l-Carnitine Supplementation in Older Women. A Pilot Study on Aging Skeletal Muscle Mass and Function
Abstract
1. Introduction
2. Materials and Methods
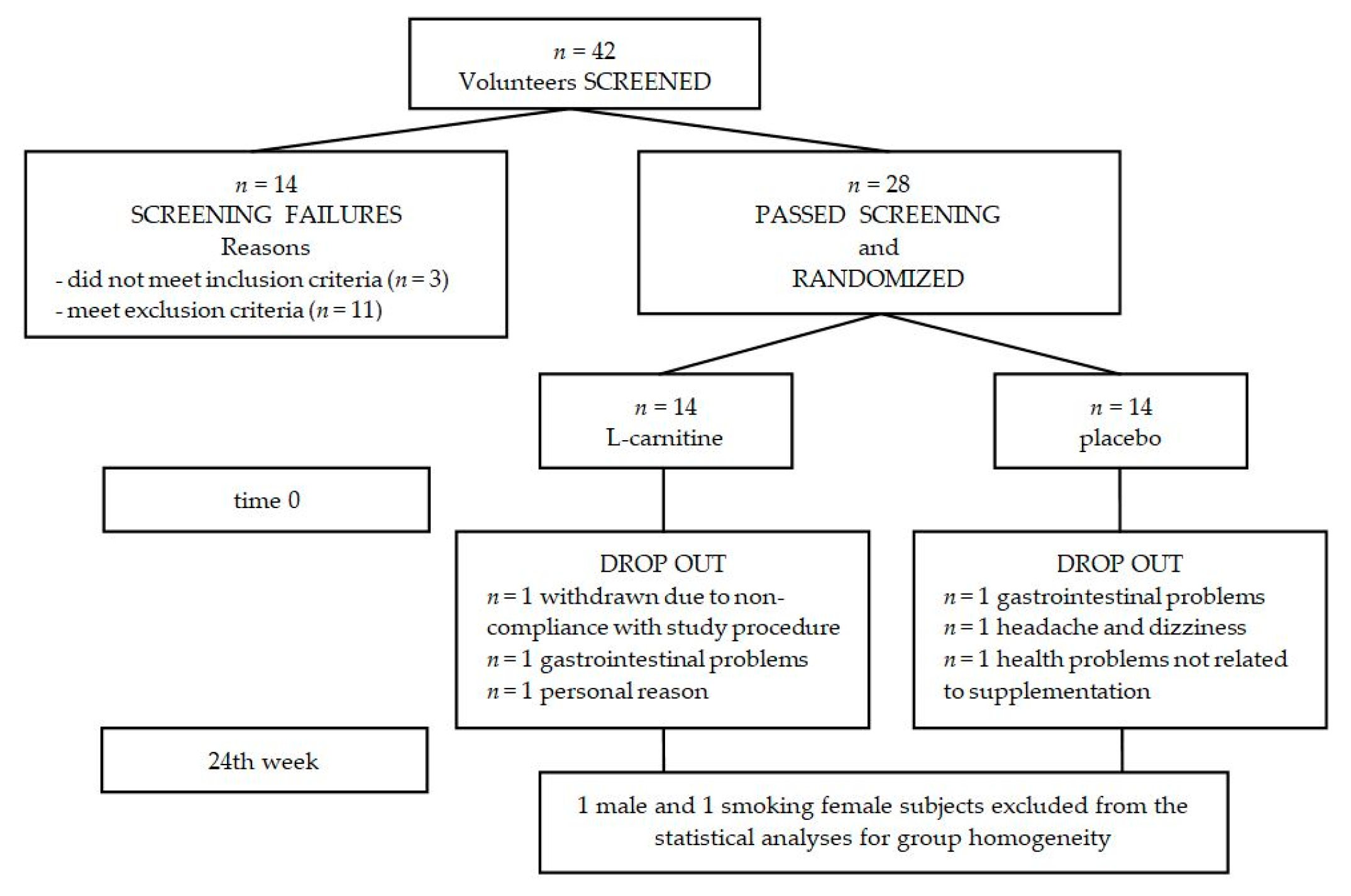
2.1. Subjects
2.2. Study Procedure
2.3. Anthropometric Measurements
2.4. Blood Sampling
2.5. Biochemical Determination
2.6. Skeletal Muscle Strength Test
2.7. Nutritional and Physical Activity Habits
- F0—never,
- F1—occasionally,
- F2—several times per year,
- F3—several times per month,
- F4—2–5 times per week,
- F5—6–7 times per week.
2.8. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zamboni, M.; Zoico, E.; Scartezzini, T.; Mazzali, G.; Tosoni, P.; Zivelonghi, A.; Gallagher, D.; de Pergola, G.; Di Francesco, V.; Bosello, O. Body composition changes in stable-weight elderly subjects: The effect of sex. Aging Clin. Exp. Res. 2003, 15, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Coppack, S.W. Pro-inflammatory cytokines and adipose tissue. Proc. Nutr. Soc. 2001, 60, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Roubenoff, R.; Harris, T.B.; Abad, L.W.; Wilson, P.W.; Dallal, G.E.; Dinarello, C.A. Monocyte cytokine production in an elderly population: Effect of age and inflammation. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1998, 53, M20–M26. [Google Scholar] [CrossRef]
- Budui, S.L.; Rossi, A.P.; Zamboni, M. The pathogenetic bases of sarcopenia. Clin. Cases Miner. Bone Metab. 2015, 12, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, D.; Costelli, P.; Sampaolesi, M.; Penna, F. Role of inflammation in muscle homeostasis and myogenesis. Med. Inflamm. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Meadows, K.A.; Holly, J.M.; Stewart, C.E. Tumor necrosis factor-alpha-induced apoptosis is associated with suppression of insulin-like growth factor binding protein-5 secretion in differentiating murine skeletal myoblasts. J. Cell. Physiol. 2000, 183, 330–337. [Google Scholar] [CrossRef]
- Sharples, A.P.; Al-Shanti, N.; Stewart, C.E. C2 and C2C12 murine skeletal myoblast models of atrophic and hypertrophic potential: Relevance to disease and ageing? J. Cell. Physiol. 2010, 225, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Girven, M.; Dugdale, H.F.; Owens, D.J.; Hughes, D.C.; Stewart, C.E.; Sharples, A.P. l-glutamine improves skeletal muscle cell differentiation and prevents myotube atrophy after cytokine (TNF-α) stress via reduced p38 MAPK signal transduction. J. Cell. Physiol. 2016, 231, 2720–2732. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Chen, Y.; John, J.; Moylan, J.; Jin, B.; Mann, D.L.; Reid, M.B. TNF-α acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. 2005, 19, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of interleukin-6 and tumor necrosis factor-α with muscle mass and muscle strength in elderly men and women: The health ABC study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, M326–M332. [Google Scholar] [CrossRef]
- Cesari, M.; Penninx, B.W.; Pahor, M.; Lauretani, F.; Corsi, A.M.; Rhys Williams, G.; Guralnik, J.M.; Ferrucci, L. Inflammatory markers and physical performance in older persons: The InCHIANTI study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2004, 59, 242–248. [Google Scholar] [CrossRef]
- Penninx, B.W.; Kritchevsky, S.B.; Newman, A.B.; Nicklas, B.J.; Simonsick, E.M.; Rubin, S.; Nevitt, M.; Visser, M.; Harris, T.; Pahor, M. Inflammatory markers and incident mobility limitation in the elderly. J. Am. Geriatr. Soc. 2004, 52, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Schaap, L.A.; Pluijm, S.M.; Deeg, D.J.; Visser, M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am. J. Med. 2006, 119, e9–e17. [Google Scholar] [CrossRef] [PubMed]
- Bucci, L.; Yani, S.L.; Fabbri, C.; Bijlsma, A.Y.; Maier, A.B.; Meskers, C.G.; Narici, M.V.; Jones, D.A.; McPhee, J.S.; Seppet, E.; et al. Circulating levels of adipokines and IGF-1 are associated with skeletal muscle strength of young and old healthy subjects. Biogerontology 2013, 14, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Bezkorovainy, A. Carnosine, carnitine, and Vladimir Gulevich. J. Chem. Educ. 1974, 51, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Winter, B.K.; Fiskum, G.; Gallo, L.L. Effects of l-carnitine on serum triglyceride and cytokine levels in rat models of cachexia and septic shock. Br. J. Cancer 1995, 72, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Izgut-Uysal, V.N.; Agac, A.; Derin, N. Effect of l-carnitine on carrageenan-induced inflammation in aged rats. Gerontology 2003, 49, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Demiroren, K.; Dogan, Y.; Kocamaz, H.; Ozercan, I.H.; Ilhan, S.; Ustundag, B.; Bahcecioglu, I.H. Protective effects of l-carnitine, N-acetylcysteine and genistein in an experimental model of liver fibrosis. Clin. Res. Hepatol. Gastroenterol. 2014, 38, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Zhang, Z.; Zhang, Y.; Wu, J.; Yu, L.; Liu, S. l-carnitine ameliorates the liver inflammatory response by regulating carnitine palmitoyltransferase I-dependent PPARγ signaling. Mol. Med. Rep. 2016, 13, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Lin, J.S.; Lin, Y.C.; Lin, P.T. Antiinflammatory effects of l-carnitine supplementation (1000 mg/d) in coronary artery disease patients. Nutrition 2015, 31, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Ringseis, R.; Koc, A.; Lukas, I.; Kluge, H.; Eder, K. Supplementation with l-carnitine downregulates genes of the ubiquitin proteasome system in the skeletal muscle and liver of piglets. Animal 2012, 6, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Couturier, A.; Haferkamp, M.; Most, E.; Eder, K. Supplementation of carnitine leads to an activation of the IGF-1/PI3K/Akt signalling pathway and down regulates the E3 ligase MuRF1 in skeletal muscle of rats. Nutr. Metab. 2013, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Costell, M.; O’Connor, J.E.; Grisolia, S. Age-dependent decrease of carnitine content in muscle of mice and humans. Biochem. Biophys. Res. Commun. 1989, 161, 1135–1143. [Google Scholar] [CrossRef]
- Stephens, F.B.; Constantin-Teodosiu, D.; Laithwaite, D.; Simpson, E.J.; Greenhaff, P.L. Insulin stimulates l-carnitine accumulation in human skeletal muscle. FASEB J. 2006, 20, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Wall, B.T.; Stephens, F.B.; Constantin-Teodosiu, D.; Marimuthu, K.; Macdonald, I.A.; Greenhaff, P.L. Chronic oral ingestion of l-carnitine and carbohydrate increases muscle carnitine content and alters muscle fuel metabolism during exercise in humans. J. Physiol. 2011, 589, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, E.; Olek, R.A.; Grzywacz, T.; Antosiewicz, J.; Kujach, S.; Luszczyk, M.; Smaruj, M.; Sledziewska, E.; Laskowski, R. Whole-body cryostimulation as an effective method of reducing low-grade inflammation in obese men. J. Physiol. Sci. 2013, 63, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Grinberga, S.; Dambrova, M.; Latkovskis, G.; Strele, I.; Konrade, I.; Hartmane, D.; Sevostjanovs, E.; Liepinsh, E.; Pugovics, O. Determination of trimethylamine-N-oxide in combination with l-carnitine and γ-butyrobetaine in human plasma by UPLC/MS/MS. BMC 2015, 29, 1670–1674. [Google Scholar] [PubMed]
- Ossowski, Z.M.; Skrobot, W.; Aschenbrenner, P.; Cesnaitiene, V.J.; Smaruj, M. Effects of short-term Nordic walking training on sarcopenia-related parameters in women with low bone mass: A preliminary study. Clin. Interv. Aging 2016, 11, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Carlson, C.L.; Visser, M.; Kelley, D.E.; Scherzinger, A.; Harris, T.B.; Stamm, E.; Newman, A.B. Attenuation of skeletal muscle and strength in the elderly: The health ABC study. J. Appl. Physiol. 2001, 90, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Steiber, A.; Kerner, J.; Hoppel, C.L. Carnitine: A nutritional, biosynthetic, and functional perspective. Mol. Asp. Med. 2004, 25, 455–473. [Google Scholar] [CrossRef] [PubMed]
- Kasielski, M.; Eusebio, M.O.; Pietruczuk, M.; Nowak, D. The relationship between peripheral blood mononuclear cells telomere length and diet-unexpected effect of red meat. Nutr. J. 2016, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.G. Spreadsheets for analysis of controlled trials, with adjustment for a subject characteristic. Sportscience 2006, 10, 46–50. [Google Scholar]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Ringseis, R.; Keller, J.; Eder, K. Mechanisms underlying the anti-wasting effect of l-carnitine supplementation under pathologic conditions: Evidence from experimental and clinical studies. Eur. J. Nutr. 2013, 52, 1421–1442. [Google Scholar] [CrossRef] [PubMed]
- Pooyandjoo, M.; Nouhi, M.; Shab-Bidar, S.; Djafarian, K.; Olyaeemanesh, A. The effect of (l-)carnitine on weight loss in adults: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2016, 17, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, F.B.; Coswig, V.S.; Galliano, L.M. Comment on ‘the effect of (l-)carnitine on weight loss in adults: A systematic review and meta-analysis of randomized controlled trials’. Obes. Rev. 2017, 18, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Maffioli, P.; Salvadeo, S.A.; Ferrari, I.; Gravina, A.; Mereu, R.; D’Angelo, A.; Palumbo, I.; Randazzo, S.; Cicero, A.F. Effects of combination of sibutramine and l-carnitine compared with sibutramine monotherapy on inflammatory parameters in diabetic patients. Metab. Exp. 2011, 60, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Maffioli, P.; Ferrari, I.; D’Angelo, A.; Fogari, E.; Palumbo, I.; Randazzo, S.; Cicero, A.F. Comparison between orlistat plus l-carnitine and orlistat alone on inflammation parameters in obese diabetic patients. Fundam. Clin. Pharmacol. 2011, 25, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Gargante, M.P.; Russo, C.; Antic, T.; Vacante, M.; Avitabile, T.; Li Volti, G.; Galvano, F. l-carnitine supplementation to diet: A new tool in treatment of nonalcoholic steatohepatitis—A randomized and controlled clinical trial. Am. J. Gastroenterol. 2010, 105, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.J. Invited review: Aging and sarcopenia. J. Appl. Physiol. 2003, 95, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Cammalleri, L.; Gargante, M.P.; Vacante, M.; Colonna, V.; Motta, M. l-carnitine treatment reduces severity of physical and mental fatigue and increases cognitive functions in centenarians: A randomized and controlled clinical trial. Am. J. Clin. Nutr. 2007, 86, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A. Effect of l-carnitine supplementation on circulating C-reactive protein levels: A systematic review and meta-analysis. J. Med. Biochem. 2015, 34, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Hakeshzadeh, F.; Tabibi, H.; Ahmadinejad, M.; Malakoutian, T.; Hedayati, M. Effects of l-carnitine supplement on plasma coagulation and anticoagulation factors in hemodialysis patients. Ren. Fail. 2010, 32, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Tabibi, H.; Hedayati, M. Effects of l-carnitine supplement on serum inflammatory cytokines, c-reactive protein, lipoprotein (a), and oxidative stress in hemodialysis patients with Lp (a) hyperlipoproteinemia. Hemodial. Int. 2010, 14, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Malek Mahdavi, A.; Mahdavi, R.; Kolahi, S. Effects of l-carnitine supplementation on serum inflammatory factors and matrix metalloproteinase enzymes in females with knee osteoarthritis: A randomized, double-blind, placebo-controlled pilot study. J. Am. Coll. Nutr. 2016, 35, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Rafraf, M.; Karimi, M.; Jafari, A. Effect of l-carnitine supplementation in comparison with moderate aerobic training on serum inflammatory parameters in healthy obese women. J. Sports Med. Phys. Fit. 2015, 55, 1363–1370. [Google Scholar]
- Calvani, R.; Joseph, A.M.; Adhihetty, P.J.; Miccheli, A.; Bossola, M.; Leeuwenburgh, C.; Bernabei, R.; Marzetti, E. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol. Chem. 2013, 394, 393–414. [Google Scholar] [CrossRef] [PubMed]
- Gordon, B.S.; Kelleher, A.R.; Kimball, S.R. Regulation of muscle protein synthesis and the effects of catabolic states. Int. J. Biochem. Cell Boil. 2013, 45, 2147–2157. [Google Scholar] [CrossRef] [PubMed]
- Doberenz, J.; Birkenfeld, C.; Kluge, H.; Eder, K. Effects of l-carnitine supplementation in pregnant sows on plasma concentrations of insulin-like growth factors, various hormones and metabolites and chorion characteristics. J. Anim. Physiol. Anim. Nutr. 2006, 90, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Rommel, C.; Bodine, S.C.; Clarke, B.A.; Rossman, R.; Nunez, L.; Stitt, T.N.; Yancopoulos, G.D.; Glass, D.J. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)k/Akt/GSK3 pathways. Nat. Cell Boil. 2001, 3, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Boil. 2001, 3, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef]
- Evans, M.; Guthrie, N.; Pezzullo, J.; Sanli, T.; Fielding, R.A.; Bellamine, A. Efficacy of a novel formulation of l-carnitine, creatine, and leucine on lean body mass and functional muscle strength in healthy older adults: A randomized, double-blind placebo-controlled study. Nutr. Metab. 2017, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Moon, A.; Heywood, L.; Rutherford, S.; Cobbold, C. Creatine supplementation: Can it improve quality of life in the elderly without associated resistance training? Curr. Aging Sci. 2013, 6, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.R.; Tan, Z.J.; Zhang, Q.; Gui, Q.F.; Yang, Y.M. The effectiveness of leucine on muscle protein synthesis, lean body mass and leg lean mass accretion in older people: A systematic review and meta-analysis. Br. J. Nutr. 2015, 113, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Petermans, J.; Reginster, J.Y.; Bruyere, O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2014, 99, 4336–4345. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Stokes, M.; Crowe, M. Size and strength of the quadriceps muscles of old and young women. Eur. J. Clin. Investing. 1984, 14, 282–287. [Google Scholar] [CrossRef]
- Hughes, V.A.; Frontera, W.R.; Wood, M.; Evans, W.J.; Dallal, G.E.; Roubenoff, R.; Fiatarone Singh, M.A. Longitudinal muscle strength changes in older adults: Influence of muscle mass, physical activity, and health. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, B209–B217. [Google Scholar] [CrossRef]
- Silverio, R.; Laviano, A.; Rossi Fanelli, F.; Seelaender, M. l-carnitine and cancer cachexia: Clinical and experimental aspects. J. Cachexia Sarcopenia Muscle 2011, 2, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Prado, R.; Esteras, R.; Perez-Gomez, M.V.; Gracia-Iguacel, C.; Gonzalez-Parra, E.; Sanz, A.B.; Ortiz, A.; Sanchez-Nino, M.D. Nutrients turned into toxins: Microbiota modulation of nutrient properties in chronic kidney disease. Nutrients 2017, 9, 489. [Google Scholar] [CrossRef] [PubMed]
| Variables | Placebo | l-Carnitine | ||
|---|---|---|---|---|
| Mean ± SD (Standard Deviation) | Mean ± SD | |||
| Age (years) | 66.4 ± 1.3 | 67.8 ± 2.3 | ||
| Height (cm) | 162 ± 5.3 | 159 ± 5.4 | ||
| BMI (kg/m2) | 26.5 ± 4.4 | 27.5 ± 4.5 | ||
| n | % | n | % | |
| Education level | ||||
| Primary | 0 | 0 | 0 | 0 |
| Secondary | 2 | 22.2 | 4 | 36.4 |
| High | 7 | 77.8 | 7 | 63.6 |
| Physical activity | ||||
| Low | 0 | 0 | 0 | 0 |
| Moderate | 4 | 44.4 | 6 | 54.5 |
| High | 5 | 55.6 | 5 | 45.5 |
| median | range | median | range | |
| Meat consumption | ||||
| Poultry | F4 | F3–F4 | F4 | F0–F4 |
| Pork | F2 | F0–F3 | F3 | F0–F4 |
| Beef | F1 | F0–F3 | F2 | F0–F4 |
| Fish | F3 | F1–F4 | F2 | F1–F4 |
| Lamb | F0 | F0–F3 | F0 | F0–F3 |
| Venison | F0 | F0–F3 | F0 | F0–F1 |
| Horseflesh | F0 | F0–F2 | F0 | F0–F1 |
| Variables | Group | Baseline Mean ± SD | Observed Change Mean ± SD | Adjusted Change a Mean ± SD | Adjusted Effect b | |
|---|---|---|---|---|---|---|
| Mean; CL | Inference | |||||
| BM (kg) | placebo | 69.7 ± 12.1 | −0.3 ± 2.8% | −0.3 ± 2.1% | 0.5%; ±1.9% | trivial † |
| l-carnitine | 69.8 ± 12.9 | 0.2 ± 2.9% | 0.2 ± 2.9% | |||
| FFM (kg) | placebo | 45.8 ± 6.7 | −1.6 ± 5.2% | −1.1 ± 3.7% | 0.7%; ±2.6% | trivial * |
| l-carnitine | 43.9 ± 4.6 | 0.0 ± 3.5% | −0.4 ± 2.8% | |||
| SMM (kg) | placebo | 24.9 ± 4.1 | −1.9 ± 6.4% | −1.3 ± 4.4% | 1.2%; ±3.2% | trivial * |
| l-carnitine | 23.9 ± 2.7 | −0.2 ± 3.8% | −0.1 ± 3.2% | |||
| Variables | Group | Baseline Mean ± SD | Observed Change Mean ± SD | Adjusted Change a Mean ± SD | Adjusted Effect b | |
|---|---|---|---|---|---|---|
| Mean; CL | Inference | |||||
| free carnitine (µmol/L) | placebo | 39.5 ± 3.7 | 10 ± 11% | 8 ± 6% | 13%; ±5.8% | moderate † |
| l-carnitine | 41.1 ± 6.4 | 22 ± 9% | 22 ± 8% | |||
| CRP (mg/L) | placebo | 1.8 ± 0.8 | −6 ± 15% | −4.7 ± 15% | 21%; ±37% | unclear |
| l-carnitine | 2.6 ± 1.1 | 8 ± 68% | 16 ± 65% | |||
| IL-6 (ng/L) | placebo | 1.8 ± 0.7 | −10 ± 23% | −13 ± 20% | 4.9%; ±22% | trivial * |
| l-carnitine | 2.2 ± 1.1 | −11 ± 42% | −8.2 ± 32% | |||
| TNF (ng/L) | placebo | 0.56 ± 0.26 | 14 ± 70% | 12 ± 68% | 9.0%; ±50% | trivial * |
| l-carnitine | 0.58 ± 0.32 | 24 ± 82% | 28 ± 38% | |||
| IGF-1 (µg/L) | placebo | 78 ± 19 | −10 ± 12% | −10 ± 13% | 1.8%; ±16% | trivial * |
| l-carnitine | 69 ± 15 | −6 ± 28% | −8 ± 28% | |||
| Variables | Group | Baseline Mean ± SD | Observed Change Mean ± SD | Adjusted Change a Mean ± SD | Adjusted Effect b | |
|---|---|---|---|---|---|---|
| Mean; CL | Inference | |||||
| TW extension (J/kg) | placebo | 76 ± 15 | 7.4 ± 26% | 5.7 ± 8.3% | 5.6%; ±7.1% | trivial * |
| l-carnitine | 78 ± 11 | 11 ± 13% | 12 ± 9.5% | |||
| TW flexion (J/kg) | placebo | 43 ± 17 | 9.4 ± 40% | 13 ± 13% | −2.9%; ±13% | trivial * |
| l-carnitine | 36 ± 9 | 14 ± 34% | 9.7 ± 23% | |||
| APT extension (Nm/kg) | placebo | 12.0 ± 2.6 | 4.7 ± 20% | 1.9 ± 9.7% | 3.0%; ±9.0% | unclear |
| l-carnitine | 12.8 ± 2.3 | 2.7 ± 18% | 5.0 ± 13% | |||
| APT flexion (Nm/kg) | placebo | 6.8 ± 2.6 | 4.9 ± 26% | 7.0 ± 7.8% | −4.2%; ±9.0% | trivial * |
| l-carnitine | 5.9 ± 1.1 | 5.0 ± 22% | 2.6 ± 16% | |||
| AP extension (W/kg) | placebo | 8.3 ± 2.0 | 6.3 ± 25% | 3.9 ± 6.2% | 1.4%; ±6.8% | trivial † |
| l-carnitine | 8.6 ± 1.4 | 3.3 ± 18% | 5.4 ± 11% | |||
| AP flexion (W/kg) | placebo | 4.4 ± 1.7 | 10 ± 40% | 14 ± 12% | −7.1%; ±9.7% | trivial † |
| l-carnitine | 3.9 ± 1.4 | 12 ± 83% | 5.8 ± 17% | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawicka, A.K.; Hartmane, D.; Lipinska, P.; Wojtowicz, E.; Lysiak-Szydlowska, W.; Olek, R.A. l-Carnitine Supplementation in Older Women. A Pilot Study on Aging Skeletal Muscle Mass and Function. Nutrients 2018, 10, 255. https://doi.org/10.3390/nu10020255
Sawicka AK, Hartmane D, Lipinska P, Wojtowicz E, Lysiak-Szydlowska W, Olek RA. l-Carnitine Supplementation in Older Women. A Pilot Study on Aging Skeletal Muscle Mass and Function. Nutrients. 2018; 10(2):255. https://doi.org/10.3390/nu10020255
Chicago/Turabian StyleSawicka, Angelika K., Dace Hartmane, Patrycja Lipinska, Ewa Wojtowicz, Wieslawa Lysiak-Szydlowska, and Robert A. Olek. 2018. "l-Carnitine Supplementation in Older Women. A Pilot Study on Aging Skeletal Muscle Mass and Function" Nutrients 10, no. 2: 255. https://doi.org/10.3390/nu10020255
APA StyleSawicka, A. K., Hartmane, D., Lipinska, P., Wojtowicz, E., Lysiak-Szydlowska, W., & Olek, R. A. (2018). l-Carnitine Supplementation in Older Women. A Pilot Study on Aging Skeletal Muscle Mass and Function. Nutrients, 10(2), 255. https://doi.org/10.3390/nu10020255





