The Nordic Prudent Diet Reduces Risk of Cognitive Decline in the Swedish Older Adults: A Population-Based Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Dementia Diagnosis and Cognitive Function
2.4. Dietary Assessment
2.5. Nordic Prudent Dietary Pattern
2.6. Scoring Dietary Indices
2.7. Statistical Analyses
3. Results
3.1. Associations between Individual Food Items and Changes in MMSE
3.2. Characteristics of the Study Population by Adherence Levels to Different Dietary Patterns
3.3. Association between Dietary Index and Changes in MMSE
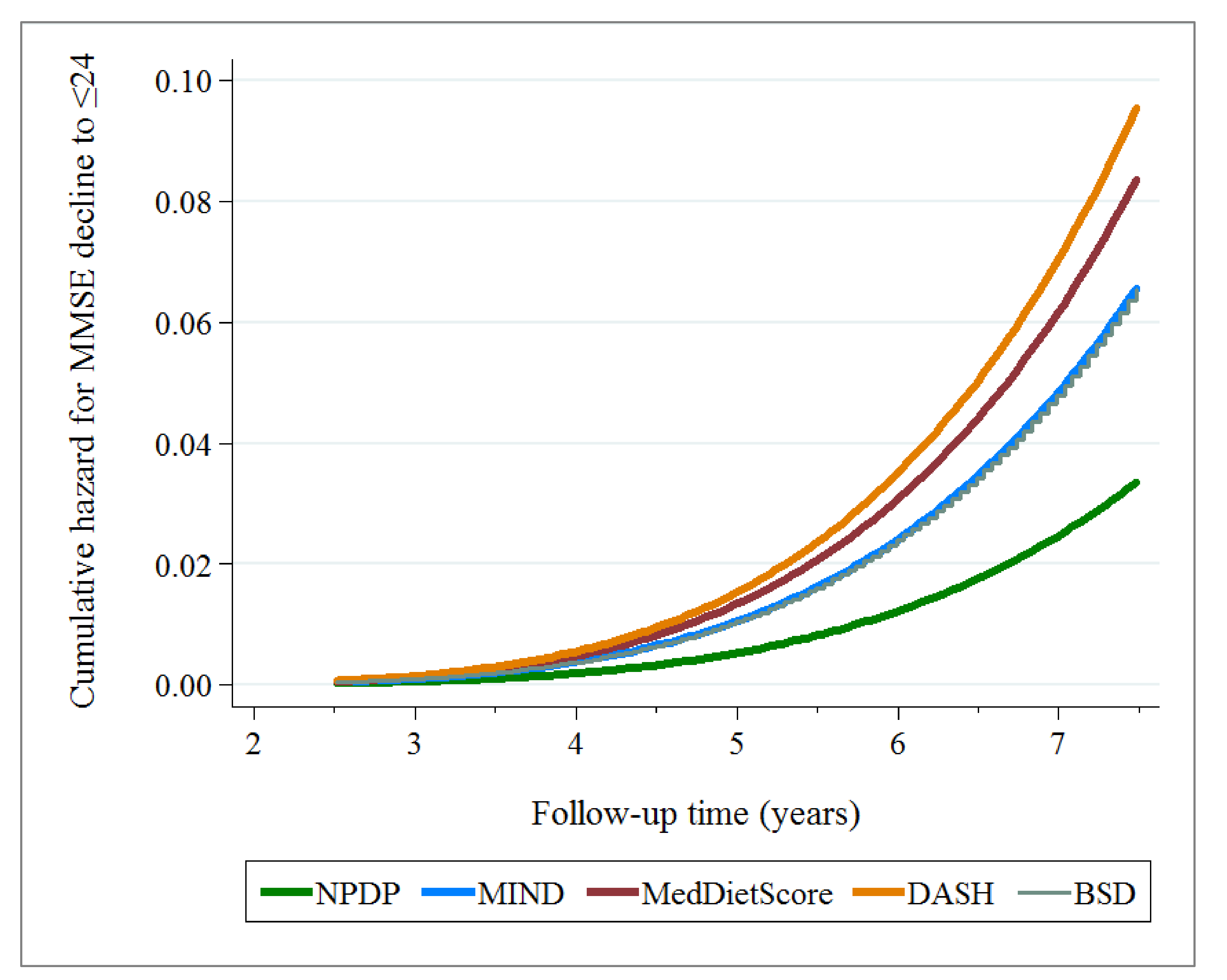
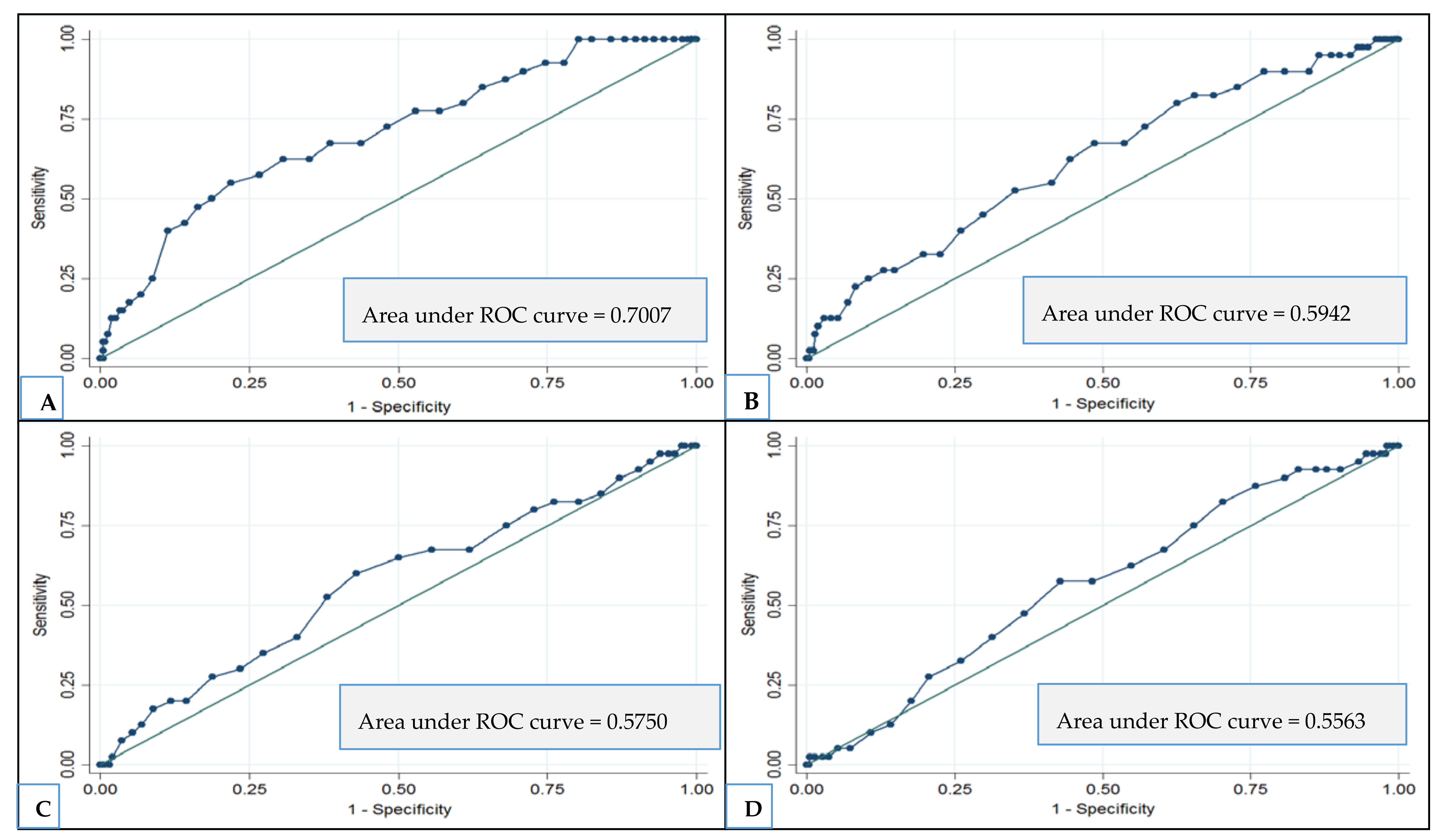
3.4. Association between Dietary Index and MMSE Decline to ≤24
3.5. Supplementary Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. The Epidemiology and Impact of Dementia: Current State and Future Trends. 2015. Available online: http://www.who.int/mental_health/neurology/dementia/dementia_thematicbrief_epidemiology.pdf (accessed on 30 May 2016).
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.J.; Blumenthal, J.A.; Babyak, M.A. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension 2010, 55, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C.; Wang, Y. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimer’s Dement 2015, 11, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Kanerva, N.; Kaartinen, N.E.; Schwab, U. The Baltic Sea Diet Score: A tool for assessing healthy eating in Nordic countries. Public Health Nutr. 2014, 17, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Mannikko, R.; Komulainen, P.; Schwab, U. The Nordic diet and cognition—The DR’s EXTRA Study. Br. J. Nutr. 2015, 114, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.; Bryan, J.; Murphy, K. Is the Mediterranean diet a feasible approach to preserving cognitive function and reducing risk of dementia for older adults in Western countries? New insights and future directions. Ageing Res. Rev. 2016, 25, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Safouris, A.; Tsivgoulis, G.; Sergentanis, T.N.; Psaltopoulou, T. Mediterranean Diet and Risk of Dementia. Curr. Alzheimer Res. 2015, 12, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Shakersain, B.; Santoni, G.; Larsson, S.C.; Fratiglioni, L.; Xu, W.L. Prudent diet may attenuate the adverse effects of Western diet on cognitive decline. Alzheimer’s Dement. 2016, 12, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Rydwik, E.; Welmer, A.K.; Kareholt, I. Adherence to physical exercise recommendations in people over 65—The SNAC-Kungsholmen study. Eur. J. Public Health 2013, 23, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T.; et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013, 36, S67–S74. [Google Scholar]
- Xu, W.L.; Pedersen, N.L.; Keller, L.; Kalpouzos, G.; Wang, H.X.; Graff, C.; Winblad, B.; Bäckman, L.; Fratiglioni, L. HHEX_23 AA Genotype Exacerbates Effect of Diabetes on Dementia and Alzheimer Disease: A Population-Based Longitudinal Study. PLoS Med. 2015, 12, e1001853. [Google Scholar] [CrossRef] [PubMed]
- Goodall, I. HbA1c standardisation destination—Global IFCC Standardisation. How, why, where and when—A tortuous pathway from kit manufacturers, via inter-laboratory lyophilized and whole blood comparisons to designated national comparison schemes. Clin. Biochem. Rev. 2005, 26, 5–19. [Google Scholar] [PubMed]
- Expert Panel on Detection, E. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar]
- Fratiglioni, L.; Viitanen, M.; Backman, L. Occurrence of dementia in advanced age: The study design of the Kungsholmen Project. Neuroepidemiology 1992, 11, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Lazek, M.D.; Howieson, D.B.; Bigler, E.D.; Tranel, D. Neuropsychological Assessment, 5th ed.; Oxford University Press: New York, NY, USA, 2012; Available online: https://books.google.se/books?hl=sv&lr=&id=FroDVkVKA2EC&oi=fnd&pg=PA3&dq=Lezak+MD+Neuropsychological+Assessment.+5th+e&ots=q6VeUQPnaU&sig=nqVLexXG-zm4sGNTs-hkFYzPwDM&redir_esc=y#v=onepage&q&f=false (accessed on 26 October 2017).
- Johansson, I.; Hallmans, G.; Wikman, A. Validation and calibration of food-frequency questionnaire measurements in the Northern Sweden Health and Disease cohort. Public Health Nutr. 2002, 5, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Pitsavos, C.; Arvaniti, F.; Stefanadis, C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev. Med. 2007, 44, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Moller, E.; Galeone, C.; Andersson, T.M. Mediterranean Diet Score and prostate cancer risk in a Swedish population-based case-control study. J. Nutr. Sci. 2013, 2, e15. [Google Scholar] [CrossRef] [PubMed]
- Livsmedelsverket (National Food Agancy). Swedish Dietary Guidelines-Risk and Benefit Management Report. 2015. Available online: https://www.livsmedelsverket.se/globalassets/rapporter/2015/rapp-hanteringsrapport-engelska-omslag--inlaga--bilagor-eng-version.pdf (accessed on 31 May 2016).
- Henry, C.J. Basal metabolic rate studies in humans: Measurement and development of new equations. Public Health Nutr. 2005, 8, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Campos, H. Dietary therapy in hypertension. N. Engl. J. Med. 2010, 362, 2102–2112. [Google Scholar] [CrossRef] [PubMed]
- Van de Rest, O.; Berendsen, A.A.; Haveman-Nies, A.; de Groot, L.C. Dietary patterns, cognitive decline, and dementia: A systematic review. Adv. Nutr. 2015, 6, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Olsson, E.; Karlstrom, B.; Kilander, L. Dietary patterns and cognitive dysfunction in a 12-year follow-up study of 70 year old men. J. Alzheimer’s Dis. 2015, 43, 109–119. [Google Scholar] [CrossRef]
- Knight, A.; Bryan, J.; Murphy, K. The Mediterranean diet and age-related cognitive functioning: A systematic review of study findings and neuropsychological assessment methodology. Nutr. Neurosci. 2017, 20, 449–468. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D. Health measurement scales: Methodological issues. Open Cardiovasc. Med. J. 2009, 3, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: A Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pinilla, F.; Tyagi, E. Diet and cognition: Interplay between cell metabolism and neuronal plasticity. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Gillette-Guyonnet, S.; Secher, M.; Vellas, B. Nutrition and neurodegeneration: Epidemiological evidence and challenges for future research. Br. J. Clin. Pharmacol. 2013, 75, 738–755. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Antoine, J.M.; Benton, D. Impact of postprandial glycaemia on health and prevention of disease. Obes. Rev. 2012, 13, 923–984. [Google Scholar] [CrossRef] [PubMed]
- Masento, N.A.; Golightly, M.; Field, D.T. Effects of hydration status on cognitive performance and mood. Br. J. Nutr. 2014, 111, 1841–1852. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, E.M. Experience of dietary assessment and validation from three Swedish studies in the elderly. Eur. J. Clin. Nutr. 2009, 63, S64–S68. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Craig, L.C.; Aucott, L.S.; Milne, A.C.; McNeill, G. Repeatability and validity of a food frequency questionnaire in free-living older people in relation to cognitive function. J. Nutr. Health Aging 2008, 12, 735–741. [Google Scholar] [PubMed]
| Dietary Items | Model 1—Group Level | Model 2—Subgroup Items |
|---|---|---|
| β (95% CI) ‡ | β (95% CI) ‡ | |
| Vegetables (total) | 0.014 (−0.0002–0.028) | |
| Non-root vegetables | 0.039 (0.021–0.056) | |
| Root vegetables | −0.071 (−0.112–−0.030) | |
| Fruits (total) | 0.006 (−0.013–0.024) | |
| Berries | −0.109 (−0.222–0.004) | |
| Apples/pears/peaches | 0.051 (0.006–0.096) | |
| Oranges/tangerines/grapefruits | −0.015 (−0.068–0.038) | |
| Bananas | −0.010 (−0.065–0.045) | |
| Grains/cereals (total) | −0.021 (−0.035–−0.007) | |
| Whole grains | −0.015 (−0.036–0.007) | |
| Refined grains/cereals | −0.037 (−0.058–−0.016) | |
| Pasta/rice | 0.197 (0.089–0.306) | |
| Legumes/beans | 0.070 (−0.085–0.225) | |
| Red/processed meat | −0.015 (−0.045–0.015) | |
| Poultry | 0.456 (0.226–0.686) | |
| Fish | 0.118 (0.013–0.223) | |
| Dairy products (total) | −0.016 (−0.031–−0.001) | |
| Low-fat dairy products | −0.004 (−0.024–0.016) | |
| Medium-fat dairy products | −0.012 (−0.036–0.013) | |
| High-fat dairy products | −0.056 (−0.093–−0.018) | |
| Milk | −0.041 (−0.066–−0.016) | |
| Low-fat milk | −0.029 (−0.062–0.005) | |
| Medium-fat milk | −0.030 (−0.067–0.005) | |
| High-fat milk | −0.105 (−0.158–−0.051) | |
| Cheese | −0.0004 (−0.028–0.028) | |
| Low-fat cheese | 0.020 (−0.019–0.059) | |
| Medium-fat cheese | 0.004 (−0.035–0.043) | |
| High-fat cheese | −0.107 (−0.208–−0.006) | |
| Yoghurt | 0.018 (−0.031–0.067) | |
| Low-fat yoghurt | −0.010 (−0.075–0.054) | |
| Medium/high-fat yoghurt | 0.038 (−0.052–0.127) | |
| Cream | 0.019 (−0.094–0.132) | 0.040 (−0.074–0.154) |
| Ice cream | 0.025 (−0.145–0.195) | |
| Butter/margarine | −0.018 (−0.032–−0.004) | |
| Butter | −0.018 (−0.041–0.004) | |
| Margarine | −0.016 (−0.032–−0.0005) | |
| Vegetable oil | 0.068 (0.034–0.103) | 0.067 (0.033–0.102) |
| Sugar/sweets/pastries | −0.027 (−0.047–−0.008) | |
| Fast/fried food | −0.042 (−0.169–0.084) | |
| Wine | 0.123 (0.054–0.191) | |
| Red wine | 0.102 (0.014–0.190) | |
| White wine | 0.172 (0.011–0.333) | |
| Beer | 0.005 (−0.046–0.055) | |
| Low-alcohol beer | −0.022 (−0.094–0.049) | |
| Medium-strong beer | 0.046 (−0.043–0.135) | |
| Strong beer | 0.006 (−0.163–0.175) | |
| Spirits | −0.055 (−0.183–0.074) | −0.056 (−0.185–0.073) |
| Tea | 0.055 (0.024–0.085) | |
| Coffee | 0.017 (−0.007–0.041) | |
| Carbonated drinks | 0.028 (−0.077–0.133) | |
| Fruit juice | −0.060 (−0.097–−0.022) | |
| Water (plain/mineral) | 0.018 (0.001–0.035) |
| Characteristics | Dietary Pattern Index Scores (Lowest vs. Highest Tertiles) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NPDP | MIND | MedDietScore | DASH | BSD | ||||||
| Low (n = 720) | High (n = 724) | Low (n = 799) | High (n = 720) | Low (n = 774) | High (n = 733) | Low (n = 812) | High (n = 655) | Low (n = 805) | High (n = 695) | |
| Age (median), years | 72.7 (66.3–81.3) | 66.2 (60.5–72.3) * | 72.2 (62.6–78.4) | 66.5 (60.6–76.5) * | 72.2 (64.6–78.4) | 66.5 (60.7–78.2) * | 66.7 (60.7–78.3) | 67.5 (60.9–78.2) | 66.8 (60.8–78.4) | 66.8 (60.8–78.2) |
| Sex, women | 437 (60.6) | 442 (61.0) | 486 (60.8) | 438 (60.8) | 475 (61.3) | 447 (61.0) | 490 (60.3) | 399 (61.0) | 491 (61.0) | 420 (60.4) |
| Education | ||||||||||
| University | 193 (26.8) | 337 (46.6) * | 225 (28.2) | 313 (43.5) * | 224 (29.0) | 304 (41.6) * | 279 (34.4) | 258 (39.4) * | 251 (31.2) | 290 (41.7) * |
| High school | 330 (45.8) | 300 (41.4) | 369 (46.1) | 308 (42.7) | 360 (46.5) | 310 (42.2) | 346 (42.5) | 287 (43.8) | 367 (45.5) | 291 (41.9) |
| Elementary school | 197 (27.4) | 87 (12.0) * | 205 (25.7) | 99 (13.8) * | 190 (24.5) | 119 (16.2) * | 187 (23.1) | 110 (16.8) * | 187 (23.3) | 114 (16.4) * |
| Civil status | ||||||||||
| Married | 315 (43.7) | 417 (57.6) * | 374 (46.8) | 390 (54.2) * | 351 (45.3) | 392 (53.4) * | 396 (48.8) | 351 (53.6) | 396 (49.1) | 352 (50.7) |
| Single | 124 (17.3) | 110 (15.1) | 146 (18.3) | 104 (14.4) | 143 (18.5) | 106 (14.5) | 139 (17.1) | 94 (14.3) | 145 (18.0) | 110 (15.8) |
| Widow/divorced | 281 (39.0) | 197 (27.3) * | 279 (34.9) | 226 (31.4) | 280 (36.2) | 235 (32.1) | 277 (34.1) | 210 (32.1) | 264 (32.9) | 233 (33.5) |
| Smoking | ||||||||||
| Never | 343 (47.7) | 291 (40.1) * | 330 (41.2) | 322 (44.7) | 337 (43.5) | 326 (44.4) | 344 (42.4) | 294 (44.9) | 335 (41.6) | 313 (45.0) |
| Former | 234 (32.5) | 347 (47.9) * | 307 (38.5) | 312 (43.4) | 279 (36.0) | 335 (45.7) | 301 (37.0) | 291 (44.4) | 306 (38.0) | 316 (45.5) |
| Current | 143 (19.8) | 87 (12.0) * | 162 (20.3) | 86 (11.9) * | 158 (20.5) | 72 (9.9) * | 167 (20.6) | 70 (10.7) * | 164 (20.4) | 66 (9.5) * |
| Physical activity | ||||||||||
| Inadequate | 194 (26.9) | 117 (16.1) * | 212 (26.5) | 110 (15.3) * | 200 (25.9) | 113 (15.5) * | 220 (27.1) | 91 (13.9) * | 209 (25.9) | 104 (15.0) * |
| Health-enhancing | 401 (55.7) | 365 (50.5) | 438 (54.8) | 360 (50.0) | 417 (53.9) | 367 (50.0) | 431 (53.1) | 349 (53.3) | 444 (55.2) | 352 (50.6) |
| Fitness-enhancing | 125 (17.4) | 242 (33.4) * | 149 (18.7) | 250 (34.7) * | 157 (20.2) | 253 (34.5) * | 161 (19.8) | 215 (32.8) * | 152 (18.9) | 239 (34.4) * |
| BMI (median), kg/m2 | 25.0 (22.9–27.7) | 26.2 (23.8–28.7) * | 25.2 (23.0–28.0) | 26.0 (23.6–28.5) * | 25.2 (23.1–27.8) | 25.7 (23.5–28.4) * | 25.4 (23.1–28.2) | 25.7 (23.5–28.1) | 25.3 (23.1–28.0) | 25.8 (23.7–28.4) |
| MMSE (median) | 29 (29–30) | 29 (29–30) | 29 (29–30) | 29 (29–30) | 29 (29–30) | 29 (29–30) | 29 (29–30) | 29 (29–30) | 29 (29–30) | 29 (29–30) |
| Vascular disorders ** | 646 (89.8) | 614 (84.8)* | 706 (88.4) | 618 (85.8) | 676 (87.3) | 638 (87.1) | 700 (86.2) | 572 (87.3) | 699 (86.9) | 612 (88.0) |
| Diabetes | 240 (33.4) | 219 (30.2) | 261 (32.6) | 228 (31.6) | 246 (31.8) | 231 (31.5) | 269 (33.1) | 208 (31.8) | 267 (33.2) | 218 (31.3) |
| Cancer | 55 (7.7) | 47 (6.5) | 55 (6.9) | 47 (6.5) | 53 (6.8) | 52 (7.0) | 57 (7.0) | 50 (7.7) | 62 (7.7) | 56 (8.0) |
| Depression | 43 (6.0) | 32 (4.4) | 55 (6.9) | 30 (4.2) | 47 (6.0) | 36 (4.9) | 52 (6.4) | 33 (5.0) | 50 (6.2) | 39 (5.6) |
| Any APOE ɛ4 | 217 (30.2) | 222 (30.6) | 234 (29.2) | 198 (27.6) | 223 (28.9) | 218 (29.8) | 234 (28.8) | 200 (30.5) | 225 (28.0) | 216 (31.1) |
| Dietary supplement use | 211 (29.3) | 197 (27.1) | 218 (27.3) | 211 (29.2) | 214 (27.6) | 231 (31.5) * | 204 (25.1) | 205 (31.3) | 206 (25.6) | 214 (30.8) |
| Dietary Index | Continuous Score | Moderate Adherence * | High Adherence * | |||
|---|---|---|---|---|---|---|
| β † (95% CI) | p | β † (95% CI) | p | β † (95% CI) | p | |
| NPDP | 0.011 (0.008–0.013) | <0.001 | 0.139 (0.077–0.201) | <0.001 | 0.238 (0.175–0.300) | <0.001 |
| MIND | 0.006 (0.003–0.009) | <0.001 | 0.075 (0.012–0.138) | 0.019 | 0.126 (0.064–0.188) | <0.001 |
| MedDietScore | 0.006 (0.002–0.009) | 0.002 | 0.063 (−0.002–0.129) | 0.057 | 0.099 (0.036–0.163) | 0.002 |
| DASH | 0.001 (−0.002–0.004) | 0.568 | 0.015 (−0.056–0.086) | 0.673 | 0.024 (−0.042–0.091) | 0.472 |
| BSD | 0.004 (0.000–0.008) | 0.049 | 0.018 (−0.060–0.097) | 0.645 | 0.053 (−0.011–0.117) | 0.103 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakersain, B.; Rizzuto, D.; Larsson, S.C.; Faxén-Irving, G.; Fratiglioni, L.; Xu, W.-L. The Nordic Prudent Diet Reduces Risk of Cognitive Decline in the Swedish Older Adults: A Population-Based Cohort Study. Nutrients 2018, 10, 229. https://doi.org/10.3390/nu10020229
Shakersain B, Rizzuto D, Larsson SC, Faxén-Irving G, Fratiglioni L, Xu W-L. The Nordic Prudent Diet Reduces Risk of Cognitive Decline in the Swedish Older Adults: A Population-Based Cohort Study. Nutrients. 2018; 10(2):229. https://doi.org/10.3390/nu10020229
Chicago/Turabian StyleShakersain, Behnaz, Debora Rizzuto, Susanna C. Larsson, Gerd Faxén-Irving, Laura Fratiglioni, and Wei-Li Xu. 2018. "The Nordic Prudent Diet Reduces Risk of Cognitive Decline in the Swedish Older Adults: A Population-Based Cohort Study" Nutrients 10, no. 2: 229. https://doi.org/10.3390/nu10020229
APA StyleShakersain, B., Rizzuto, D., Larsson, S. C., Faxén-Irving, G., Fratiglioni, L., & Xu, W.-L. (2018). The Nordic Prudent Diet Reduces Risk of Cognitive Decline in the Swedish Older Adults: A Population-Based Cohort Study. Nutrients, 10(2), 229. https://doi.org/10.3390/nu10020229






