Single Nucleotide Polymorphisms in Taste Receptor Genes Are Associated with Snacking Patterns of Preschool-Aged Children in the Guelph Family Health Study: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. SNP Genotyping
2.3. Snack Frequency, Quantity, Quality and Composition
2.4. Data Analysis
3. Results
3.1. Participant Characteristics and Snacking Patterns
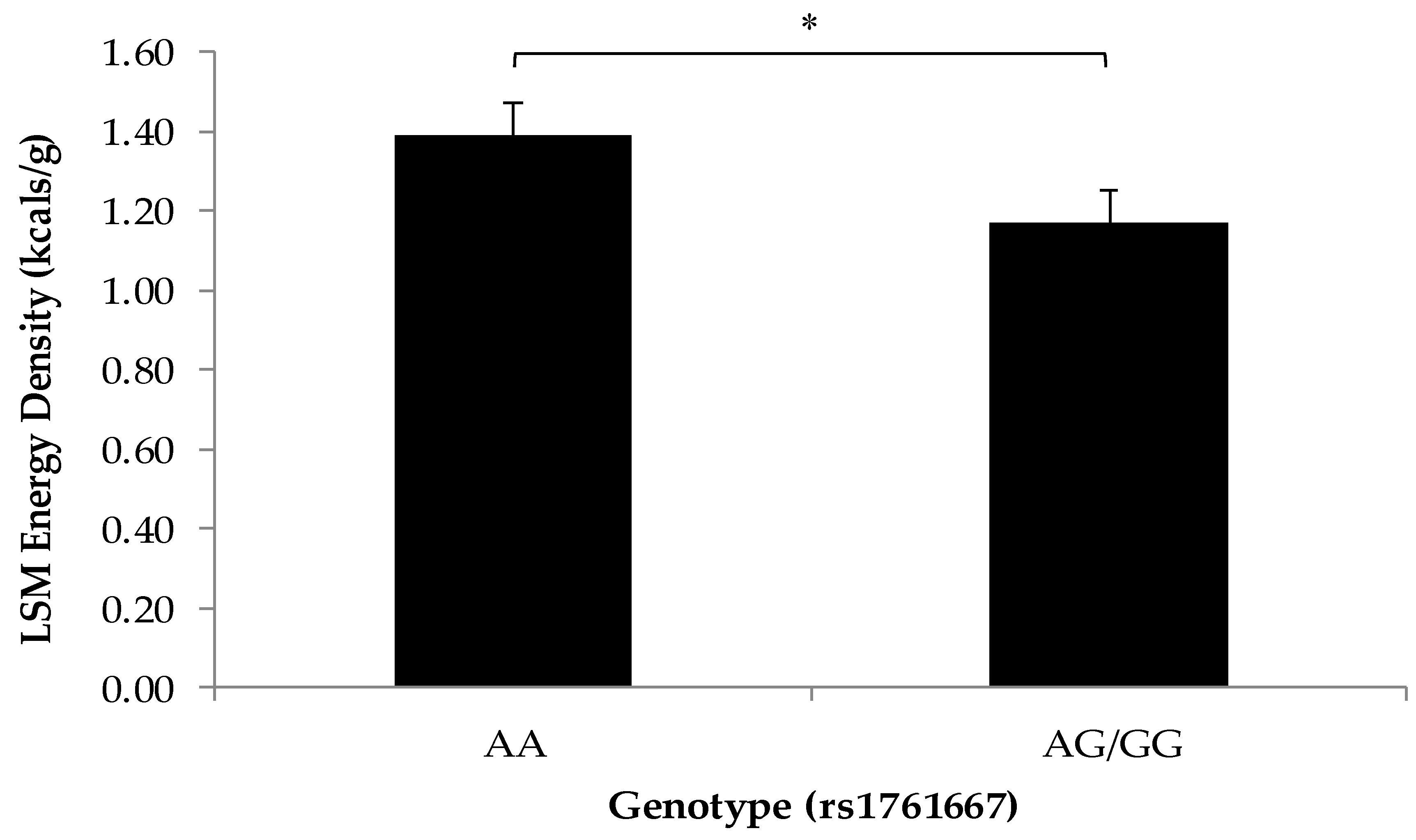
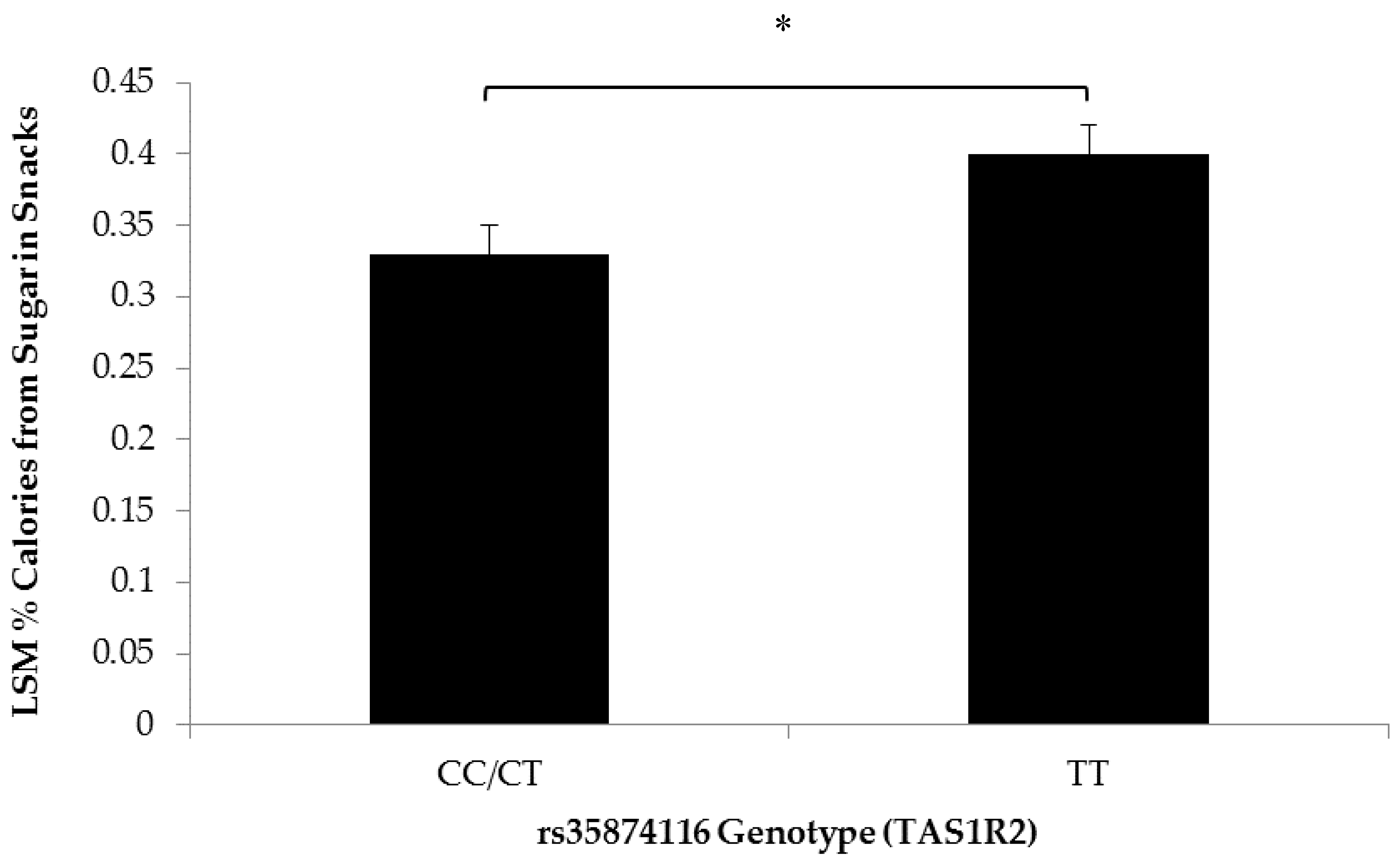
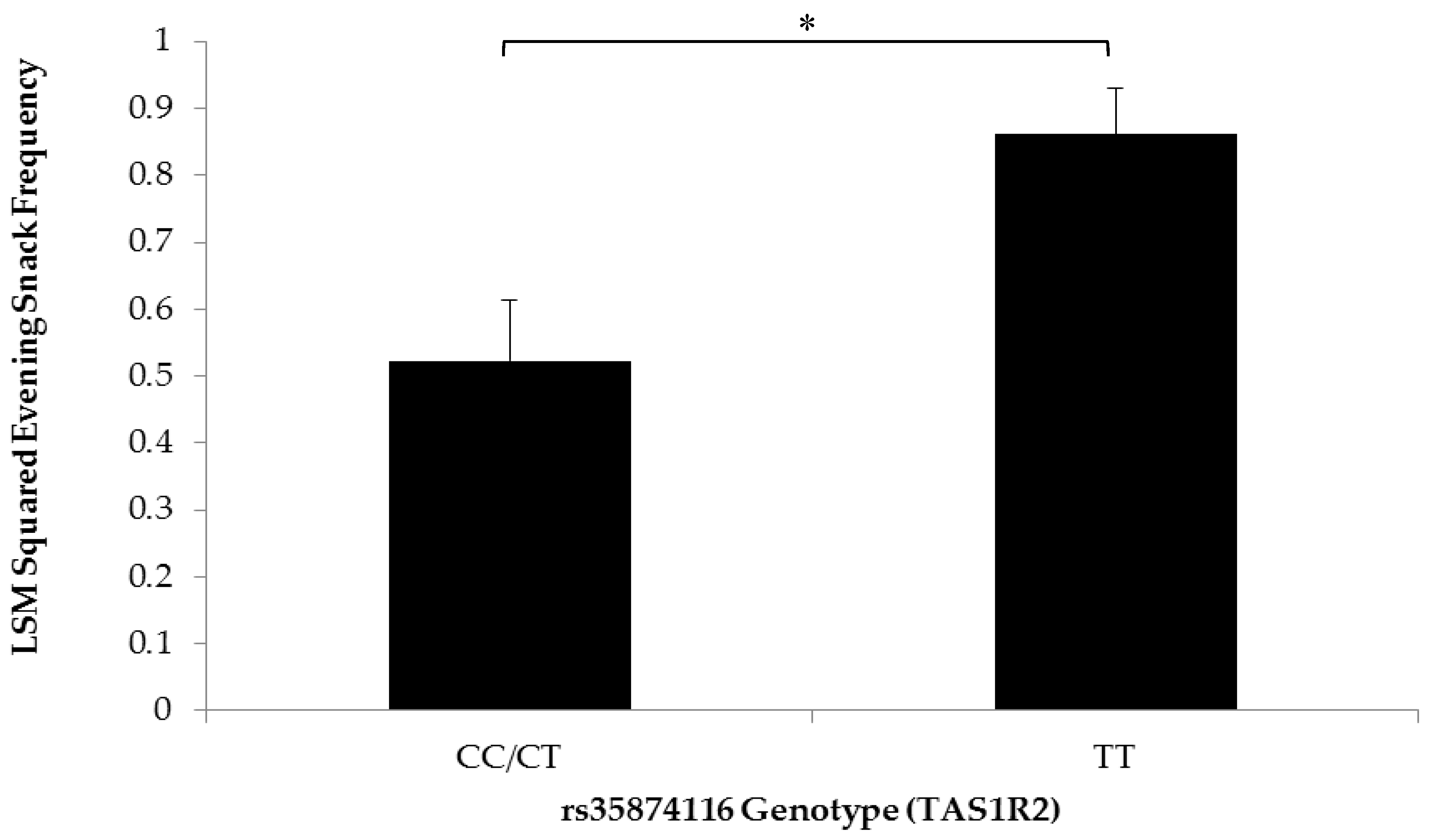
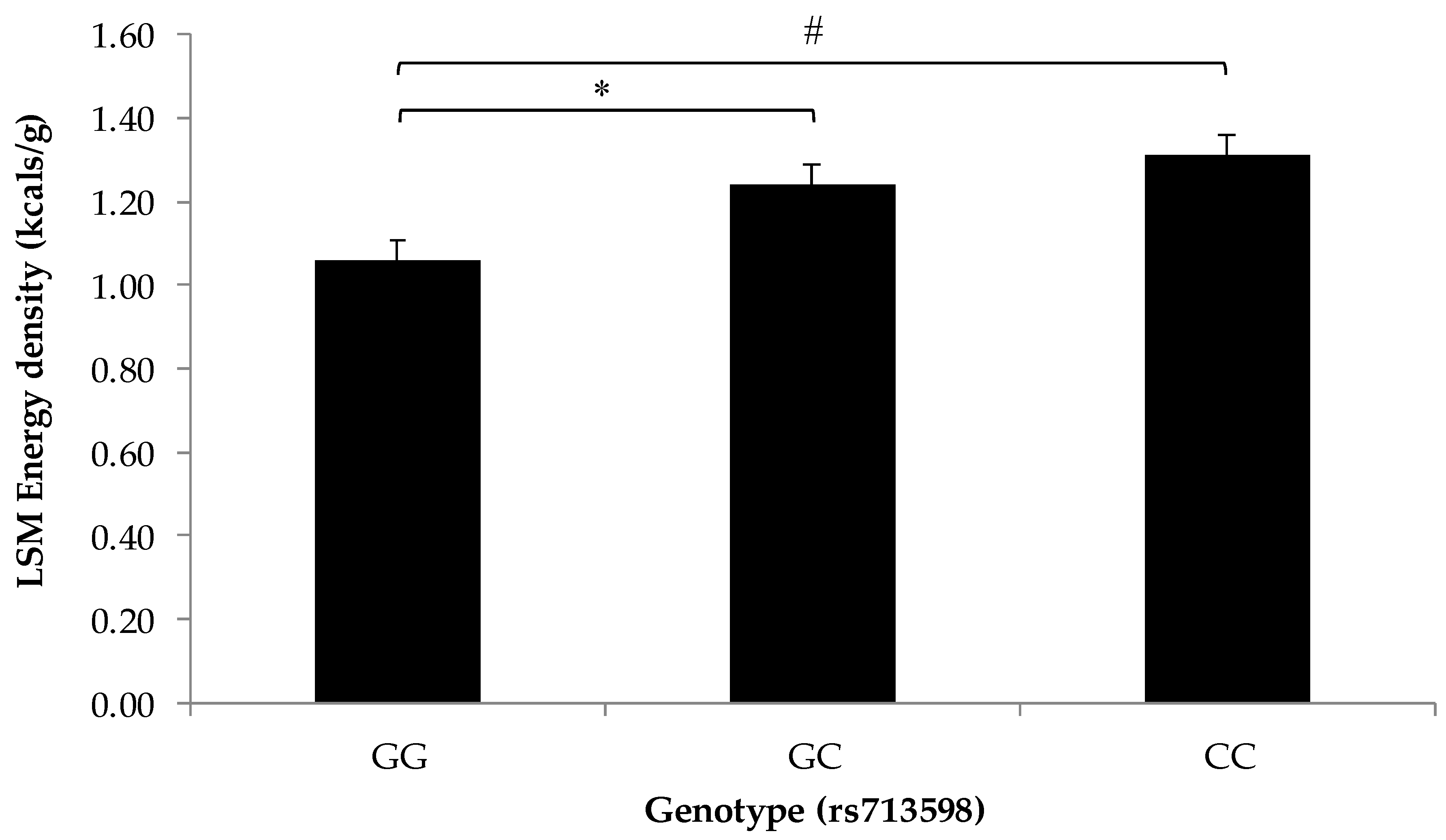
3.2. Association of SNPs at the CD36, T1R2, and T2R38 Genes with Snacking Patterns
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Guideline: Sugar Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Chamoun, E.; Mutch, D.M.; Allen-Vercoe, E.; Buchholz, A.C.; Duncan, A.M.; Spriet, L.L.; Haines, J.; Ma, D.W.L. A review of the associations between single nucleotide polymorphisms in taste receptors, eating behaviors, and health. Crit. Rev. Food Sci. Nutr. 2018, 58, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Kral, T.V.; Rauh, E.M. Eating behaviors of children in the context of their family environment. Physiol. Behav. 2010, 100, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Pepino, M.Y.; Reed, D.R. Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics 2005, 115, e216–e222. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.R.; Xia, M.B. Recent advances in fatty acid perception and genetics. Adv. Nutr. 2015, 6, 353S–360S. [Google Scholar] [CrossRef] [PubMed]
- Pepino, M.Y.; Love-Gregory, L.; Klein, S.; Abumrad, N.A. The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J. Lipid Res. 2012, 53, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.E.; Keast, R.S. Recent fat intake modulates fat taste sensitivity in lean and overweight subjects. Int. J. Obes. 2012, 36, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Staszewski, L.; Xu, H.; Durick, K.; Zoller, M.; Adler, E. Human receptors for sweet and umami taste. Proc. Natl. Acad. Sci. USA 2002, 99, 4692–4696. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, J.; Hoon, M.A.; Ryba, N.J.; Zuker, C.S. The receptors and cells for mammalian taste. Nature 2006, 444, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.R.; McDaniel, A.H. The human sweet tooth. BMC Oral Health 2006, 6, S17. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.R.; Tanaka, T.; McDaniel, A.H. Diverse tastes: Genetics of sweet and bitter perception. Physiol. Behav. 2006, 88, 215–226. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, A.H.; Reed, D.R. Genomics and Proteomics in Nutrition; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- Reed, D.R.; Li, X.; Bachmanov, A.A.; Mascioli, K.; Beauchamp, G.K. Progress in Obesity Research; John Libbey Eurotext Ltd.: London, UK, 2003; Volume 9. [Google Scholar]
- Reed, D.R.; Bachmanov, A.A.; Beauchamp, G.K.; Tordoff, M.G.; Price, R.A. Heritable variation in food preferences and their contribution to obesity. Behav. Genet. 1997, 27, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Drayna, D. Human taste genetics. Annu. Rev. Genom. Hum. Genet. 2005, 6, 217–235. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.K.; Breslin, P.A.; Reed, D.; Drayna, D. Genetics of human taste perception. J. Dent. Res. 2004, 83, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Keskitalo, K.; Tuorila, H.; Spector, T.D.; Cherkas, L.F.; Knaapila, A.; Silventoinen, K.; Perola, M. Same genetic components underlie different measures of sweet taste preference. Am. J. Clin. Nutr. 2007, 86, 1663–1669. [Google Scholar] [PubMed]
- Keskitalo, K.; Knaapila, A.; Kallela, M.; Palotie, A.; Wessman, M.; Sammalisto, S.; Peltonen, L.; Tuorila, H.; Perola, M. Sweet taste preferences are partly genetically determined: Identification of a trait locus on chromosome 16. Am. J. Clin. Nutr. 2007, 86, 55–63. [Google Scholar] [PubMed]
- Keskitalo, K.; Tuorila, H.; Spector, T.D.; Cherkas, L.F.; Knaapila, A.; Kaprio, J.; Silventoinen, K.; Perola, M. The Three-Factor Eating Questionnaire, body mass index, and responses to sweet and salty fatty foods: A twin study of genetic and environmental associations. Am. J. Clin. Nutr. 2008, 88, 263–271. [Google Scholar] [PubMed]
- Eny, K.M.; Wolever, T.M.; Corey, P.N.; El-Sohemy, A. Genetic variation in TAS1R2 (Ile191Val) is associated with consumption of sugars in overweight and obese individuals in 2 distinct populations. Am. J. Clin. Nutr. 2010, 92, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.V.; Agnes, G.; Vitolo, M.R.; Mattevi, V.S.; Campagnolo, P.D.B.; Almeida, S. Evaluation of the association between the TAS1R2 and TAS1R3 variants and food intake and nutritional status in children. Genet. Mol. Biol. 2017, 40, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Weisberger, D. A role of glucose in the production of artificial caries. J. Dent. Res. 1950, 29, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, G.V.; Chng, T.; Eny, K.M.; Nielsen, D.; Wessman, C.; El-Sohemy, A. Association of GLUT2 and TAS1R2 genotypes with risk for dental caries. Caries Res. 2013, 47, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Glendenning, J.I. Is the bitter ejective response always adaptive? Physiol. Behav. 1994, 56, 1217–1227. [Google Scholar] [CrossRef]
- Hladik, C.M.; Simmen, B. Taste percpetion and feeding behavior in nonhuman primates and human populations. Evolut. Anthropol. 1996, 5, 58–71. [Google Scholar] [CrossRef]
- Pawellek, I.; Grote, V.; Theurich, M.; Closa-Monasterolo, R.; Stolarczyk, A.; Verduci, E.; Xhonneux, A.; Koletzko, B. Factors associated with sugar intake and sugar sources in European children from 1 to 8 years of age. Eur. J. Clin. Nutr. 2017, 71, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Hoppu, U.; Laitinen, K.; Jaakkola, J.; Sandell, M. The hTAS2R38 genotype is associated with sugar and candy consumption in preschool boys. J. Hum. Nutr. Diet. 2015, 28, S45–S51. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, G.L.; Daun, H.; Tepper, B.J. Adiposity in middle-aged women is associated with genetic taste blindness to 6-n-propylthiouracil. Obes. Res. 2005, 13, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.K.; Jorgenson, E.; Coon, H.; Leppert, M.; Risch, N.; Drayna, D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science 2003, 299, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Bufe, B.; Breslin, P.A.; Kuhn, C.; Reed, D.R.; Tharp, C.D.; Slack, J.P.; Kim, U.K.; Drayna, D.; Meyerhof, W. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr. Biol. 2005, 15, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Piernas, C.; Popkin, B.M. Trends in snacking among U.S. children. Health Aff. 2010, 29, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Davison, K.K.; Blake, C.E.; Blaine, R.E.; Younginer, N.A.; Orloski, A.; Hamtil, H.A.; Ganter, C.; Bruton, Y.P.; Vaughn, A.E.; Fisher, J.O. Parenting around child snacking: Development of a theoretically-guided, empirically informed conceptual model. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J.M.; Watterworth, J.; Haines, J.; Duncan, A.M.; Mirotta, J.; Ma, D.W.L.; Buchholz, A.C. Guelph Family Health Study Snacking patterns of preschool-aged children: Opportunity for improvement. Can. J. Diet. Pract. Res. 2017, 78, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Duffy, V.B.; Davidson, A.C.; Kidd, J.R.; Kidd, K.K.; Speed, W.C.; Pakstis, A.J.; Reed, D.R.; Snyder, D.J.; Bartoshuk, L.M. Bitter receptor gene (TAS2R38), 6-n-propylthiouracil (PROP) bitterness and alcohol intake. Alcohol. Clin. Exp. Res. 2004, 28, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Duffey, K.J.; Pereira, R.A.; Popkin, B.M. Prevalence and energy intake from snacking in Brazil: Analysis of the first nationwide individual survey. Eur. J. Clin. Nutr. 2013, 67, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.L.; Liang, L.C.; Sakimura, J.; May, D.; van Belle, C.; Breen, C.; Driggin, E.; Tepper, B.J.; Lanzano, P.C.; Deng, L.; et al. Common variants in the CD36 gene are associated with oral fat perception, fat preferences, and obesity in African Americans. Obesity 2012, 20, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C. Dietary fats and coronary heart disease. J. Intern. Med. 2012, 272, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Fushan, A.A.; Simons, C.T.; Slack, J.P.; Manichaikul, A.; Drayna, D. Allelic polymorphism within the TAS1R3 promoter is associated with human taste sensitivity to sucrose. Curr. Biol. 2009, 19, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Lustig, R.H.; Schmidt, L.A.; Brindis, C.D. Public health: The toxic truth about sugar. Nature 2012, 482, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.L.; Tepper, B.J. Inherited taste sensitivity to 6-n-propylthiouracil in diet and body weight in children. Obes. Res. 2004, 12, 904–912. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.A.; Feeney, E.L.; Scannell, A.G.; Markey, A.; Gibney, E.R. Bitter taste perception and dietary intake patterns in irish children. J. Nutrigenet. Nutrigenom. 2013, 6, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Feeney, E.L.; O’Brien, S.A.; Scannell, A.G.; Markey, A.; Gibney, E.R. Suprathreshold measures of taste perception in children—Association with dietary quality and body weight. Appetite 2017, 113, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.E.; Bartoshuk, L.M.; Kidd, J.R.; Duffy, V.B. Supertasting and PROP bitterness depends on more than the TAS2R38 gene. Chem. Senses 2008, 33, 255–265. [Google Scholar] [CrossRef] [PubMed]
| Sex | |
| Male | n = 22, 47% |
| Female | n = 25, 53% |
| Age (years, mean (SD)) | 3.47 (1.15) |
| BMI Z-Score (mean (SD)) | 0.43 (0.99) |
| Ethnicity (%) | |
| Caucasian | 87.5 |
| Other | 12.5 |
| Parent annual household income (Canadian $), n = 37 * (%) | |
| ≤$49,999 | 18.9 |
| $50,000–$79,999 | 21.6 |
| $80,000–$99,999 | 21.6 |
| ≥$100,000 | 37.8 |
| Snacking Measure | Mean (SD) (n = 47) |
|---|---|
| Total daily energy intake (kcals/day) | 1407 (347) |
| Total energy intake from snacks (kcals/day) | 456 (213) |
| % Daily energy from snacking | 32 (14) |
| Total energy density of snacks (kcals/g) | 1.13 (0.43) |
| Total snack consumption frequency (%) | 78 (39) * |
| Morning (%) | 100 (33) * |
| Afternoon (%) | 100 (33) * |
| Evening (%) | 67 (67) * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamoun, E.; Hutchinson, J.M.; Krystia, O.; Mirotta, J.A.; Mutch, D.M.; Buchholz, A.C.; Duncan, A.M.; Darlington, G.; Haines, J.; Ma, D.W.L.; et al. Single Nucleotide Polymorphisms in Taste Receptor Genes Are Associated with Snacking Patterns of Preschool-Aged Children in the Guelph Family Health Study: A Pilot Study. Nutrients 2018, 10, 153. https://doi.org/10.3390/nu10020153
Chamoun E, Hutchinson JM, Krystia O, Mirotta JA, Mutch DM, Buchholz AC, Duncan AM, Darlington G, Haines J, Ma DWL, et al. Single Nucleotide Polymorphisms in Taste Receptor Genes Are Associated with Snacking Patterns of Preschool-Aged Children in the Guelph Family Health Study: A Pilot Study. Nutrients. 2018; 10(2):153. https://doi.org/10.3390/nu10020153
Chicago/Turabian StyleChamoun, Elie, Joy M. Hutchinson, Owen Krystia, Julia A. Mirotta, David M. Mutch, Andrea C. Buchholz, Alison M. Duncan, Gerarda Darlington, Jess Haines, David W. L. Ma, and et al. 2018. "Single Nucleotide Polymorphisms in Taste Receptor Genes Are Associated with Snacking Patterns of Preschool-Aged Children in the Guelph Family Health Study: A Pilot Study" Nutrients 10, no. 2: 153. https://doi.org/10.3390/nu10020153
APA StyleChamoun, E., Hutchinson, J. M., Krystia, O., Mirotta, J. A., Mutch, D. M., Buchholz, A. C., Duncan, A. M., Darlington, G., Haines, J., Ma, D. W. L., & Guelph Family Health Study. (2018). Single Nucleotide Polymorphisms in Taste Receptor Genes Are Associated with Snacking Patterns of Preschool-Aged Children in the Guelph Family Health Study: A Pilot Study. Nutrients, 10(2), 153. https://doi.org/10.3390/nu10020153





