E.A.; Vagin, O.; Tokhtaeva, E.; Sachs, G.; Scott, D.R. Helicobacter pylori impedes acid-induced tightening of gastric epithelial junctions. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.; Wild, C.P. World Cancer Report 2014, 2014 International Agency for Research on Cancer. Available online: http://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-2014 (accessed on 18 October 2017).
- Holtmann, G.; Talley, N.J. Functional dyspepsia. Curr. Opin. Gastroen. 2015, 31, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, S.; Boyko, T.; Filippov, Y.; Torda, T. Further evidence on the effectiveness of potato juice in dyspeptic complaints. Phytomedicine 2006, 13, 596–597. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, S.; Chrubasik, C.; Torda, T.; Madisch, A. Efficacy and tolerability of potato juice in dyspeptic patients: A pilot study. Phytomedicine 2006, 13, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Vlachojannis, J.E.; Cameron, M.; Chrubasik, S. Medicinal use of potato-derived products: A systematic review. Phytother. Res. 2010, 24, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, R.; Jayathilake, C.; Chaminda Jayawardana, B.; Liyanage, R. Health-beneficial properties of potato and compounds of interest. J. Sci. Food Agric. 2016, 96, 4850–4860. [Google Scholar] [CrossRef] [PubMed]
- Ruseler-van Embden, J.G.; van Lieshout, L.M.; Smits, S.A.; van Kessel, I.; Laman, J.D. Potato tuber proteins efficiently inhibit human faecal proteolytic activity: Implications for treatment of peri-anal dermatitis. Eur. J. Clin. Investig. 2004, 34, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, K.L.; Park, J.S.; Brown, C.R.; Mathison, B.D.; Navarre, D.A.; Chew, B.P. Pigmented potato consumption alters oxidative stress and inflammatory damage in men. J. Nutr. 2011, 141, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, L.M.; Cheon, K.; Nansel, T.R.; Albert, P.S. Candidate measures of whole plant food intake are related to biomarkers of nutrition and health in the US population (National Health and Nutrition Examination Survey 1999–2002). Nutr. Res. 2012, 32, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Camire, M.E.; Kubow, S.; Donnelly, D.J. Potatoes and human health. Crit. Rev. Food Sci. Nutr. 2009, 49, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Vomero, N.D.; Colpo, E. Nutritional care in peptic ulcer. Arq. Bras. Cir. Dig. 2014, 27, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, H.M.; Masewicz, Ł.; Kowalczewski, P.; Grażyna Lewandowicz, G.; Piątek, M.; Kubiak, P. Water properties in pâtés enriched with potato juice. Eur. Food Res. Technol. 2017, 244, 387–393. [Google Scholar] [CrossRef]
- Kowalczewski, P.; Lewandowicz, G.; Krzywdzińska-Bartkowiak, M.; Piątek, M.; Baranowska, H.M.; Białas, W.; Jeziorna, M.; Kubiak, P. Finely comminuted frankfurters fortified with potato juice—Quality and structure. J. Food Eng. 2015, 167, 183–188. [Google Scholar] [CrossRef]
- Kowalczewski, P.; Lewandowicz, G.; Makowska, A.; Knoll, I.; Błaszczak, W.; Białas, W.; Kubiak, P. Pasta Fortified with Potato Juice: Structure, Quality, and Consumer Acceptance. J. Food Sci. 2015, 80, S1377–S1382. [Google Scholar] [CrossRef] [PubMed]
- Lewandowicz, G.; Kowalczewski, P.; Olejnik, A.; Jodynis-Liebert, J.; Kujawska, M.; Lesiecki, M. Method for Obtaining the Preparation from Potato Juice and Its Application. WO2015112034-A1, 30 July 2015. [Google Scholar]
- Biswas, A.K.; Sahoo, J.; Chatli, M.K. A simple UV-Vis spectrophotometric method for determination of β-carotene content in raw carrot, sweet potato and supplemented chicken meat nuggets. LWT Food Sci. Technol. 2011, 44, 1809–1813. [Google Scholar] [CrossRef]
- Stobiecki, M.; Matysiak-Kata, I.; Frański, R.; Skała, J.; Szopa, J. Monitoring changes in anthocyanin and steroid alkaloid glycoside content in lines of transgenic potato plants using liquid chromatography/mass spectrometry. Phytochemistry 2003, 62, 959–969. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Bio. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, M.; Sun, Y.; Sun, J. How to improve bayberry (Myrica rubra Sieb. et Zucc.) juice color quality: Effect of juice processing on bayberry anthocyanins and polyphenolics. J. Agric. Food Chem. 2006, 54, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, A.; Kowalska, K.; Olkowicz, M.; Rychlik, J.; Juzwa, W.; Myszka, K.; Dembczyński, J.; Białas, W. Anti-inflammatory effects of gastrointestinal digested Sambucus nigra L. fruit extract analysed in co-cultured intestinal epithelial cells and lipopolysaccharide-stimulated macrophages. J. Funct. Foods 2015, 19, 649–660. [Google Scholar] [CrossRef]
- Caldas, G.F.; do Amaral Costa, I.M.; da Silva, J.B.; da Nóbrega, R.F.; Rodrigues, F.F.; da Costa, J.G.; Wanderley, A.G. Antiulcerogenic activity of the essential oil of Hyptis martiusii Benth. (Lamiaceae). J. Ethnopharmacol. 2011, 137, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Banerjee, R.K. Effect of stress on the antioxidant enzymes and gastric ulceration. Mol. Cell Biochem. 1993, 125, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Vanheel, H.; Vicario, M.; Vanuytsel, T.; Van Oudenhove, L.; Martinez, C.; Keita, Å.V.; Pardon, N.; Santos, J.; Söderholm, J.D.; Tack, J.; Farré, R. Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia. Gut 2014, 63, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Miranda, L.; Deußer, H.; Evers, D. The impact of in vitro digestion on bioaccessibility of polyphenols from potatoes and sweet potatoes and their influence on iron absorption by human intestinal cells. Food Funct. 2013, 4, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Liu, S.; Zhou, Y.; Mi, S.; Liu, G.; Wu, X.; Yao, K.; Assaad, H.; Deng, Z.; Hou, Y.; et al. Chlorogenic acid decreases intestinal permeability and increases expression of intestinal tight junction proteins in weaned rats challenged with LPS. PLoS ONE 2014, 9, e97815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lian, F.; Zhu, Y.; Xia, M.; Wang, Q.; Ling, W.; Wang, X.D. Cyanidin-3-O-beta-glucoside inhibits LPS-induced expression of inflammatory mediators through decreasing Ikappa Balpha phosphorylation in THP-1 cells. Inflamm. Res. 2010, 59, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Sugata, M.; Lin, C.Y.; Shih, Y.C. Anti-Inflammatory and Anticancer Activities of Taiwanese Purple-Fleshed Sweet Potatoes (Ipomoea batatas L. Lam) Extracts. Biomed. Res. Int. 2015, 2015, 768093. [Google Scholar] [CrossRef] [PubMed]
- Kenny, O.M.; McCarthy, C.M.; Brunton, N.P.; Hossain, M.B.; Rai, D.K.; Collins, S.G.; Jones, P.W.; Maguire, A.R.; O’Brien, N.M. Anti-inflammatory properties of potato glycoalkaloids in stimulated Jurkat and Raw 264.7 mouse macrophages. Life Sci. 2013, 92, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Korpan, Y.I.; Nazarenko, E.A.; Skryshevskaya, M.C.; Jaffrezic-Renault, N.; El’skaya, A.V. Potato glycoalkaloids: True safety or false sense of security. Trends Biotechnol. 2004, 22, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Nema, P.K.; Ramayya, N.; Duncan, E.; Niranjan, K. Review Potato glycoalkaloids: Formation and strategies for mitigation. J. Sci. Food Agric. 2008, 88, 1869–1881. [Google Scholar] [CrossRef]
- Andreo, M.A.; Ballesteros, K.V.; Hiruma-Lima, C.A.; Machado da Rocha, L.R.; Souza Brito, A.R.; Vilegas, W. Effect of Mouriri pusa extracts on experimentally induced gastric lesions in rodents: Role of endogenous sulfhydryls compounds and nitric oxide in gastroprotection. J. Ethnopharmacol. 2006, 107, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Shin, J.S.; Choi, H.E.; Lee, K.G.; Cho, Y.W.; An, H.J.; Jang, D.S.; Jeong, J.C.; Kwon, O.K.; Nam, J.H.; et al. Chloroform fraction of Solanum tuberosum L. cv Jayoung epidermis suppresses LPS-induced inflammatory responses in macrophages and DSS-induced colitis in mice. Food Chem. Toxicol. 2014, 63, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.H.; Shen, J.; Sun, P.; Yang, M.L.; Zhao, P.W.; Niu, Y.; Lu, J.K.; Wang, Z.Q.; Gao, C.; Han, X.; et al. Anti-inflammatory effects of potato extract on a rat model of cigarette smoke-induced chronic obstructive pulmonary disease. Food Nutr. Res. 2015, 59, 28879. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shim, E.H.; Choung, S.Y. Inhibitory effects of Solanum tuberosum L. var. vitelotte extract on 2,4-dinitrochlorobenzene-induced atopic dermatitis in mice. J. Pharm. Pharmacol. 2014, 66, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Sadar, S.S.; Vyawahare, N.S.; Bodhankar, S.L. Ferulic acid ameliorates TNBS-induced ulcerative colitis through modulation of cytokines, oxidative stress, iNOs, COX-2, and apoptosis in laboratory rats. EXCLI J. 2016, 15, 482–499. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Satsu, H.; Bae, M.J.; Zhao, Z.; Ogiwara, H.; Totsuka, M.; Shimizu, M. Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 cells and the dextran sulphate sodium-induced colitis symptoms in C57BL/6 mice. Food Chem. 2015, 168, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Birringer, M. Hormetics: Dietary triggers of an Adaptive Stress Response. Pharm. Res. 2011, 28, 2680–2694. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Guha, P.; Chattopadhyay, S.; Bandyopadhyay, S.K. Biphasic activity of resveratrol on indomethacin-induced gastric ulcers. Biochem. Biophys. Res. Commun. 2009, 381, 90–95. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Unit | Amount |
|---|---|---|
| Dry matter | g/100 g FM | 90.1 ± 0.5 |
| Protein | g/100 g DM | 49.22 ± 0.40 |
| Ash | g/100 g DM | 16.34 ± 0.09 |
| Total phenolic compounds: | ||
| by Folin-Ciocalteu | mg CAE/100g DM | 366 ± 35 |
| by HPLC | mg CAE/100g DM | 330.2 ± 12.0 |
| Individual phenolic compound: | ||
| Chlorogenic acid | mg/100g DM | 13.3 ± 1.8 |
| Ferulic acid | mg/100g DM | 17.8 ± 2.3 |
| Caffeic acid | mg/100g DM | 22.2 ± 1.4 |
| β-carotene | mg/100g DM | 0.020 ± 0.008 |
| Glycoalkaloids: | ||
| α-solanine | mg/100 g DM | 59.1 ± 1.1 |
| α-chaconine | mg/100g DM | 99.0 ± 2.0 |
| Antioxidant activity * | mmol TEAC/100g DM | 26 ± 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kujawska, M.; Olejnik, A.; Lewandowicz, G.; Kowalczewski, P.; Forjasz, R.; Jodynis-Liebert, J. Spray-Dried Potato Juice as a Potential Functional Food Component with Gastrointestinal Protective Effects. Nutrients 2018, 10, 259. https://doi.org/10.3390/nu10020259
Kujawska M, Olejnik A, Lewandowicz G, Kowalczewski P, Forjasz R, Jodynis-Liebert J. Spray-Dried Potato Juice as a Potential Functional Food Component with Gastrointestinal Protective Effects. Nutrients. 2018; 10(2):259. https://doi.org/10.3390/nu10020259
Chicago/Turabian StyleKujawska, Małgorzata, Anna Olejnik, Grażyna Lewandowicz, Przemysław Kowalczewski, Renata Forjasz, and Jadwiga Jodynis-Liebert. 2018. "Spray-Dried Potato Juice as a Potential Functional Food Component with Gastrointestinal Protective Effects" Nutrients 10, no. 2: 259. https://doi.org/10.3390/nu10020259
APA StyleKujawska, M., Olejnik, A., Lewandowicz, G., Kowalczewski, P., Forjasz, R., & Jodynis-Liebert, J. (2018). Spray-Dried Potato Juice as a Potential Functional Food Component with Gastrointestinal Protective Effects. Nutrients, 10(2), 259. https://doi.org/10.3390/nu10020259








